Functional Cognitive Disorders (FCD): How Is Metacognition Involved?
Abstract
:1. Introduction: Functional Cognitive Disorders (FCD)
“It has been suggested … that the word ‘functional’ should be used instead of ‘hysterical’. This … is an example of the fallacy of Molière’s physician [in the play Le Malade imaginaire (The Imaginary Invalid) of 1673] in that it pretends to explain by a learned circumlocution a condition which to date neither doctors nor patients understand”.([3], p. 270)
More recent commentators have also opined that “functional” is “no more enlightening” than other options, although serviceable and “accommodating in theoretical terms”.([4], p. 3496)
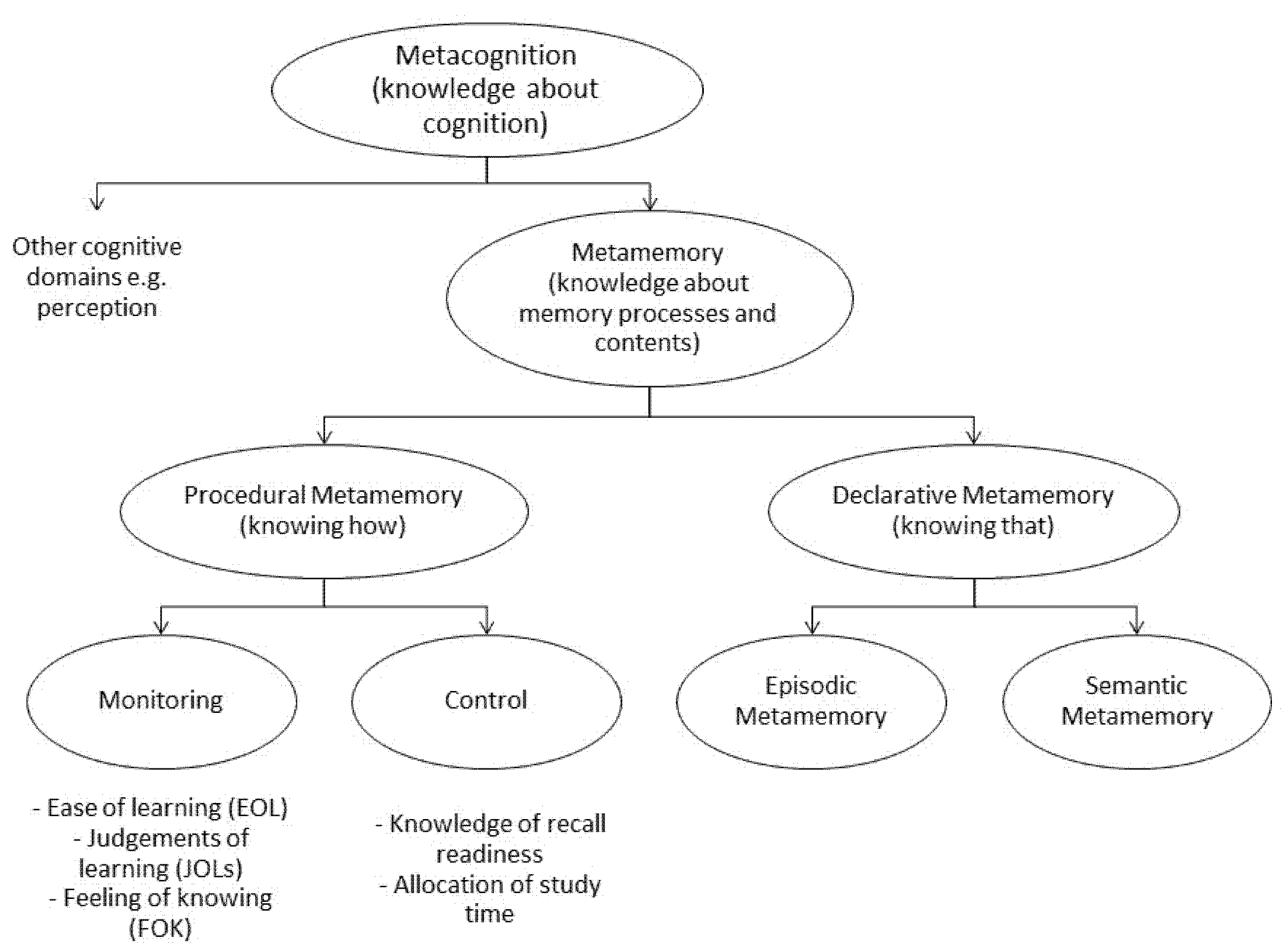
2. Metamemory and Metacognition
3. FCD: Evidence for Impaired Metacognition
4. FND Models and Their Possible Relation to FCD
4.1. Integrative Cognitive Model (ICM)
4.2. Bayesian Model
4.3. Unifying Theory Model
5. Metacognitive Models and Their Possible Relation to FCD
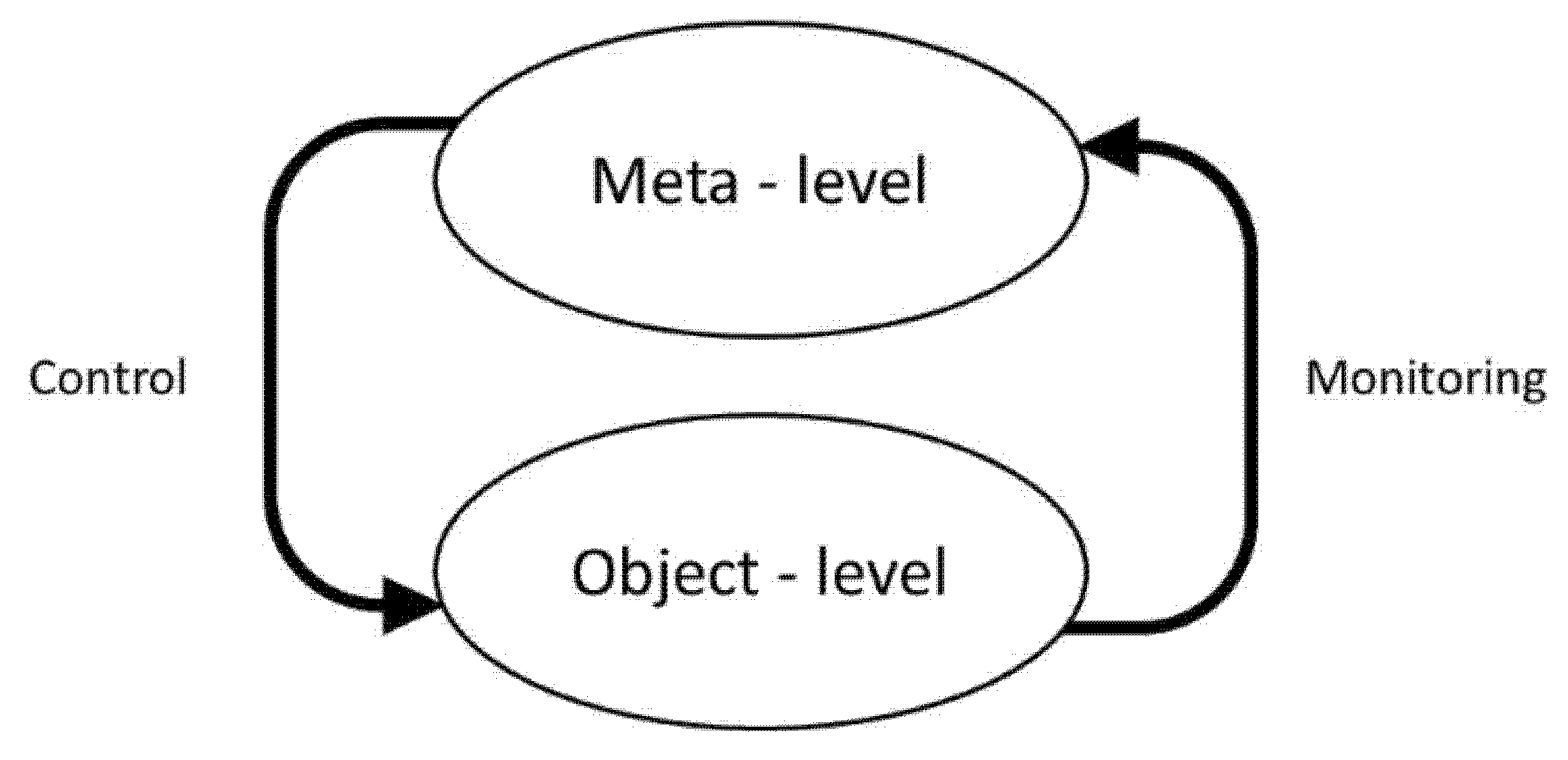
5.1. Metamemory “Monitoring and Control” Model of Nelson and Narens
5.2. Proposed Adaptation of the Nelson and Narens’ Metamemory Model to FCD
5.3. Other Metacognitive Models, including the Cognitive Awareness Model (CAM)
5.4. Metamemory Model Limitations and Comparisons with FND Models
6. Summary: FCD, Anosognosia, and Wittgenstein
7. Future Prospects and Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallett, M.; Stone, J.; Carson, A. (Eds.) Functional Neurologic Disorders (Handbook of Clinical Neurology Volume 139, 3rd Series); Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Stone, J.; Wojcik, W.; Durrance, D.; Carson, A.; Lewis, S.; MacKenzie, L.; Warlow, C.P.; Sharpe, M. What should we say to patients with symptoms unexplained by disease? The “number needed to offend”. BMJ 2002, 325, 1449–1450. [Google Scholar] [CrossRef]
