Reduction of Sniff Nasal Inspiratory Pressure (SNIP) as an Early Indicator of the Need of Enteral Nutrition in Patients with Amyotrophic Lateral Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
Retrospective Observational Study
2.2. Procedures of Assessment
2.3. Nutritional Management
2.4. Ethics
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowland, L.P.; Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001, 344, 1688–1700. [Google Scholar] [CrossRef] [PubMed]
- Pupillo, E.; Messina, P.; Logroscino, G.; Beghi, E. Long-term survival in amyotrophic lateral sclerosis: A population-based study. Ann. Neurol. 2014, 75, 287–297. [Google Scholar] [CrossRef]
- Logroscino, G.; Traynor, B.J.; Hardiman, O.; Chio, A.; Couratier, P.; Mitchell, J.D.; Swingler, R.J.; Beghi, E. Descriptive epidemiology of amyotrophic lateral sclerosis: New evidence and unsolved issues. J. Neurol. Neurosurg. Psychiatry 2008, 79, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Marin, B.; Desport, J.C.; Kajeu, P.; Jesus, P.; Nicolaud, B.; Nicol, M.; Preux, P.M.; Couratier, P. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J. Neurol. Neurosurg. Psychiatry 2011, 82, 628–634. [Google Scholar] [CrossRef] [Green Version]
- López-Gómez, J.J.; Ballesteros-Pomar, M.D.; Torres-Torres, B.; De la Maza, B.P.; Penacho-Lázaro, M.; Palacio-Mures, J.M.; Abreu-Padín, C.; López-Guzmán, A.; De Luis-Román, D.A. Malnutrition at diagnosis in amyotrophic lateral sclerosis (als) and its influence on survival: Using glim criteria. Clin. Nutr. 2021, 40, 237–244. [Google Scholar] [CrossRef]
- Plowman, E.K.; Tabor, L.C.; Wymer, J.; Pattee, G. The evaluation of bulbar dysfunction in amyotrophic lateral sclerosis: Survey of clinical practice patterns in the United States. Amyotroph. Lateral Scler. Frontotemporal Degener. 2017, 18, 351–357. [Google Scholar] [CrossRef]
- Genton, L.; Viatte, V.; Janssens, J.P.; Héritier, A.C.; Pichard, C. Nutritional state, energy intakes and energy expenditure of amyotrophic lateral sclerosis (ALS) patients. Clin. Nutr. 2011, 30, 553–559. [Google Scholar] [CrossRef]
- Nunes, G.; Santos, C.A.; Grunho, M.; Fonseca, J. Enteral feeding through endoscopic gastrostomy in amyotrophic lateral sclerosis patients. Nutr. Hosp. 2016, 33, 561. [Google Scholar] [CrossRef] [PubMed]
