Existence of Interhemispheric Inhibition between Foot Sections of Human Primary Motor Cortices: Evidence from Negative Blood Oxygenation-Level Dependent Signal
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. fMRI Task
2.2.1. Active Task
2.2.2. Vibration Task
2.2.3. Trunk Task for Mapping Trunk Sections
2.2.4. Bimanual Task for Mapping Hand Sections
2.2.5. Tongue Task for Mapping Face Sections
2.3. MRI Data Acquisition
2.4. fMRI Data Preprocessing
2.5. Single-Subject Analysis
2.6. Group Analysis for Foot Tasks
2.7. Region-of-Interest (ROI) Analysis of Foot Sections of M1s
2.8. Mapping of the Trunk, Hand, and Face Sections as Well as Their ROI Analysis
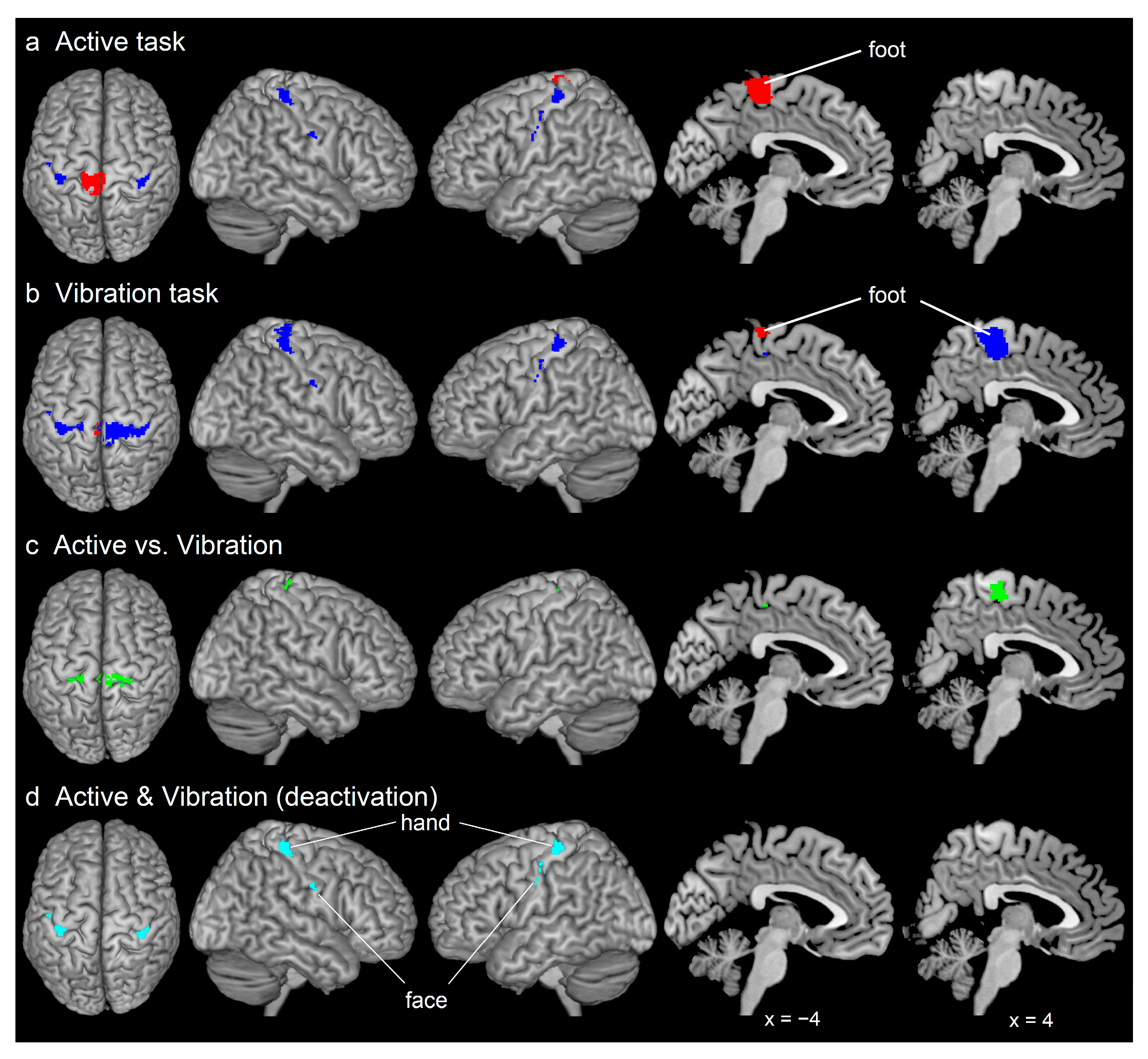
3. Results
3.1. Brain Deactivation and Activation during Both Foot Tasks
3.2. Between-Task Differences and Similarities in the Deactivation
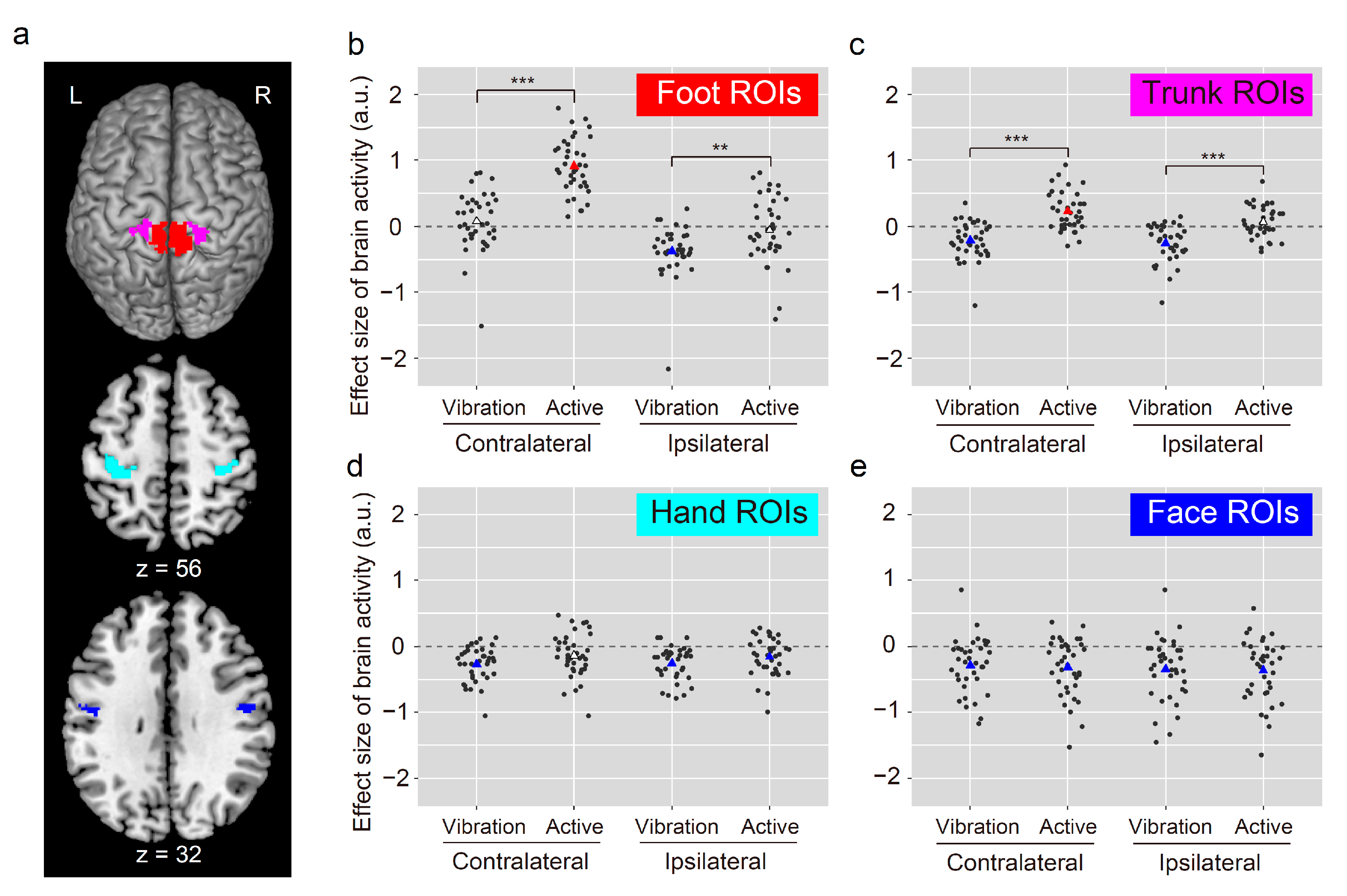
3.3. ROI Analysis
4. Discussion
4.1. Negative BOLD Phenomenon
4.2. Between-Task Differences in Foot Sections
4.3. Between-Task Differences and Similarities in the Trunk, Hand, and Face Sections
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talelli, P.; Ewas, A.; Waddingham, W.; Rothwell, J.C.; Ward, N.S. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage 2008, 40, 1772–1781. [Google Scholar] [CrossRef]
- Allison, J.D.; Meador, K.J.; Loring, D.W.; Figueroa, R.E.; Wright, J.C. Functional MRI cerebral activation and deactivation during finger movement. Neurology 2000, 54, 135–142. [Google Scholar] [CrossRef]
- Newton, J.M.; Sunderland, A.; Gowland, P.A. fMRI signal decreases in ipsilateral primary motor cortex during unilateral hand movements are related to duration and side of movement. NeuroImage 2005, 24, 1080–1087. [Google Scholar] [CrossRef]
- Hayashi, M.J.; Saito, D.N.; Aramaki, Y.; Asai, T.; Fujibayashi, Y.; Sadato, N. Hemispheric asymmetry of frequency-dependent suppression in the ipsilateral primary motor cortex during finger movement: A functional magnetic resonance imaging study. Cereb Cortex 2008, 18, 2932–2940. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Asada, M.; Naito, E. Developmental changes in task-induced brain deactivation in humans revealed by a motor task. Dev. Neurobiol. 2019, 79, 536–558. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Domoto, R.; Mizuguchi, N.; Sakamoto, K.; Kanosue, K. Negative BOLD responses during hand and foot movements: An fMRI study. PLoS ONE 2019, 14, e0215736. [Google Scholar] [CrossRef] [PubMed]
