Abstract
Although motor coordination and imitation are important factors affecting motor skill learning, few studies have examined the relationship between them in healthy adults. In order to address this in the present study, we used fNIRS to analyze the relationship between motor coordination and imitation in college students. Our results showed that: (1) motor coordination in female students was positively correlated with the average time taken to perform an imitation; (2) the mean imitation time was negatively correlated with the activation level of the supplementary motor cortex, primary somatosensory cortex, and angular gyrus of the mirror neuron system; (3) motor coordination in female students moderated mirror neuron system (MNS) activation and imitation. For women with low rather than high motor coordination, higher MNS activation was associated with a stronger imitation ability. These results demonstrate that motor coordination in female students is closely related to action imitation, and that it moderates the activation of the MNS, as measured via fNIRS.
1. Introduction
Action imitation emerges in infancy [1] and is present in many aspects of life, especially in the process of motor skill learning. Imitation is the repetition of observed physical movements [2]. In motor skill learning, a learner first observes the movement of a demonstrator, and is then gradually able to produce movements that are consistent with the demonstrator’s movements through repeated exercise [3]. When learning new motor skills, an individual’s motor coordination plays an important role. Motor coordination refers to the timing of interactions between different physical systems, body parts, and organs in order to achieve a unified action. Motor coordination is an important basis for the formation of motor skills, and requires comprehensive interactions between different neural systems [4]. Human motor coordination is involved in generating reflexive responses, spatial orientation, ontological perception, rhythm perception and production, balance, action cognition, and so on [4].
Despite the close connection between imitation and motor coordination, few studies have examined this relationship. Further, these studies have been limited to infants and children with developmental coordination disorder (DCD). Researchers examining the development of movement in infants have found that those with better movement coordination are more quickly able to imitate and complete grasping movements [5,6]. Compared with healthy children, children with DCD may have difficultly imitating simple and complex gestures [7]. Thus, there is a close relationship between motor coordination and imitation in infants and children. However, whether this connection is also present in healthy adults has not yet been established.
At the end of the 20th century, researchers began to examine the neural mechanisms of imitation using neuroimaging technology (magnetic resonance imaging, electroencephalogram, etc.). When people observe, imagine, perform, and imitate actions, the mirror neuron system (MNS), comprising multimodal neurons in the frontal and parietal lobes, is triggered [8,9]. The MNS was first identified in the premotor cortex (F5) and the rostral side (PFG) of the inferior parietal lobule (IPL) in non-human primates [10]. Since then, researchers have explored the MNS in humans. MNS activity responsible for human imitation has been found to include the dorsal premotor cortex (PMD), ventral premotor cortex (PMV), intraparietal sulcus (AIPS), and superior marginal gyrus (SMG), which together constitute the core circuit of visual–motor transmission. In addition, sensorimotor regions, including the inferior frontal gyrus (IFG), supplementary motor area (SMA), and angular gyrus (ANG), also play a role [11]. Reynolds et al. examined brain activation when both children with DCD and healthy children observed, performed, and imitated finger tasks, and found differences in the activation of brain regions related to mirror neurons between children with DCD and healthy children during imitation. Thus, MNS dysfunction may be the basis of imitation and movement disorders in children [12]. Another study explored the activation of brain regions in children with DCD and healthy children during imitation, and found that posterior inferior frontal gyrus activation was lower in children with DCD [7]. Current studies have shown that, compared with healthy children, those with DCD not only exhibit defects in imitation, but also show altered MNS activity in regions responsible for imitation.
Most previous studies examining the neural basis of imitation have used magnetic resonance imaging (MRI) [8,9]. However, subjects must lie down during data collection, which limits the range of actions that can be performed in an imitation task. Functional near-infrared spectroscopy (fNIRS) has the advantages of a high temporal resolution and spatial resolution, and is not as sensitive as MRI to the motor actions of subjects during scanning [13,14,15]. Thus, it is a more convenient choice for the collection of neural data from healthy adults during an action simulation task.