- Bond, L.; Ganguly, P.; Khamankar, N.; Mallet, N.; Bowen, G.; Green, B.; Mitchell, C.S. A Comprehensive Examination of Percutaneous Endoscopic Gastrostomy and Its Association with Amyotrophic Lateral Sclerosis Patient Outcomes. Brain Sci. 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, R.H.; Allison, M.C.; Lang, J.; Spence, E.; Morris, A.J.; Danesh, B.J.; Russell, R.I.; Mills, P.R. Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ 1992, 304, 1406–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, P.M.; Abrahams, S.; Borasio, G.D.; de Carvalho, M.; Chio, A.; Van Damme, P.; Hardiman, O.; Kollewe, K.; Morrison, K.E.; Petri, S.; et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)—Revised report of an EFNS task force. Eur. J. Neurol. 2012, 19, 360–375. [Google Scholar] [CrossRef]
- Andersen, P.M.; Borasio, G.D.; Dengler, R.; Hardiman, O.; Kollewe, K.; Leigh, P.N.; Pradat, P.F.; Silani, V.; Tomik, B. Good practice in the management of amyotrophic lateral sclerosis: Clinical guidelines. An evidence-based review with good practice points. EALSC Working Group. Amyotroph. Lateral Scler. 2007, 8, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Radunović, A.; Mitsumoto, H.; Leigh, P.N. Clinical care of patients with amyotrophic lateral sclerosis. Lancet Neurol. 2007, 6, 913–925. [Google Scholar] [CrossRef]
- Miller, R.G.; Jackson, C.E.; Kasarskis, E.J.; England, J.D.; Forshew, D.; Johnston, W.; Kalra, S.; Katz, J.S.; Mitsumoto, H.; Rosenfeld, J.; et al. Practice parameter update: The care of the patient with amyotrophic lateral sclerosis: Drug, nutritional, and respiratory therapies (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009, 73, 1218–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbe, P.; Markus, K.; Valente, R.; Ingre, C.; Tsolakis, A.V.; Vujasinovic, M. Effectiveness of percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis. Minerva Gastroenterol. Dietol. 2020, 66, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; de Carvalho, M. Sniff nasal inspiratory pressure (SNIP) in amyotrophic lateral sclerosis: Relevance of the methodology for respiratory function evaluation. Clin. Neurol. Neurosurg. 2018, 171, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Black, L.F.; Hyatt, R.E. Maximal static respiratory pressures in generalized neuromuscular disease. Am. Rev. Respir. Dis. 1971, 103, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Fitting, J.W.; Paillex, R.; Hirt, L.; Aebischer, P.; Schluep, M. Sniff nasal pressure: A sensitive respiratory test to assess progression of amyotrophic lateral sclerosis. Ann. Neurol. 1999, 46, 887–893. [Google Scholar] [CrossRef]
- Polkey, M.I.; Green, M.; Moxham, J. Measurement of respiratory muscle strength. Thorax 1995, 50, 1131–1135. [Google Scholar] [CrossRef] [Green Version]
