An ID-Associated Application to Facilitate Patient-Tailored Management of Multiple Sclerosis
Abstract
1. Introduction
2. Materials
3. Results
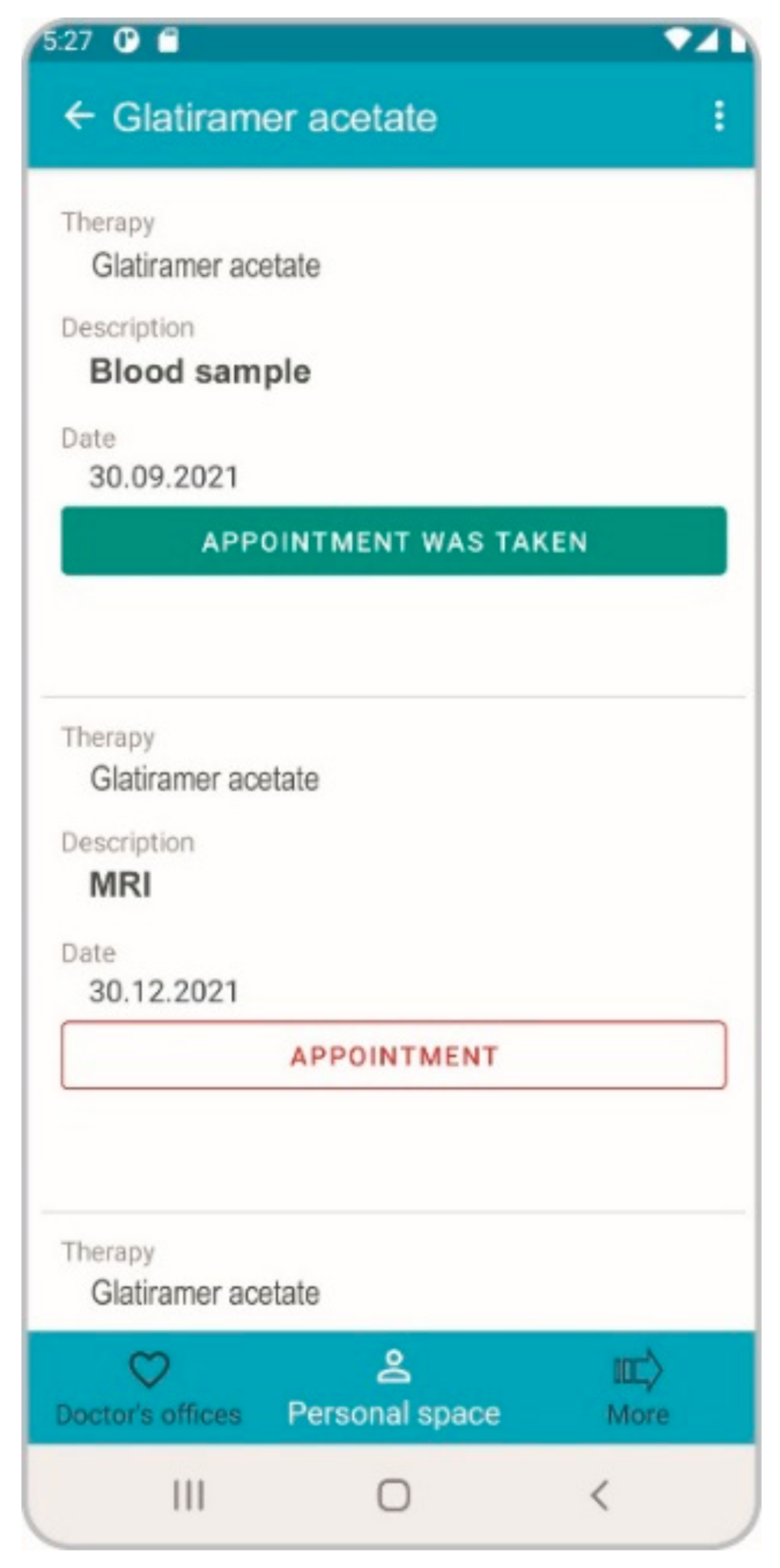
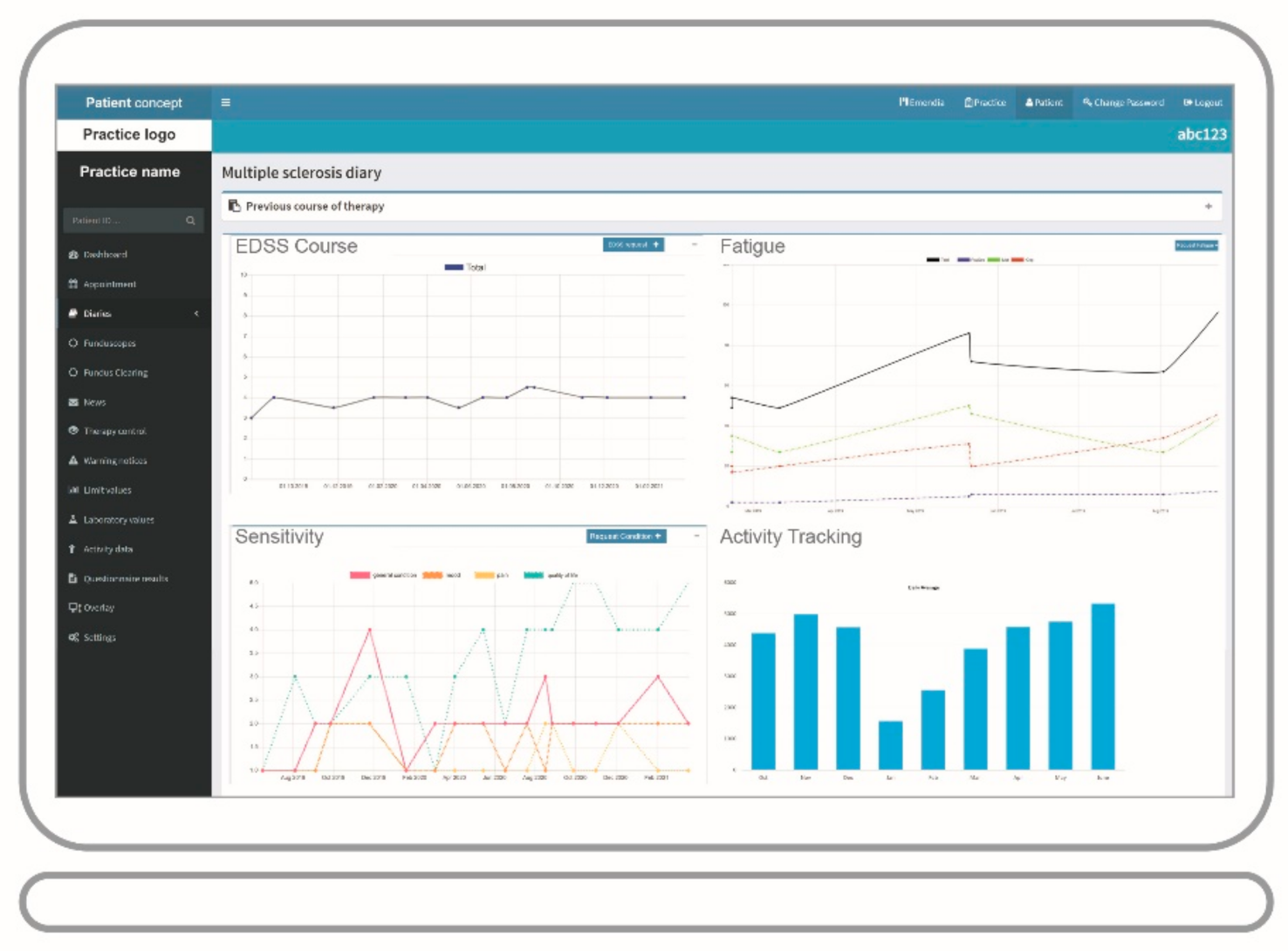
3.1. Application in the Field of MS
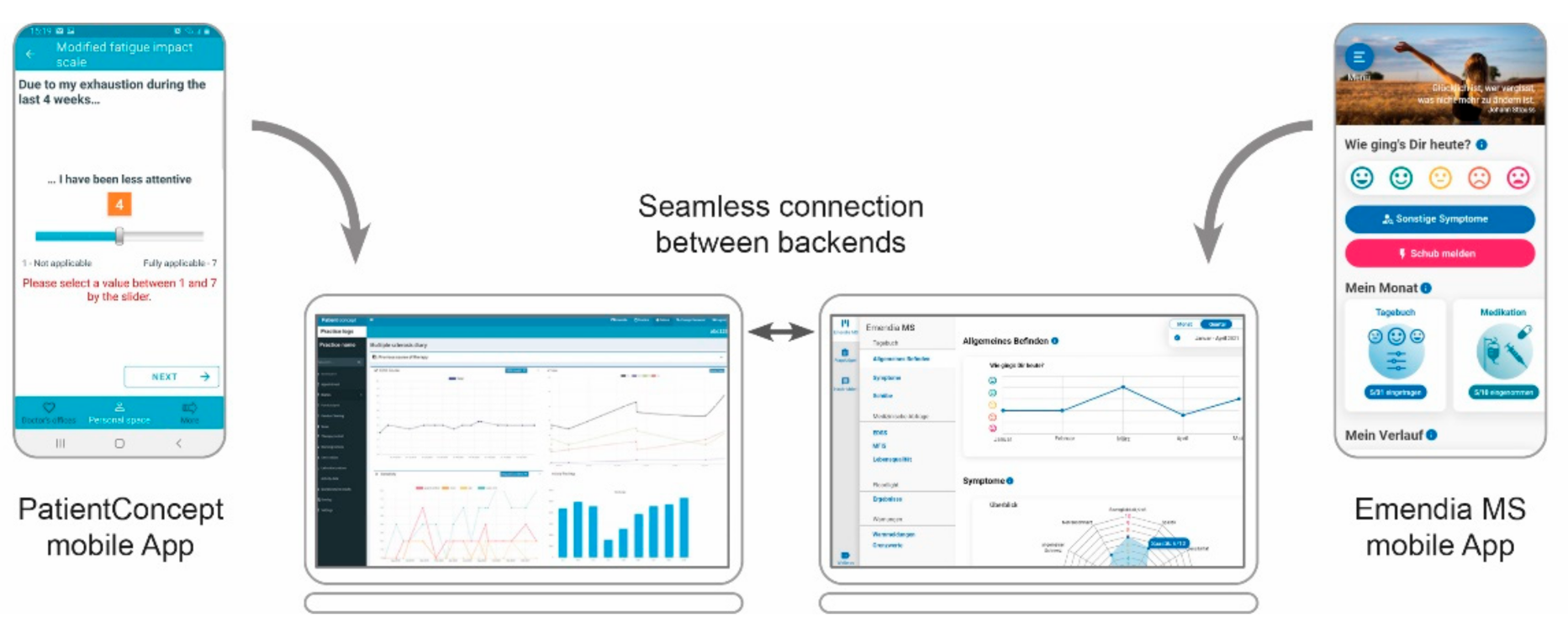
3.2. Further Applications beyond MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Multiple Sclerosis International Federation. Atlas of MS 2020, 3rd edition. Part 1: Mapping Multiple Sclerosis around the World. Available online: https://www.msif.org/wp-content/uploads/2020/10/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf (accessed on 19 May 2021).
- Coetzee, T.; Thompson, A.J. Atlas of MS 2020: Informing global policy change. Mult. Scler. 2020, 26, 1807–1808. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Ziemssen, T. Multiple sclerosis beyond EDSS: Depression and fatigue. J. Neurol. Sci. 2009, 277, S37–S41. [Google Scholar] [CrossRef]
- Kip, M.; Zimmermann, A. Krankheitsbild Multiple Sklerose. Weißbuch Multiple Sklerose; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Reynolds, R.; Dennis, S.; Hasan, I.; Slewa, J.; Chen, W.; Tian, D.; Bobba, S.; Zwar, N. A systematic review of chronic disease management interventions in primary care. BMC Fam. Pract. 2018, 19, 11. [Google Scholar] [CrossRef]
- WHO: mHealth. New Horizons for Health through Mobile Technologies. Available online: https://www.who.int/goe/publications/goe_mhealth_web.pdf (accessed on 12 August 2021).