- Hayes, J. (Ed.) The Sele Cted Writings of Maurice O’Connor Drury. On Wittgenstein, Philosophy, Religion and Psychiatry; Bloomsbury Academic: London, UK, 2019. [Google Scholar] [CrossRef]
- Edwards, M.J.; Adams, R.A.; Brown, H.; Pareés, I.; Friston, K.J. A Bayesian account of “hysteria”. Brain 2012, 135, 3495–3512. [Google Scholar] [CrossRef]
- Bailey, C.; Bell, S.M.; Blackburn, D.M. How the UK describes functional memory symptoms. Psychogeriatrics 2017, 17, 336–337. [Google Scholar] [CrossRef] [Green Version]
- Stone, J.; Pal, S.; Blackburn, D.; Reuber, M.; Thekkumpurath, P.; Carson, A. Functional (psychogenic) cognitive disorders: A perspective from the neurology clinic. J. Alzheimers Dis. 2015, 48 (Suppl. 1), S5–S17. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.; Blackburn, D.; Reuber, M. Patients’ accounts of memory lapses in interactions between neurologists and patients with functional memory disorders. Sociol. Health Illn. 2019, 41, 249–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larner, A.J. Michael Faraday’s “loss of memory” revisited. J. Hist. Neurosci. 2021, 30, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tagai, K.; Nagata, T.; Shinagawa, S.; Shigeta, M. Anosognosia in patients with Alzheimer’s disease: Current perspectives. Psychogeriatrics 2020, 20, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Pennington, C.; Hayre, A.; Newsom, M.; Coulthard, E. Functional cognitive disorder: A common cause of subjective cognitive symptoms. J. Alzheimers Dis. 2015, 48 (Suppl. 1), S19–S24. [Google Scholar] [CrossRef]
- Elsey, C.; Drew, P.; Jones, D.; Blackburn, D.; Wakefield, S.; Harkness, K.; Venneri, A.; Reuber, M. Towards diagnostic conversational profiles of patients presenting with dementia or functional memory disorders to memory clinics. Patient Educ. Couns. 