- Naito, E.; Morita, T.; Asada, M. Importance of the primary motor cortex in development of human hand/finger dexterity. Cereb. Cortex Commun. 2020. [Google Scholar] [CrossRef]
- Morita, T.; Asada, M.; Naito, E. Examination of the development and aging of brain deactivation using a unimanual motor task. Adv. Robot. 2021. [Google Scholar] [CrossRef]
- Luft, A.R.; Smith, G.V.; Forrester, L.; Whitall, J.; Macko, R.F.; Hauser, T.-K.; Goldberg, A.P.; Hanley, D.F. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum. Brain Mapp. 2002, 17, 131–140. [Google Scholar] [CrossRef]
- Kapreli, E.; Athanasopoulos, S.; Papathanasiou, M.; Van Hecke, P.; Strimpakos, N.; Gouliamos, A.; Peeters, R.; Sunaert, S. Lateralization of brain activity during lower limb joints movement. An fMRI study. Neuroimage 2006, 32, 1709–1721. [Google Scholar] [CrossRef]
- Volz, L.J.; Eickhoff, S.B.; Pool, E.-M.; Fink, G.R.; Grefkes, C. Differential modulation of motor network connectivity during movements of the upper and lower limbs. NeuroImage 2015, 119, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Kelso, J.A. Phase transitions and critical behavior in human bimanual coordination. Am. J. Physiol. 1984, 246, R1000–R1004. [Google Scholar] [CrossRef]
- Naito, E.; Nakashima, T.; Kito, T.; Aramaki, Y.; Okada, T.; Sadato, N. Human limb-specific and non-limb-specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur. J. Neurosci. 2007, 25, 3476–3487. [Google Scholar] [CrossRef] [PubMed]
- Naito, E.; Morita, T.; Amemiya, K. Body representations in the human brain revealed by kinesthetic illusions and their essential contributions to motor control and corporeal awareness. Neurosci. Res. 2016, 104, 16–30. [Google Scholar] [CrossRef]
- Zeharia, N.; Hertz, U.; Flash, T.; Amedi, A. Negative blood oxygenation level dependent homunculus and somatotopic information in primary motor cortex and supplementary motor area. Proc. Natl. Acad. Sci. USA 2012, 109, 18565–18570. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Stephan, K.E.; Mohlberg, H.; Grefkes, C.; Fink, G.R.; Amunts, K.; Zilles, K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 2005, 25, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Van Melick, N.; Meddeler, B.M.; Hoogeboom, T.J.; Nijhuis-van der Sanden, M.W.; van Cingel, R.E. How to determine leg dominance: The agreement between self-reported and observed performance in healthy adults. PLoS ONE 2017, 12, e0189876. [Google Scholar] [CrossRef] [PubMed]