In this study, we used fNIRS to examine changes in oxyhemoglobin (HbO) in the frontal and parietal cortex during imitation. Our goal was to elucidate the relationship between motor coordination and imitation in healthy adults in terms of behavior and neural activation. We hypothesized that there would be a correlation between motor coordination and imitation in healthy adults, that the MNS would be the neural basis of imitation-related behaviors, and that different types of motor coordination would have different effects on MNS activation.
2. Materials and Methods
2.1. Subjects
We recruited 53 college students (24 men and 29 women). All subjects were right-handed, had no diseases affecting brain function or structure, had no mental health conditions (no anxiety or depression in the last month), a visual acuity (including corrected vision) of >0.8, and had no color blindness or color weakness. The participants were asked not to drink coffee or other drinks containing stimulants during the 24 h before the study. The experimental protocol was approved by the university ethics committee (approval number: HR 125-2019), and all subjects provided written informed consent.
2.2. Stimuli
2.2.1. Imitation Task
The imitation task used in this study involved single gesture imitation. According to Makuuchi and Pizzamiglio et al. [16,17], we prepared 70 different hand gestures, including those involving the left hand, right hand, and both hands. We used finger coordination and palm orientation as variables, and generated 70 gesture images (25 for the left hand, 25 for the right hand, and 20 for both hands). We conducted a pilot study in which we asked four subjects to imitate the gestures and evaluate the pictures, and as a result, 10 difficult gesture pictures were excluded. The remaining 60 gesture pictures (including 18 for the left hand, 23 for the right hand, and 9 for both hands) were used in the imitation task.
We used the E-Prime software to administer the action simulation task. The task consisted of 60 10-s trials presented on a screen in a pseudo-random order. Each trial included a fixation point (6S) and a gesture imitation task map (4S). When a new gesture imitation task appeared, the subjects were required to imitate the movement as fast as possible and to then press the ‘confirm’ key on a keyboard. After all of the 60 tasks were completed, the screen indicated that the participants could rest for 30 s, and the task ended (as shown in Figure 1). The hand gesture imitation task was divided into three experimental conditions: left hand imitation, right hand imitation, and double hand imitation. In order to facilitate the supervision of the participants, as well as the evaluation of task performance, video recording was conducted during the experiment.
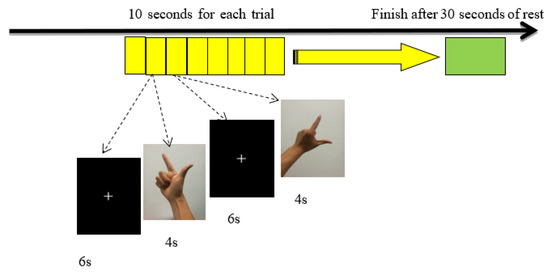
Figure 1.
Flow chart of the imitation tasks.
In previous studies on imitation, the speed of imitation [18], accuracy [19], error (spatial error, body part error, etc.) [19,20] have been used to evaluate imitation, along with auxiliary tools (such as motion capture instruments) [21]. Due to the fact that previous studies have indicated that accuracy and imitation speed are effective measures of imitation, we evaluated these variables in the present study.
2.2.2. Motor Coordination Test—Harre Circuit Test (HCT)
The HCT is a measure of dynamic motor coordination, coordinative ability, and cognitive capabilities, which is general and effective in measuring coordination ability. Specifically, subjects are required to move through a circuit (Figure 2) as quickly as possible. After completing a 10-min warm-up activity, the subjects were asked to start running 0.7 m from a starting point, and to move through the circuit as directed. Timing began when the participants arrived at the starting point. They were instructed to enter the circuit at the fastest speed possible, and to move through the circuit in a particular pattern to reach the end point. If the subjects made mistakes (stepping on the center point, crossing the obstacles in the wrong order, etc.) or touched any of the obstacles during the test (e.g., marker posts), they were considered to have failed the test, and were asked to repeat the test after an interval of 5 min. Two valid scores were recorded (the interval between the two tests was 5 min) for each subject, and the shortest score was selected for statistical analysis [22,23].