- Morgan, R.K.; McNally, S.; Alexander, M.; Conroy, R.; Hardiman, O.; Costello, R.W. Use of Sniff nasal-inspiratory force to predict survival in amyotrophic lateral sclerosis. Am. J. Respir. Crit. Care Med. 2005, 171, 269–274. [Google Scholar] [CrossRef]
- Capozzo, R.; Quaranta, V.N.; Pellegrini, F.; Fontana, A.; Copetti, M.; Carratù, P.; Panza, F.; Cassano, A.; Falcone, V.A.; Tortelli, R.; et al. Sniff nasal inspiratory pressure as a prognostic factor of tracheostomy or death in amyotrophic lateral sclerosis. J. Neurol. 2015, 262, 593–603. [Google Scholar] [CrossRef]
- Brooks, B.R. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J. Neurol. Sci. 1994, 124, 96–107. [Google Scholar] [CrossRef]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef]
- Vergonjeanne, M.; Fayemendy, P.; Marin, B.; Penoty, M.; Lautrette, G.; Sourisseau, H.; Preux, P.M.; Desport, J.C.; Couratier, P.; Jésus, P. Predictive factors for gastrostomy at time of diagnosis and impact on survival in patients with amyotrophic lateral sclerosis. Clin. Nutr. 2020, 39, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care. Med. 2002, 166, 518–624. [CrossRef]
- Gauderer, M.W.; Ponsky, J.L.; Izant, R.J., Jr. Gastrostomy without laparotomy: A percutaneous endoscopic technique. J. Pediatr. Surg. 1980, 15, 872–875. [Google Scholar] [CrossRef]
- Traynor, B.J.; Zhang, H.; Shefner, J.M.; Schoenfeld, D.; Cudkowicz, M.E. Functional outcome measures as clinical trial endpoints in ALS. Neurology 2004, 63, 1933–1935. [Google Scholar] [CrossRef]
- Dupuis, L.; Oudart, H.; René, F.; Gonzalez de Aguilar, J.L.; Loeffler, J.P. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: Benefit of a high-energy diet in a transgenic mouse model. Proc. Natl. Acad. Sci. USA 2004, 101, 11159–11164. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Baek, H.; Kim, S.H.; Park, Y. Association between estimated total daily energy expenditure and stage of amyotrophic lateral sclerosis. Nutrition 2017, 33, 181–186. [Google Scholar] [CrossRef]
- Kasarskis, E.J.; Mendiondo, M.S.; Matthews, D.E.; Mitsumoto, H.; Tandan, R.; Simmons, Z.; Bromberg, M.B.; Kryscio, R.J. Estimating daily energy expenditure in individuals with amyotrophic lateral sclerosis. Am. J. Clin. Nutr. 2014, 99, 792–803. [Google Scholar] [CrossRef] [Green Version]
- Mathus-Vliegen, L.M.; Louwerse, L.S.; Merkus, M.P.; Tytgat, G.N.; Vianney de Jong, J.M. Percutaneous endoscopic gastrostomy in patients with amyotrophic lateral sclerosis and impaired pulmonary function. Gastrointest. Endosc. 1994, 40, 463–469. [Google Scholar] [CrossRef]