- Ehrsson, H.H.; Geyer, S.; Naito, E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part–specific motor representations. J. Neurophysiol. 2003, 90, 3304–3316. [Google Scholar] [CrossRef]
- Amemiya, K.; Morita, T.; Hirose, S.; Ikegami, T.; Hirashima, M.; Naito, E. Neurological and behavioral features of locomotor imagery in the blind. Brain Imag. Behav. 2020, 15, 656–676. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.C.; Kamber, M.; Collins, D.L.; MacDonald, D. An MRI-based probabilistic atlas of neuroanatomy. In Magnetic Resonance Scanning and Epilepsy; Shorvon, S.D., Fish, D.R., Andermann, F., Bydder, G.M., Stefan, H., Eds.; NATO ASI Series; Springer US: Boston, MA, USA, 1994; pp. 263–274. ISBN 978-1-4615-2546-2. [Google Scholar]
- Friston, K.J.; Holmes, A.P.; Poline, J.B.; Grasby, P.J.; Williams, S.C.; Frackowiak, R.S.; Turner, R. Analysis of fMRI time-series revisited. Neuroimage 1995, 2, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Worsley, K.J.; Friston, K.J. Analysis of fMRI time-series revisited—Again. NeuroImage 1995, 2, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, G.K.; Zarahn, E.; D’Esposito, M. The inferential impact of global signal covariates in functional neuroimaging analyses. NeuroImage 1998, 8, 302–306. [Google Scholar] [CrossRef]
- Holmes, A.P.; Friston, K.J. Generalisability, random effects & population inference. NeuroImage 1998, 7, S754. [Google Scholar] [CrossRef]
- Price, C.J.; Friston, K.J. Cognitive conjunction: A new approach to brain activation experiments. Neuroimage 1997, 5, 261–270. [Google Scholar] [CrossRef]
- Moraschi, M.; DiNuzzo, M.; Giove, F. On the origin of sustained negative BOLD response. J. Neurophysiol. 2012, 108, 2339–2342. [Google Scholar] [CrossRef]
- Kim, S.-G.; Ogawa, S. Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals. J. Cereb. Blood Flow Metab. 2012, 32, 1188–1206. [Google Scholar] [CrossRef]
- Shmuel, A.; Yacoub, E.; Pfeuffer, J.; Van de Moortele, P.-F.; Adriany, G.; Hu, X. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 2002, 36, 1195–1210. [Google Scholar] [CrossRef]
- Smith, A.; Williams, A.; Singh, K. Negative BOLD in the visual cortex: Evidence against blood stealing. Hum. Brain Mapp. 2004, 21, 213–220. [Google Scholar] [CrossRef]