2015, 98, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.; Drew, P.; Elsey, C.; Blackburn, D.; Wakefield, S.; Harkness, K.; Reuber, M. Conversational assessment in memory clinic encounters: Interactional profiling for differentiating dementia from functional memory disorders. Aging Ment. Health 2016, 20, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Bharambe, V.; Larner, A.J. Functional cognitive disorders: Memory clinic study. Prog. Neurol. Psychiatry 2018, 22, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Bharambe, V.; Larner, A.J. Functional cognitive disorders: Demographic and clinical features contribute to a positive diagnosis. Neurodegener. Dis. Manag. 2018, 8, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Bhome, R.; Huntley, J.D.; Price, G.; Howard, R.J. Clinical presentation and neuropsychological profiles of Functional Cognitive Disorder patients with and without co-morbid depression. Cogn. Neuropsychiatry 2019, 24, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Pennington, C.; Ball, H.; Swirski, M. Functional cognitive disorder: Diagnostic challenges and future directions. Diagnostics 2019, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakefield, S.J.; Blackburn, D.J.; Harkness, K.; Khan, A.; Reuber, M.; Venneri, A. Distinctive neuropsychological profiles differentiate patients with functional memory disorder from patients with amnestic mild cognitive impairment. Acta Neuropsychiatr. 2019, 30, 90–96. [Google Scholar] [CrossRef]
- Pennington, C.; Newson, M.; Hayre, A.; Coulthard, E. Functional cognitive disorder: What is it and what to do about it? Pract. Neurol. 2015, 15, 436–444. [Google Scholar] [CrossRef]
- McWhirter, L.; Ritchie, C.; Stone, J.; Carson, A. Functional cognitive disorders: A systematic review. Lancet Psychiatry 2020, 7, 191–207. [Google Scholar] [CrossRef]
- Larner, A.J. Functional cognitive disorders: Update on diagnostic status. Neurodegener. Dis. Manag. 2020, 10, 67–72. [Google Scholar] [CrossRef]