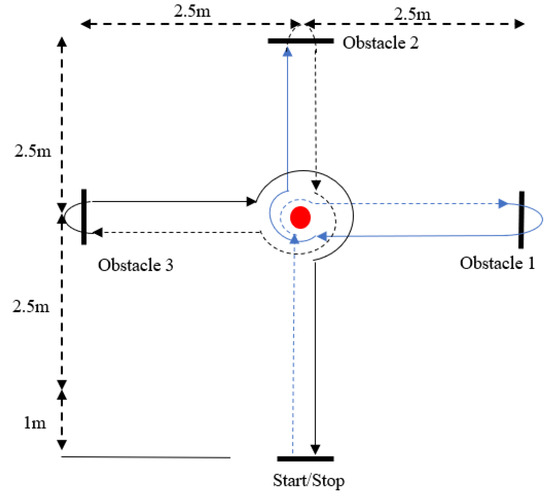
Figure 2.
Harre circuit test (HCT).
2.3. Procedure
After arriving at the laboratory, the subjects completed a form to collect their demographic information and signed an informed consent form. After describing the detailed experimental process and related task requirements, the experiment began.
The subjects sat at a table in front of a computer, and were fitted with an fNIRS cap. After adjusting the channel signals in order to ensure a strong connection, the action simulation task began. When the subjects performed the imitation tasks, they were required to confine their actions to a designated area, which was indicated by a yellow circle positioned in front of the computer on the experimental table (as shown in Figure 3). Requiring the subjects to complete the imitation task in the designated place minimized the difference in the imitation time caused by different imitation positions. When the subjects completed the imitation task, they conducted the HCT in a specified test site.
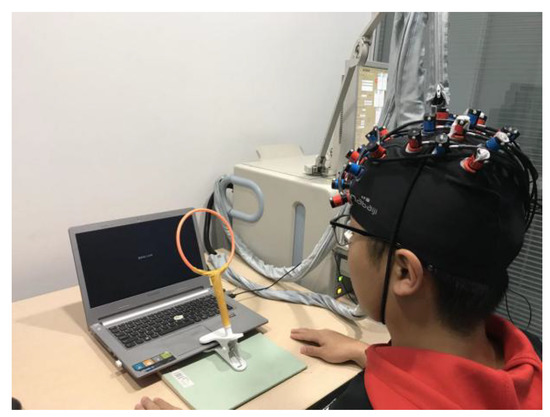
Figure 3.
Experimental settings.
2.4. fNIRS Data Acquisition
While the subjects were performing the imitation task, we used fNIRS (ETG-4000, Hitachi Medical Coopteration, Tokyo, Japan) to measure changes in signals representing oxyhemoglobin and deoxyhemoglobin concentrations in the brain. The sampling rate was 10 Hz. We used two 3 × 5 light pole probe patches, each with 8 emitters and 7 receivers. The probe spacing was 3 cm, and there were 44 channels in total, covering the frontal and parietal lobes, as shown in Figure 4. According to the international standard 10–20 system, the probe at the middle point of the lowest line of the plate was placed over FPZ, and the paths were placed horizontally. The specific spatial position of each channel was determined using a 3D locator.
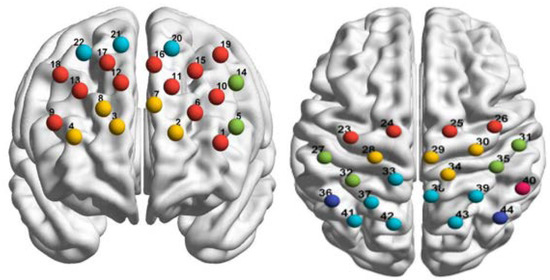
Figure 4.
Schematic diagram of 44 channels over the frontal lobe and parietal lobe (left: frontal lobe; right: parietal lobe).