- Stefanovic, B.; Warnking, J.M.; Pike, G.B. Hemodynamic and metabolic responses to neuronal inhibition. NeuroImage 2004, 22, 771–778. [Google Scholar] [CrossRef]
- Shmuel, A.; Augath, M.; Oeltermann, A.; Logothetis, N.K. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 2006, 9, 569–577. [Google Scholar] [CrossRef]
- Pasley, B.N.; Inglis, B.A.; Freeman, R.D. Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage 2007, 36, 269–276. [Google Scholar] [CrossRef]
- Boorman, L.; Kennerley, A.; Johnston, D.; Jones, M.; Zheng, Y.; Redgrave, P.; Berwick, J. Negative blood oxygen level dependence in the rat: A model for investigating the role of suppression in neurovascular coupling. J. Neurosci. 2010, 30, 4285–4294. [Google Scholar] [CrossRef]
- Wade, A.R.; Rowland, J. Early suppressive mechanisms and the negative blood oxygenation level-dependent response in human visual cortex. J. Neurosci. 2010, 30, 5008–5019. [Google Scholar] [CrossRef] [PubMed]
- Sten, S.; Lundengård, K.; Witt, S.T.; Cedersund, G.; Elinder, F.; Engström, M. Neural inhibition can explain negative BOLD responses: A mechanistic modelling and fMRI study. Neuroimage 2017, 158, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Jiang, H.; Vo, T.T.; Jung, W.B.; Vazquez, A.L.; Kim, S.-G. Contribution of excitatory and inhibitory neuronal activity to BOLD fMRI. Cereb. Cortex 2021. [Google Scholar] [CrossRef]
- Hummel, F.; Saur, R.; Lasogga, S.; Plewnia, C.; Erb, M.; Wildgruber, D.; Grodd, W.; Gerloff, C. To act or not to act. Neural correlates of executive control of learned motor behavior. Neuroimage 2004, 23, 1391–1401. [Google Scholar] [CrossRef]
- Mullinger, K.J.; Mayhew, S.D.; Bagshaw, A.P.; Bowtell, R.; Francis, S.T. Evidence that the negative BOLD response is neuronal in origin: A simultaneous EEG-BOLD-CBF study in humans. Neuroimage 2014, 94, 263–274. [Google Scholar] [CrossRef]
- Ferbert, A.; Priori, A.; Rothwell, J.C.; Day, B.L.; Colebatch, J.G.; Marsden, C.D. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992, 453, 525–546. [Google Scholar] [CrossRef]
- Kobayashi, M.; Hutchinson, S.; Schlaug, G.; Pascual-Leone, A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. NeuroImage 2003, 20, 2259–2270. [Google Scholar] [CrossRef]