- Larner, A.J. The “attended alone” and “attended with” signs in the assessment of cognitive impairment: A revalidation. Postgrad. Med. 2020, 132, 595–600. [Google Scholar] [CrossRef]
- Randall, A.; Larner, A.J. La maladie du petit papier: A sign of functional cognitive disorder? Int. J. Geriatr. Psychiatry 2018, 33, 800. [Google Scholar] [CrossRef]
- Elhadd, K.; Bharambe, V.; Larner, A.J. Functional cognitive disorders: Can sleep disturbance contribute to a positive diagnosis? J. Sleep Disord. Ther. 2018, 7, 291. [Google Scholar] [CrossRef]
- Elhadd, K.; Bharambe, V.; Larner, A.J. Functional cognitive disorders: Prognosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, A19–A20. [Google Scholar] [CrossRef]
- Schwilk, N.; Klöppel, S.; Schmidtke, K.; Metternich, B. Functional cognitive disorder in subjective cognitive decline—a 10-year follow-up. Int. J. Geriatr. Psychiatry 2021, 36, 677–683. [Google Scholar] [CrossRef]
- Hamid, S.; Larner, A.J. For how long should patients with FCD be followed up? Prog. Neurol. Psychiatry 2019, 23, 16–18. [Google Scholar] [CrossRef] [Green Version]
- Ball, H.; McWhirter, L.; Ballard, C.; Bhome, R.; Blackburn, D.J.; Edwards, M.J.; Fleming, S.M.; Fox, N.C.; Howard, R.; Huntley, J.; et al. Functional cognitive disorder: Dementia’s blind spot. Brain 2020, 143, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, T.; Edwards, M.J.; Isaacs, J.D. A unifying theory for cognitive abnormalities in functional neurological disorders, fibromyalgia and chronic fatigue syndrome: Systematic review. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1308–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharambe, V.; Williamson, J.C.; Larner, A.J. Re: A Unifying Theory for Cognitive Abnormalities in Functional Neurological Disorders, Fibromyalgia and Chronic Fatigue Syndrome. 2018. Available online: https://jnnp.bmj.com/content/89/12/1308.responses (accessed on 7 May 2021).