Using the probability registration method, the fNIRS channel positions were reg-istered with the Montreal Neurological Institute spatial coordinates in order to obtain the corresponding locations of the Brodmann areas in Talairach coordinates. In this study, we examined activity in the dorsolateral prefrontal cortex (DLPFC, Left-DLPFC: ch1, ch 6, ch 10, ch 11, ch 15, ch 16, ch 19; Right-DLPFC: ch 9, ch 12, ch 13, ch 17, ch 18), frontopolar area (Left: ch 2, ch 7; Right: ch3, ch 4, ch 8), pars triangularis Broca’s area (PTR, Left-PTR: ch 5, ch 14), prefrontal eye movement area (Left: ch 20; Right: ch 21, ch 22), supplementary motor cortex (SMA, Left-SMA: ch 23, ch 24; Right-SMA: ch 25, ch 26), primary motor cortex (PMC, Left-PMC: ch 28; Right-PMC: ch 29, ch 30, ch 34), primary somatosensory cortex (S1, Left-S1: ch 27, ch 32; Right-S1: ch 31, ch 35), soma-tosensory association cortex (SSAC, Left-SSAC: ch 33, ch 37, ch 41, ch 42; Right-SSAC: ch 38, ch 39,ch 43), angular gyrus (ANG, Left-ANG: ch 36; Right-ANG: ch 44), and su-pramarginal gyrus (SMG, Left-SMG: ch 40), as shown in Figure 4.
As per previous studies on the neural mechanisms of imitation, we only analyzed activity in the MNS, including the SMA, PMC, S1, SSAC, ANG, and SMG.
2.5. Data Processing and Analysis
2.5.1. Behavioral Data Processing
We used the E-Prime software to evaluate performance in the imitation tasks. We calculated the accuracy of gesture imitation and the average time for each trial under the right hand, left hand, and double hand imitation. A trial was scored as ‘correct’ if the gesture was completed accurately within 4 s of the trial onset. Otherwise, the trial was scored as ‘incorrect’. We calculated the reaction time as the average time taken for the subjects to press the ‘confirm’ key in ‘correct’ trials.
2.5.2. fNIRS Data Processing and Analysis
We used the NIRS_SPM toolbox in MATLAB (Math-Works Inc., Natick, MA, USA) to process changes in blood-oxygen-dependent signals in the fNIRS data [24]. NIRS_SPM mainly uses the general linear model (GLM) to analyze fNIRS data. Data preprocessing included wavelet analysis and low-pass filtering in order to remove drift and noise, thus eliminating the influence of movements and physiological artifacts (such as heartbeats) on the fNIRS data. Second, according to the GLM model, we set the reference wave of oxygenated hemoglobin (HbO), deoxygenated hemoglobin (HbR) and total hemoglobin (HbT) changes in each channel. The time-dependent hemodynamic response function included three conditions: left hand imitation, right hand imitation, and double hand imitation. Finally, the model estimated the changes in HbO, HbR, and HbT concentration in the 44 channels, expressed as beta values. In order to obtain the neural activity in the cerebral cortex associated with imitation, we only analyzed brain activation in successful trials.
2.5.3. Statistical Analysis
SPSS 26.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the relationship between imitation and motor coordination. Independent sample t tests were used to compare motor coordination between the male and female subjects. Pearson correlation analysis was used to calculate the correlation between the data of imitation behavior and the activation and motor coordination of imitation-related brain areas.
3. Results
We recruited a total of 53 subjects (24 men and 29 women). As obesity will affect the performance of the coordination ability, the results will cause great interference, so the obese subjects were deleted. According to the World Health Organization definition of obesity [25] (BMI values ≥ 24.0), seven subjects (six men and one woman) with BMI values ≥ 24.0 were excluded from the analysis. Finally, data from 46 subjects were analyzed. The demographic information is given in Table 1.

Table 1.
Demographic characteristics of subjects (M ± SD).
3.1. Behavioral Results
The descriptive statistics regarding motor coordination and imitation ability are given in Table 2 below.

Table 2.
Descriptive statistics of motor coordination and imitation (M ± SD).
The results showed a significantly positive correlation between the average time taken to complete the imitation tasks in the right hand (r = 0.30, p = 0.04), left hand (r = 0.32, p = 0.02), and double hand trials (r = 0.39, p = 0.01). A correlation test revealed a significant correlation between motor coordination and imitation ability, such that higher motor coordination was associated with a shorter imitation time.