- Marziniak, M.; Brichetto, G.; Feys, P.; Meyding-Lamade, U.; Vernon, K.; Meuth, S.G. The Use of Digital and Remote Communication Technologies as a Tool for Multiple Sclerosis Management: Narrative Review. JMIR Rehabil. Assist. Technol. 2018, 5, e5. [Google Scholar] [CrossRef]
- Lavorgna, L.; Brigo, F.; Moccia, M.; Leocani, L.; Lanzillo, R.; Clerico, M.; Abbadessa, G.; Schmierer, K.; Solaro, C.; Prosperini, L.; et al. e-Health and multiple sclerosis: An update. Mult. Scler. 2018, 24, 1657–1664. [Google Scholar] [CrossRef]
- Kern, R.; Haase, R.; Eisele, J.C.; Thomas, K.; Ziemssen, T. Designing an Electronic Patient Management System for Multiple Sclerosis: Building a Next Generation Multiple Sclerosis Documentation System. Interact. J. Med. Res. 2016, 5, e2. [Google Scholar] [CrossRef] [PubMed]
- Haase, R.; Wunderlich, M.; Dillenseger, A.; Kern, R.; Akgun, K.; Ziemssen, T. Improving multiple sclerosis management and collecting safety information in the real world: The MSDS3D software approach. Expert Opin. Drug Saf. 2018, 17, 369–378. [Google Scholar] [CrossRef]
- Matthews, P.M.; Block, V.J.; Leocani, L. E-health and multiple sclerosis. Curr. Opin. Neurol. 2020, 33, 271–276. [Google Scholar] [CrossRef]
- Bundesministerium für Gesundheit. Available online: https://www.bundesgesundheitsministerium.de/digital-healthcare-act.html (accessed on 25 May 2021).
- Lang, M.; Mayr, M.; Ringbauer, S.; Cepek, L. PatientConcept App: Key Characteristics, Implementation, and its Potential Benefit. Neurol. Ther. 2019, 8, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; ATTRv. Abstract Submitted to DGN Congress 2021. University of Luxembourg. CON-VINCE Study. Available online: https://wwwde.uni.lu/universitaet/aktuelles/diashow/erste_ergebnisse_der_con_vince_studie (accessed on 27 May 2021).
- Klawonn, F.; Lechner, W.; Lechner, C.; Mayr, M.; Lang, M.; Grigull, L. Neuromuskuläre Erkrankungen: Mit künstlicher Intelligenz zur schnelleren Diagnose; Abstract Submitted to DGN Congress 2021. Available online: https://dgnkongress.org/home.html (accessed on 12 August 2021).
- Cepek, L.; Guggenmoos, F.; Ringbauer, S.; Mayr, M.; Lang, M. Telemetrische Untersuchung des Augenhintergrunds; Abstract Sumitted to DGN Congress 2021. Available online: https://dgnkongress.org/home.html (accessed on 12 August 2021).