- Caspi, A.; Houts, R.M.; Belsky, D.W.; Goldman-Mellor, S.J.; Harrington, H.; Israel, S.; Meier, M.H.; Ramrakha, S.; Shalev, I.; Poulton, R.; et al. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin. Psychol. Sci. 2014, 2, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Larner, A. Rethinking Mental Health and Functional Disorders. New Sci. 2020, 245, 26. Available online: https://www.newscientist.com/letter/mg24532690-100-rethinking-mental-health-and-functional-disorders/ (accessed on 7 May 2021).
- Larner, A.J. Dementia screening: A different proposal. Future Neurol. 2018, 13, 177–179. [Google Scholar] [CrossRef]
- Bhome, R.; McWilliams, A.; Huntley, J.D.; Fleming, S.M.; Howard, R.J. Metacognition in functional cognitive disorder—A potential mechanism and treatment target. Cogn. Neuropsychiatry 2019, 24, 311–321. [Google Scholar] [CrossRef]
- Flavell, J.H. First discussant’s comments: What is memory development the development of? Hum. Dev. 1971, 14, 272–278. [Google Scholar] [CrossRef]
- Flavell, J.H. Metacognitive aspects of problem solving. In The Nature of Intelligence; Resnick, L.B., Ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1976; pp. 231–235. [Google Scholar]
- Hart, J.T. Memory and the feeling-of-knowing experience. J. Educ. Psychol. 1965, 56, 208–216. [Google Scholar] [CrossRef]
- Dixon, R.A. The concept of metamemory: Cognitive, developmental, and clinical issues. In Memory Disorders in Psychiatric Practice; Berrios, G.E., Hodges, J.R., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 47–57. [Google Scholar] [CrossRef]
- Mazancieux, A.; Fleming, S.M.; Souchay, C.; Moulin, C.J.A. Is there a g factor for metacognition? Correlations in retrospective metacognitive sensitivity across tasks. J. Exp. Psychol. Gen. 2020, 149, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Hultsch, D.F.; Hertzog, C. The Metamemory in Adulthood (MIA) Questionnaire. Psychopharmacol. Bull. 1988, 24, 671–688. [Google Scholar]
- Gilewski, M.J.; Zelinski, E.M.; Schaie, K.W. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol. Aging 1990, 5, 482–490. [Google Scholar] [CrossRef]
- Sehulster, J.R. Structure and pragmatics of a self-theory of memory. Mem. Cognit. 1981, 9, 263–276. [Google Scholar] [CrossRef]
- Hertzog, C.; Hultsch, D.F.; Dixon, R.A. Evidence for the convergent validity of two self-report metamemory questionnaires. Dev. Psychol. 1989, 25, 687–700. [Google Scholar] [CrossRef]
- Pannu, J.K.; Kaszniak, A.W. Metamemory experiments in neurological populations: A review. Neuropsychol. Rev. 2005, 15, 105–130. [Google Scholar] [CrossRef]
- Moulin, C. Sense and sensitivity: Metacognition in Alzheimer’s disease. In Applied Metacognition; Perfect, T.J., Schwartz, B.L., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 197–223. [Google Scholar] [CrossRef]
- Souchay, C. Metamemory in Alzheimer’s disease. Cortex 2007, 43, 987–1003. [Google Scholar] [CrossRef]
- Cosentino, S. Metacognition in Alzheimer’s disease. In The Cognitive Neuroscience of Metacognition; Fleming, S.M., Frith, C.D., Eds.; Springer: London, UK, 2014; pp. 389–407. [Google Scholar] [CrossRef]
- Rosen, H.J.; Alcantar, O.; Zakrzewski, J.; Shimamura, A.P.; Neuhaus, J.; Miller, B.L. Metacogntion in the behavioral variant of frontotemporal dementia and Alzheimer’s disease. Neuropsychology 2014, 28, 436–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazancieux, A.; Souchay, C.; Casez, O.; Moulin, C.J.A. Metacognition and self-awareness in multiple sclerosis. Cortex 2019, 111, 238–255. [Google Scholar] [CrossRef]