On this basis, we used gender as a grouping variable, and conducted an independent sample t test to identify differences in motor coordination and imitation between male and female participants. The results showed significant differences in motor coordination between men and women (t(44) = −3.18, p = 0.003, Cohen’s d = −0.97) such that motor coordination in men was significantly better than that in women.
Given the significant gender differences in motor coordination, we analyzed the data from male and female participants separately. The results showed that, in female participants, there was a significantly positive correlation between motor coordination and the average time in the right hand imitation trials (rright hand = 0.47, p = 0.01), left hand imitation trials (rleft hand = 0.45, p = 0.02), and double hand imitation trials (rdouble hands = 0.53, p = 0.004). Thus, better motor coordination was linked with shorter imitation time in female students, as shown in Figure 5. Since we found no correlation between motor coordination and imitation in the male students, we did not further examine the data from this group.
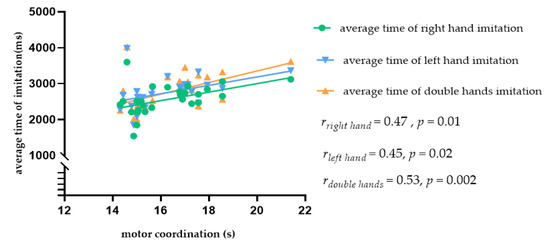
Figure 5.
Scatter plot showing the relationship between motor coordination and the average imitation time in female students.
3.2. Correlation Test between Imitation and Activation of Region of Interest (ROI)
In order to observe the neural response to the task intuitively, we presented time series across all subjects from ROIs (Figure 6). In this figure, we can obviously see the task period yielded an increased hemodynamic response, as the amplitude of the HbO signal is higher than that of the HbR and HbT signal, so next we only analyzed HbO.
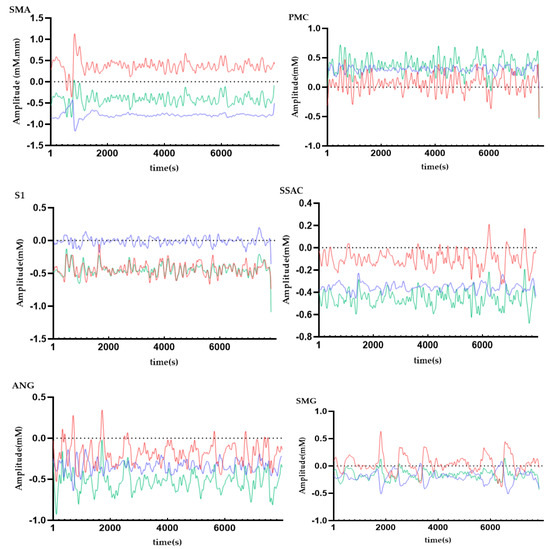
Figure 6.
Time series of hemodynamic response for HbO (red line), HbR (blue line), and HbT (green line) across all subjects FAbbreviations: SMA = supplementary motor cortex; PMC = primary motor cortex; S1 = primary somatosensory cortex; SSAC = somatosensory association cortex; ANG = angular gyrus; SMG = supramarginal gyrus.
Due to the fact that we found no significant correlation between the accuracy of imitation and motor coordination in the behavioral results, we did not further analyze the imitation accuracy data. The correlation results are shown in Table 3. The average imitation time in women was negatively correlated with the activation degree in most regions of interest (ROIs), such that higher activation in the relevant ROI was associated with a stronger imitation ability.

Table 3.
Correlation between average imitation time and MNS activation in female students.
3.3. The Moderating Effect of Motor Coordination
In order to understand whether motor coordination modulated the relationship between imitation and ROI activation in the MNS, we first examined the correlation between motor coordination and ROIs in the MNS in the male and female participants. The results showed that motor coordination in women was significantly negatively correlated with PMC activation during right hand imitation (rright hand-PMC = −0.48, p = 0.01) and double hand imitation trials (rdouble hands-PMC = −0.39, p = 0.04), as shown in Figure 7. Given the significant correlation between motor coordination, imitation, and MNS activity, as well as the data indicating that different types of motor coordination may have different effects on imitation, we speculated regarding the ways in which motor coordination might influence the relationship between imitation and MNS activation.