- Mazzini, L.; Corrà, T.; Zaccala, M.; Mora, G.; Del Piano, M.; Galante, M. Percutaneous endoscopic gastrostomy and enteral nutrition in amyotrophic lateral sclerosis. J. Neurol. 1995, 242, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.; Siderowf, A.; Golaszewski, A.L.; McCluskey, L. Gastrostomy insertion in ALS patients with low vital capacity: Respiratory support and survival. Neurology 2002, 58, 485–487. [Google Scholar] [CrossRef]
- Yang, B.; Shi, X. Percutaneous endoscopic gastrostomy versus fluoroscopic gastrostomy in amyotrophic lateral sclerosis (ALS) sufferers with nutritional impairment: A meta-analysis of current studies. Oncotarget 2017, 8, 102244–102253. [Google Scholar] [CrossRef] [Green Version]
- Lyall, R.A.; Donaldson, N.; Polkey, M.I.; Leigh, P.N.; Moxham, J. Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain 2001, 124, 2000–2013. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.P.; Drachman, D.B.; Wiener, C.M.; Clawson, L.; Kimball, R.; Lechtzin, N. Pulmonary predictors of survival in amyotrophic lateral sclerosis: Use in clinical trial design. Muscle Nerve 2006, 33, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Carratù, P.; Cassano, A.; Gadaleta, F.; Tedone, M.; Dongiovanni, S.; Fanfulla, F.; Resta, O. Association between low sniff nasal-inspiratory pressure (SNIP) and sleep disordered breathing in amyotrophic lateral sclerosis: Preliminary results. Amyotroph. Lateral Scler. 2011, 12, 458–463. [Google Scholar] [CrossRef]
- Quaranta, V.N.; Carratù, P.; Damiani, M.F.; Dragonieri, S.; Capozzolo, A.; Cassano, A.; Resta, O. The Prognostic Role of Obstructive Sleep Apnea at the Onset of Amyotrophic Lateral Sclerosis. Neurodegener. Dis. 2017, 17, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Héritier, F.; Rahm, F.; Pasche, P.; Fitting, J.W. Sniff nasal inspiratory pressure. A noninvasive assessment of inspiratory muscle strength. Am. J. Respir. Crit. Care. Med. 1994, 150, 1678–1683. [Google Scholar] [CrossRef]
- Carratù, P.; Spicuzza, L.; Cassano, A.; Maniscalco, M.; Gadaleta, F.; Lacedonia, D.; Scoditti, C.; Boniello, E.; Di Maria, G.; Resta, O. Early treatment with noninvasive positive pressure ventilation prolongs survival in Amyotrophic Lateral Sclerosis patients with nocturnal respiratory insufficiency. Orphanet J. Rare Dis. 2009, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, P.M.; Kuzma-Kozakiewicz, M.; Keller, J.; Aho-Oezhan, H.E.A.; Ciecwierska, K.; Szejko, N.; Vázquez, C.; Böhm, S.; Badura-Lotter, G.; Meyer, T.; et al. Therapeutic decisions in ALS patients: Cross-cultural differences and clinical implications. J. Neurol. 