- Kaszniak, A.W.; Edmonds, E.C. Anosognosia and Alzheimer’s disease: Behavioral studies. In The Study of Anosognosia; Prigatano, G.P., Ed.; Oxford University Press: Oxford, UK, 2010; pp. 189–227. [Google Scholar]
- Ernst, A.; Moulin, C.J.A.; Souchay, C.; Mograbi, D.C.; Morris, R. Anosognosia and metacognition in Alzheimer’s disease: Insights from experimental psychology. In The Oxford Handbook of Metamemory; Dunlosky, J., Tauber, S.K., Eds.; Oxford University Press: Oxford, UK, 2016; pp. 451–472. [Google Scholar] [CrossRef]
- Metternich, B.; Schmidtke, K.; Hüll, M. How are memory complaints in functional memory disorder related to measures of affect, metamemory and cognition? J. Psychosom. Res. 2009, 66, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Paradise, M.B.; Glozier, N.S.; Naismith, S.L.; Davenport, T.A.; Hickie, I.B. Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: A cross-sectional study. BMC Psychiatry 2011, 11, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larner, A.J. Metamemory: A construct with diagnostic utility in a cognitive disorders clinic? Int. J. Geriatr. Psychiatry 2018, 33, 553–554. [Google Scholar] [CrossRef]
- Carson, A.; Ludwig, L.; Welch, K. Psychologic theories in functional neurologic disorders. Handb. Clin. Neurol. 2016, 139, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Reuber, M. Towards an integrative theory of psychogenic non-epileptic seizures (PNES). Clin. Psychol. Rev. 2016, 47, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.J. Psychological mechanisms of medically unexplained symptoms: An integrative conceptual model. Psychol. Bull. 2004, 130, 793–812. [Google Scholar] [CrossRef]
- Mumford, D. On the computational architecture of the neocortex. II. The role of cortico-cortical loops. Biol. Cybern. 1992, 66, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.O.; Narens, L. Metamemory: A theoretical framework and new findings. In Psychology of Learning and Motivation; Bower, G.H., Ed.; Academic Press: New York, NY, USA, 1990; Volume 26, pp. 125–173. [Google Scholar]
- Heilman, K. Anosognosia: Possible neuropsychological mechanisms. In Awareness of Deficit after Brain Injury. Clinical and Theoretical Issues; Prigatano, G.P., Schacter, D.L., Eds.; Oxford University Press: New York, NY, USA, 1991; pp. 53–62. [Google Scholar]
- Herzog, C. Metacognition in older adults: Implications for application. In Applied Metacognition; Perfect, T.J., Schwartz, B.L., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 169–196. [Google Scholar] [CrossRef]
- Benjamin, A.S.; Bjork, R.A.; Schwartz, B.L. The mismeasure of memory: When retrieval fluency is misleading as a metamnemonic index. J. Exp. Psychol. Gen. 1998, 127, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Jónsdóttir, M.K.; Adólfsdóttir, S.; Cortez, R.D.; Gunnarsdóttir, M.; Gústafsdóttir, A.H. A diary study of action slips in healthy individuals. Clin. Neuropsychol. 2007, 21, 875–883. [Google Scholar] [CrossRef]
- McWhirter, L.; King, L.; McClure, E.; Ritchie, C.; Stone, J.; Carson, A. The frequency and framing of cognitive lapses in healthy adults. CNS Spectr. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J. Subjective memory complaints: Is family history of dementia a risk factor? J. Neurol. Sci. 2013, 333, e295. [Google Scholar] [CrossRef]
- Ginó, S.; Mendes, T.; Maroco, J.; Ribeiro, F.; Schmand, B.A.; de Mendonça, A.; Guerreiro, M. Memory complaints are frequent but qualitatively different in young and elderly healthy people. Gerontology 2010, 56, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.; Pearson, D. Memory symptoms and memory performance of neurological patients. Br. J. Psychol. 1983, 74, 409–415. [Google Scholar] [CrossRef] [PubMed]