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Eng. J. Med. 2018, 378, 169–180. [Google Scholar] [CrossRef]
- Giovannoni, G.; Butzkueven, H.; Dhib-Jalbut, S.; Hobart, J.; Kobelt, G.; Pepper, G.; Sormani, M.P.; Thalheim, C.; Traboulsee, A.; Vollmer, T. Brain health: Time matters in multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 9, S5–S48. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Ray, P. mHealth—an Ultimate Platform to Serve the Unserved. Yearb. Med. Inform. 2010, 19, 94–100. [Google Scholar] [CrossRef]
- Tachakra, S.; Wang, X.H.; Istepanian, R.S.; Song, Y.H. Mobile e-health: The unwired evolution of telemedicine. Telemed J. E Health 2003, 9, 247–257. [Google Scholar] [CrossRef]
- Ziemssen, T.; Thomas, K. Treatment optimization in multiple sclerosis: How do we apply emerging evidence? Expert Rev. Clin. Immunol. 2017, 13, 509–511. [Google Scholar] [CrossRef][Green Version]
- D’Amico, E.; Haase, R.; Ziemssen, T. Review: Patient-reported outcomes in multiple sclerosis care. Mult. Scler. Relat. Disord. 2019, 33, 61–66. [Google Scholar] [CrossRef]
- Brichetto, G.; Zaratin, P. Measuring outcomes that matter most to people with multiple sclerosis: The role of patient-reported outcomes. Curr. Opin. Neurol. 2020, 33, 295–299. [Google Scholar] [CrossRef]
- Scholz, M.; Haase, R.; Trentzsch, K.; Stoelzer-Hutsch, H.; Ziemssen, T. Improving Digital Patient Care: Lessons Learned from Patient-Reported and Expert-Reported Experience Measures for the Clinical Practice of MultidimensionalWalking Assessment. Brain Sci. 2021, 11, 786. [Google Scholar] [CrossRef]
- Yeandle, D.; Rieckmann, P.; Giovannoni, G.; Alexandri, N.; Langdon, D. Patient Power Revolution in Multiple Sclerosis: Navigating the New Frontier. Neurol. Ther. 2018, 7, 179–187. [Google Scholar] [CrossRef]
- Rieckmann, P.; Centonze, D.; Elovaara, I.; Giovannoni, G.; Havrdova, E.; Kesselring, J.; Kobelt, G.; Langdon, D.; Morrow, S.A.; Oreja-Guevara, C.; et al. Unmet needs, burden of treatment, and patient engagement in multiple sclerosis: A combined perspective from the MS in the 21st Century Steering Group. Mult. Scler. Relat. Disord 2018, 19, 153–160. [Google Scholar] [CrossRef]
- Heesen, C.; Bruce, J.; Feys, P.; Sastre-Garriga, J.; Solari, A.; Eliasson, L.; Matthews, V.; Hausmann, B.; Ross, A.P.; Asano, M.; et al. Adherence in multiple sclerosis (ADAMS): Classification, relevance, and research needs. A meeting report. Mult. Scler. 2014, 20, 1795–1798. [Google Scholar] [CrossRef]
- Col, N.F.; Solomon, A.J.; Springmann, V.; Garbin, C.P.; Ionete, C.; Pbert, L.; Alvarez, E.; Tierman, B.; Hopson, A.; Kutz, C.; et al. Whose Preferences Matter? A Patient-Centered Approach for Eliciting Treatment Goals. Med. Decis. Mak. 2018, 38, 44–55. [Google Scholar] [CrossRef]
- Rieckmann, P.; Boyko, A.; Centonze, D.; Elovaara, I.; Giovannoni, G.; Havrdova, E.; Hommes, O.; Kesselring, J.; Kobelt, G.; Langdon, D.; et al. Achieving patient engagement in multiple sclerosis: A perspective from the multiple sclerosis in the 21st Century Steering Group. Mult. Scler. Relat. Disord. 2015, 4, 202–218. [Google Scholar] [CrossRef]
- Treadaway, K.; Cutter, G.; Salter, A.; Lynch, S.; Simsarian, J.; Corboy, J.; Jeffery, D.; Cohen, B.; Mankowski, K.; Guarnaccia, J.; et al. Factors that influence adherence with disease-modifying therapy in MS. J. Neurol. 2009, 256, 568–576. [Google Scholar] [CrossRef]