2018, 265, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Hesters, A.; Amador, M.D.M.; Debs, R.; Le Forestier, N.; Lenglet, T.; Pradat, P.F.; Salachas, F.; Faure, M.; Jimenez, M.G.; Gonzalez-Bermejo, J.; et al. Predictive factors for prognosis after gastrostomy placement in routine non-invasive ventilation users ALS patients. Sci. Rep. 2020, 10, 15117. [Google Scholar] [CrossRef] [PubMed]
- Logroscino, G.; Marin, B.; Piccininni, M.; Arcuti, S.; Chiò, A.; Hardiman, O.; Rooney, J.; Zoccolella, S.; Couratier, P.; Preux, P.M.; et al. Referral bias in ALS epidemiological studies. PLoS ONE 2018, 13, e0195821. [Google Scholar] [CrossRef] [PubMed]
| Variable (n) | M ± SD, or n (%) |
|---|---|
| Age (179) (years) | 66.87 ± 13.33 |
| Sex M (179) | 102 (57.0) |
| Type of Onset (179) | |
| 128 (71.5) |
| 51 (28.5) |
| CCI (179) | |
| 0 | 41 (22.9) |
| 1 | 96 (53.6) |
| 2 | 29 (16.2) |
| 3 | 10 (5.6) |
| 4 | 3 (1.7) |
| Time onset—1st visit (179) (months) | 24.3 ± 12.9 |
| ODI (179) (months) | 15.7 ± 11.6 |
| Time diagnosis—1st visit (179) (months) | 8.7 ± 8.2 |
| Follow-up time (PEG or last clinical follow-up) (179) (months) | 38.1 ± 24.3 |
| Time 1st visit—PEG (n = 75) (months) | 16.6 ± 15.7 |
| PEG(179) | |
| Yes | 75 (41.9) |
| No | 105 (58.1) |
| PaO2 (179) (mmHg) | 89.7 ± 13.6 |
| PaCO2 (179) (mmHg) | 39 ± 5.4 |
| pH (179) | 7.3 ± 0.8 |
| SatO2 (179) (%) | 97.1 ± 1.7 |
| ALSFSRr (127) | 36.3 ± 8 |
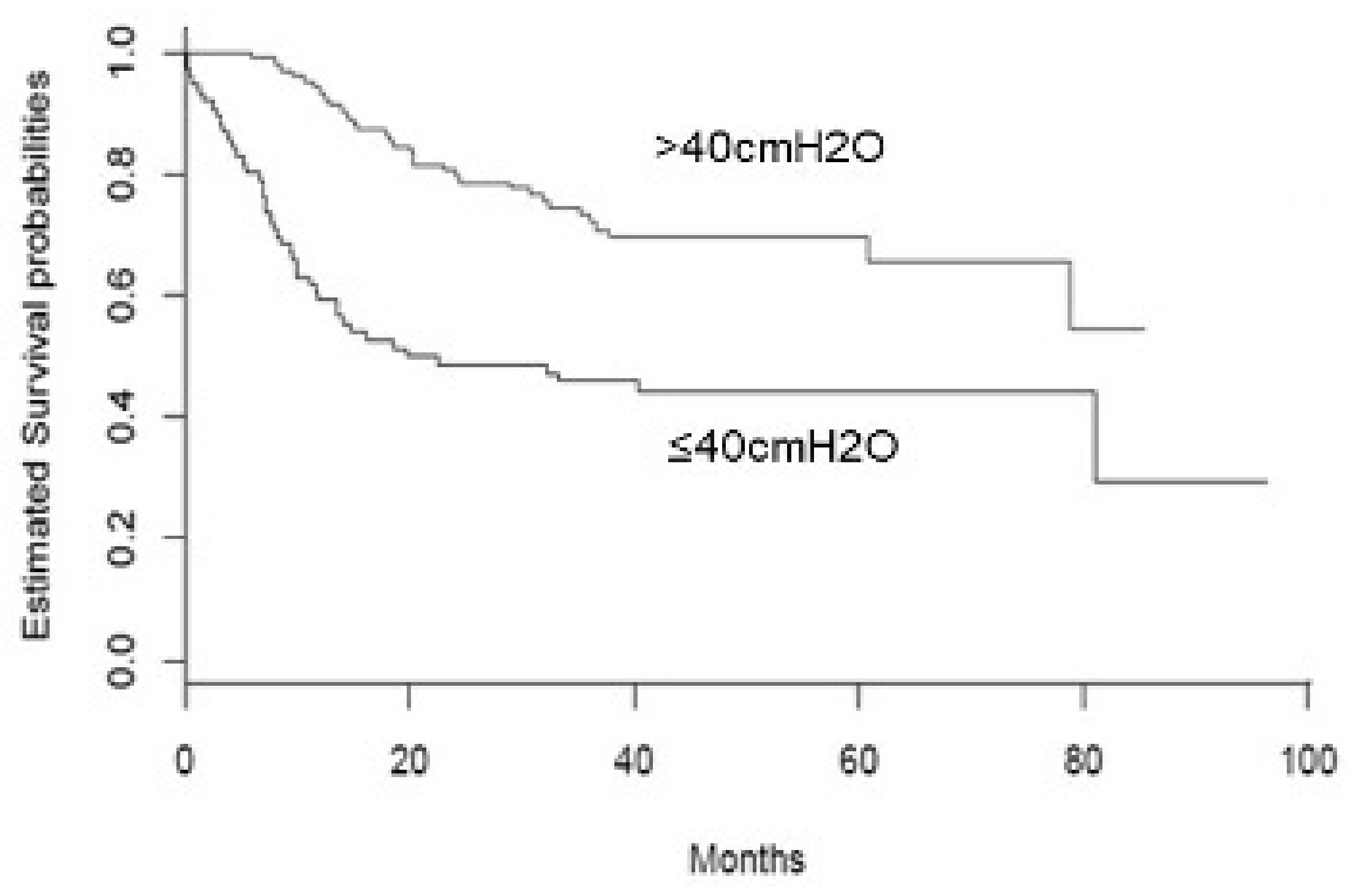
| SNIP (179) (cmH2O) | 49.4 ± 27.2 |
| SNIP categories (179) | |
| >40 cmH2O | 103 (57.5) |
| ≤40 cmH2O | 76 (42.5) |
| FVC (174) (%) | 80. 46 ±27 |
| BMI (179) (kg/m2) | 25.3 ± 4.4 |
| Variables (n) | SNIP ≤ 40 cmH2O (n = 76) M ± SD, or n (%) | SNIP > 40 cmH2O (n = 103) M ± SD, or n (%) | p-Value |
|---|---|---|---|
| Age (179) (years) | 69.6 ± 14.0 | 64.9 ± 12.6 | 0.02 |
| Sex (179) | 0.25 | ||
| M | 39 (51) | 63 (61) | |
| F | 37 (49) | 40 (39) | |
| Type of onset (179) | 0.2 | ||
| Spinal | 26 (34) | 25 (24) | |
| Bulbar | 50 (66) | 78 (76) | |
| CCI (179) | 0.7 | ||
| 0 | 18 (24) | 23 (22) | |
| 1 | 37 (49) | 57 (59) | |
| 2 | 14 (18) | 15 (15) | |
| 3 | 6 (8) | 4 (4) | |
| 4 | 1 (1) | 2 (2) | |
| Time onset—1st visit (179) (months) | 23.2 ± 12.7 | 25.2 ± 13.0 | 0.3 |
| ODI (179) (months) | 15.0 ± 11.0 | 16.1 ± 12 | 0.5 |