- McWhirter, L.; Ritchie, C.W.; Stone, J.; Carson, A. Performance validity test failure in clinical populations—A systematic review. J. Neurol. Neurosurg. Psychiatry 2020, 91, 945–952. [Google Scholar] [CrossRef]
- Drigas, A.; Mitsea, E. The 8 pillars of metacognition. Int. J. Emerg. Technol. Learn. 2020, 15, 162–178. [Google Scholar] [CrossRef]
- Agnew, S.K.; Morris, R.G. The heterogeneity of anosognosia for memory impairment in Alzheimer’s disease: A review of the literature and a proposed model. Aging Ment. Health 1998, 2, 7–19. [Google Scholar] [CrossRef]
- Morris, R.G.; Hannesdottir, K. Loss of “Awareness” in Alzheimer’s disease. In Cognitive Neuropsychology of Alzheimer’s Disease, 2nd ed.; Morris, R., Becker, J.T., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 275–296. [Google Scholar]
- Morris, R.G.; Mograbi, D.C. Anosognosia, autobiographical memory and self knowledge in Alzheimer’s disease. Cortex 2013, 49, 1553–1565. [Google Scholar] [CrossRef]
- Son, L.K.; Schwartz, B.L. The relation between metacognitive monitoring and control. In Applied Metacognition; Perfect, T.J., Schwartz, B.L., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 15–38. [Google Scholar] [CrossRef]
- Friston, K. The history of the future of the Bayesian brain. Neuroimage 2012, 62, 1230–1233. [Google Scholar] [CrossRef] [Green Version]
- Stephan, K.E.; Manjaly, Z.M.; Mathys, C.D.; Weber, L.A.E.; Paliwal, S.; Gard, T.; Tittgemeyer, M.; Fleming, S.M.; Haker, H.; Seth, A.K.; et al. Allostatic self-efficacy: A metacognitive theory of dyshomeostasis-induced fatigue and depression. Front. Hum. Neurosci. 2016, 10, 550. [Google Scholar] [CrossRef] [Green Version]
- Hallam, B.; Chan, J.; Costafreda, S.G.; Bhome, R.; Huntley, J. What are the neural correlates of meta-cognition and anosognosia in Alzheimer’s disease? A systematic review. Neurobiol. Aging 2020, 94, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S. The Power of Self-Reflection. New Sci. 2021, 250, 36–40. Available online: https://www.newscientist.com/article/mg25033332-300-how-to-boost-your-self-awareness-and-make-better-decisions/ (accessed on 8 May 2021). [CrossRef]
| Memory Expectations | Memory Performance | Comparator | Inference or Phenomenological Sense | Relation of Inference to Clinical Context | Behavioural Outcome |
|---|---|---|---|---|---|
| T | T | No mismatch | “Everything’s OK” | Appropriate (Inference true) | Well and aware |
| T | F | Mismatch (=prediction error) | “Something’s wrong” | Appropriate (Inference true) | Unwell and aware = amnesia |
| F | T | Mismatch (=prediction error) | “Something’s wrong” | Inappropriate (Inference false) | Well and unaware = FCD |
| F | F | No mismatch | “Everything’s OK” | Inappropriate (Inference false) | Unwell and unaware = anosognosia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larner, A.J. Functional Cognitive Disorders (FCD): How Is Metacognition Involved? Brain Sci. 2021, 11, 1082. https://doi.org/10.3390/brainsci11081082
Larner AJ. Functional Cognitive Disorders (FCD): How Is Metacognition Involved? Brain Sciences. 2021; 11(8):1082. https://doi.org/10.3390/brainsci11081082
Chicago/Turabian StyleLarner, Andrew J. 2021. "Functional Cognitive Disorders (FCD): How Is Metacognition Involved?" Brain Sciences 11, no. 8: 1082. https://doi.org/10.3390/brainsci11081082
APA StyleLarner, A. J. (2021). Functional Cognitive Disorders (FCD): How Is Metacognition Involved? Brain Sciences, 11(8), 1082. https://doi.org/10.3390/brainsci11081082