- Lugaresi, A.; Ziemssen, T.; Oreja-Guevara, C.; Thomas, D.; Verdun, E. Improving patient-physician dialog: Commentary on the results of the MS Choices survey. Patient Prefer. Adherence 2012, 6, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ammenwerth, E.; Schnell-Inderst, P.; Hoerbst, A. The impact of electronic patient portals on patient care: A systematic review of controlled trials. J. Med. Internet Res. 2012, 14, e162. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, I.C.; van Uden-Kraan, C.F.; Eerenstein, S.E.; Rinkel, R.N.; Aalders, I.J.; van den Berg, K.; de Goede, C.J.; van Stijgeren, A.J.; Cruijff-Bijl, Y.; de Bree, R.; et al. A Participatory Design Approach to Develop a Web-Based Self-Care Program Supporting Early Rehabilitation among Patients after Total Laryngectomy. Folia Phoniatr. Logop. 2015, 67, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.S.; Brown, T.L.; Williams, A.; Hasnain-Wynia, R.; Thompson, J.A.; Baker, D.W. Disparities in enrollment and use of an electronic patient portal. J. Gen. Intern. Med. 2011, 26, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.S.; Bolton, K.; Freriks, G. The effect of patient portals on quality outcomes and its implications to meaningful use: A systematic review. J. Med. Internet Res. 2015, 17, e44. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Ringbauer, S.; Mayr, M.; Cepek, L. How to improve disease management of chronically ill patients? Perception of telemetric ECG recording and a novel software application. Clin. Res. Trials 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Kornhuber, A.; Lang, M. Interne und externe Einflussfaktoren auf die Adhärenz bei Multipler Sklerose—eine retrospektive und prospektive Analyse mit der Medication Possession Ratio. In Proceedings of the 87. Kongress der Deutschen Gesellschaft für Neurologie, München, Germany, 15–19 September 2014. [Google Scholar]
- Radić, M.; Donner, I.; Waak, M.; Brinkmann, C.; Stein, L.; Radić, D. Digitale Gesundheitsanwendungen: Die Akzeptanz steigern. Dtsch. Arztebl. 2021, 118, A-286–B-250. [Google Scholar]
- BfArM. DiGa-Verzeichnis. Available online: https://diga.bfarm.de/de/verzeichnis (accessed on 12 August 2021).
- Feys, P.; Giovannoni, G.; Dijsselbloem, N.; Centonze, D.; Eelen, P.; Lykke Andersen, S. The importance of a multi-disciplinary perspective and patient activation programmes in MS management. Mult. Scler. 2016, 22, 34–46. [Google Scholar] [CrossRef]
- Gallien, P.; Gich, J.; Sanchez-Dalmau, B.F.; Feneberg, W. Multidisciplinary management of multiple sclerosis symptoms. Eur. Neurol. 2014, 72, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Messmer, U.M.; Battaglia, M.A.; Zagami, P.; Solaro, C. The interdisciplinary approach to the treatment of multiple sclerosis patients in Italy: An aspiration or a reality? Mult. Scler. 2002, 8, 36–39. [Google Scholar] [CrossRef]
- Birnbaum, F.; Lewis, D.; Rosen, R.K.; Ranney, M.L. Patient engagement and the design of digital health. Acad. Emerg. Med. 2015, 22, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, B.; de la Torre-Díez, I.; López-Coronado, M. Privacy and Security in Mobile Health Apps: A Review and Recommendations. J. Med. Syst. 2015, 39, 181. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, M.; Rau, D.; Cepek, L.; Cürten, F.; Ringbauer, S.; Mayr, M. An ID-Associated Application to Facilitate Patient-Tailored Management of Multiple Sclerosis. Brain Sci. 2021, 11, 1061. https://doi.org/10.3390/brainsci11081061
Lang M, Rau D, Cepek L, Cürten F, Ringbauer S, Mayr M. An ID-Associated Application to Facilitate Patient-Tailored Management of Multiple Sclerosis. Brain Sciences. 2021; 11(8):1061. https://doi.org/10.3390/brainsci11081061
Chicago/Turabian StyleLang, Michael, Daniela Rau, Lukas Cepek, Fia Cürten, Stefan Ringbauer, and Martin Mayr. 2021. "An ID-Associated Application to Facilitate Patient-Tailored Management of Multiple Sclerosis" Brain Sciences 11, no. 8: 1061. https://doi.org/10.3390/brainsci11081061
APA StyleLang, M., Rau, D., Cepek, L., Cürten, F., Ringbauer, S., & Mayr, M. (2021). An ID-Associated Application to Facilitate Patient-Tailored Management of Multiple Sclerosis. Brain Sciences, 11(8), 1061. https://doi.org/10.3390/brainsci11081061