| Time diagnosis—1st visit (179) (months) | 8.1 ± 7.3 | 9.09 ± 8.91 | 0.4 |
| Follow-up time (PEG or last clinical follow-up) (179) (months) | 31.8 ± 28.2 | 42.7 ± 19.9 | 0.004 |
| Time 1st visit—PEG (75) (months) | 11.6 ± 14.0 | 23.3 ± 15.5 | 0.001 |
| PEG (179) | 0.001 | ||
| Yes | 33 (43) | 71 (69) | |
| No | 43 (57) | 32 (31) | |
| PaO2 (179) (mmHg) | 85.8 ± 14.9 | 92.59 ± 11.8 | <0.0001 |
| PaCO2 (179) (mmHg) | 39.8 ± 6.2 | 38.4 ± 4.6 | 0.1 |
| pH (179) | 7.4 ± 0.82 | 7.3 ± 0.8 | 0.9 |
| SatO2 (179) (%) | 96.8 ± 2.1 | 97.3 ± 1.4 | 0.1 |
| ALSFSRr (127) | 31.9 ± 8.6 | 39.4 ± 5.8 | <0.0001 |
| FVC (174) (%) | 63.4 ± 24.9 | 92.8 ± 21.2 | <0.0001 |
| BMI (179) (kg/m2) | 24.2 ± 5.1 | 26.0 ± 3.7 | 0.008 |
| HR | 95% CI | p | |
|---|---|---|---|
| Sex (males vs. females) | 0.48 | 0.25–0.95 | 0.03 |
| ALSFRSr | 0.95 | 0.92–0.99 | 0.03 |
| SNIP | 0.98 | 0.96–0.99 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoccolella, S.; Capozzo, R.; Quaranta, V.N.; Castellana, G.; Marra, L.; Liotino, V.; Giorgio, V.; Simone, I.L.; Resta, O.; Piccininni, M.; et al. Reduction of Sniff Nasal Inspiratory Pressure (SNIP) as an Early Indicator of the Need of Enteral Nutrition in Patients with Amyotrophic Lateral Sclerosis. Brain Sci. 2021, 11, 1091. https://doi.org/10.3390/brainsci11081091
Zoccolella S, Capozzo R, Quaranta VN, Castellana G, Marra L, Liotino V, Giorgio V, Simone IL, Resta O, Piccininni M, et al. Reduction of Sniff Nasal Inspiratory Pressure (SNIP) as an Early Indicator of the Need of Enteral Nutrition in Patients with Amyotrophic Lateral Sclerosis. Brain Sciences. 2021; 11(8):1091. https://doi.org/10.3390/brainsci11081091
Chicago/Turabian StyleZoccolella, Stefano, Rosa Capozzo, Vitaliano N. Quaranta, Giorgio Castellana, Lorenzo Marra, Vito Liotino, Vincenza Giorgio, Isabella L. Simone, Onofrio Resta, Marco Piccininni, and et al. 2021. "Reduction of Sniff Nasal Inspiratory Pressure (SNIP) as an Early Indicator of the Need of Enteral Nutrition in Patients with Amyotrophic Lateral Sclerosis" Brain Sciences 11, no. 8: 1091. https://doi.org/10.3390/brainsci11081091
APA StyleZoccolella, S., Capozzo, R., Quaranta, V. N., Castellana, G., Marra, L., Liotino, V., Giorgio, V., Simone, I. L., Resta, O., Piccininni, M., Tortelli, R., & Logroscino, G. (2021). Reduction of Sniff Nasal Inspiratory Pressure (SNIP) as an Early Indicator of the Need of Enteral Nutrition in Patients with Amyotrophic Lateral Sclerosis. Brain Sciences, 11(8), 1091. https://doi.org/10.3390/brainsci11081091