- Hübers, A.; Orekhov, Y.; Ziemann, U. Interhemispheric motor inhibition: Its role in controlling electromyographic mirror activity. Eur. J. Neurosci. 2008, 28, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Swayne, O.B.; Vandermeeren, Y.; Camus, M.; Dimyan, M.A.; Harris-Love, M.; Perez, M.A.; Ragert, P.; Rothwell, J.C.; Cohen, L.G. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol. 2008, 586, 325–351. [Google Scholar] [CrossRef] [PubMed]
- Uehara, K.; Funase, K. Contribution of ipsilateral primary motor cortex activity to the execution of voluntary movements in humans: A review of recent studies. J. Phys. Fit. Sports Med. 2014, 3, 297–306. [Google Scholar] [CrossRef]
- Jacobs, K.M.; Donoghue, J.P. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 1991, 251, 944–947. [Google Scholar] [CrossRef]
- Ni, Z.; Gunraj, C.; Nelson, A.J.; Yeh, I.-J.; Castillo, G.; Hoque, T.; Chen, R. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb. Cortex 2009, 19, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Kai, S.; Yoshimoto, R.; Nakahara, M.; Murakami, S.; Watari, K.; Takahashi, S. Trunk muscle activity in two-leg standing to one-leg standing in healthy elderly adults. J. Phys. Therapy Sci. 2008, 20, 77–80. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naito, E.; Morita, T.; Kimura, N.; Asada, M. Existence of Interhemispheric Inhibition between Foot Sections of Human Primary Motor Cortices: Evidence from Negative Blood Oxygenation-Level Dependent Signal. Brain Sci. 2021, 11, 1099. https://doi.org/10.3390/brainsci11081099
Naito E, Morita T, Kimura N, Asada M. Existence of Interhemispheric Inhibition between Foot Sections of Human Primary Motor Cortices: Evidence from Negative Blood Oxygenation-Level Dependent Signal. Brain Sciences. 2021; 11(8):1099. https://doi.org/10.3390/brainsci11081099
Chicago/Turabian StyleNaito, Eiichi, Tomoyo Morita, Nodoka Kimura, and Minoru Asada. 2021. "Existence of Interhemispheric Inhibition between Foot Sections of Human Primary Motor Cortices: Evidence from Negative Blood Oxygenation-Level Dependent Signal" Brain Sciences 11, no. 8: 1099. https://doi.org/10.3390/brainsci11081099
APA StyleNaito, E., Morita, T., Kimura, N., & Asada, M. (2021). Existence of Interhemispheric Inhibition between Foot Sections of Human Primary Motor Cortices: Evidence from Negative Blood Oxygenation-Level Dependent Signal. Brain Sciences, 11(8), 1099. https://doi.org/10.3390/brainsci11081099