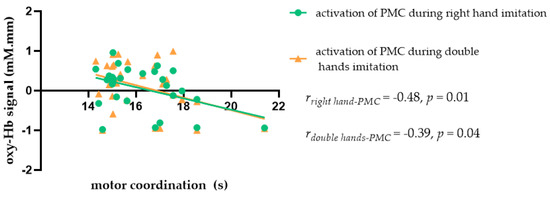
Figure 7.
Scatter plot showing the relationship between motor coordination and MNS activation during imitation in female students.
We regarded MNS activation as an independent variable, motor coordination as a moderating variable, and imitation as a dependent variable. Our data indicate that motor coordination influenced the relationship between ROI activation and imitation in female participants only. See Table 4 for details regarding the moderating effect and simple slope analysis results.

Table 4.
Motor coordination moderates the relationship between MNS activity and imitation behavior in female students.
From the above table, one can see that when low motor coordination (M = 17.53) was associated with higher MNS activation (PMC and S1 for right hand, left hand, and double hand trials and SMA, ANG, and SMG for left hand trials), female students exhibited a better imitation ability. Further, when motor coordination was high (M = 14.96), MNS activation did not significantly affect imitation.
4. Discussion
In this study, we explored the relationship between motor coordination and imitation, as well as the moderating role of motor coordination in terms of behavior and surface neural activation. Our results revealed a significant positive correlation between motor coordination and behavioral performance in the right hand, left hand, and double hand trials in female students. Further analysis indicated that motor coordination in female students moderated the relationship between MNS activation and imitation behavior. For female students with low motor coordination, ROI activation was significantly correlated with imitation performance. Thus, higher MNS activation was associated with greater action imitation in this population. However, we found no relationship between ROI activation and action imitation in women with higher motor coordination.
We found a positive correlation between motor coordination and the average imitation time in female students. Thus, women with better motor coordination had a stronger action imitation ability. This is consistent with the results of Kamandulis et al. [26], who examined basketball skills in 17-year-old participants, and found that basketball players with better motor coordination acquired new basketball skills faster. This phenomenon may be related to a strong imitation ability, because motor coordination can facilitate the integration between different systems, body parts, and organs during imitation. Thus, individuals with good motor coordination are likely to have a strong imitation ability. In the present study, this result was only observed in female subjects, and not in male subjects. We speculate that this may be due to the increased likelihood of boys participating in sports activities, which can improve motor coordination during development.
We also found that the PMC, ANG, SMG, and other MNS ROIs were significantly activated during imitation. Previous studies have indicated that the MNS is activated during imitation, mainly in the PMC, ANG, SMG, and SSAC [11,27]. In our participants, the average imitation time was negatively correlated with the degree of MNS activation, which indicated that the subjects achieved a better imitation performance if they had stronger internal processing. Thus, our study verified that brain regions associated with the MNS were activated during imitation.
This study found that higher motor coordination in healthy female college students was associated with higher activation in the PMC and a stronger action imitation ability. Kiyama et al. also found that motor coordination was related to the activation of the PMC during imitation [28], and that motor coordination was closely related to the function of the PMC. The PMC is an important brain area for action control and execution [29]. As the core brain area of the MNS, it is the neural basis of action imitation [30]. Our behavioral and neuroimaging findings supported the close relationship between motor coordination, action imitation, and PMC activity in healthy female subjects. As a next step, future studies should test the regulatory role of motor coordination on the interaction between ROI activation and the action imitation.
In this study, we found that motor coordination in female students moderated the interaction between brain activation and imitation ability. Specifically, in female students with lower motor coordination, higher activation in regions involved in the MNS, such as the PMC, SMA, S1, ANG, and SMG, when they imitated actions with their right hand, left hand, and both hands, was associated with better action imitation. However, this relationship was not found in women with higher motor coordination. We speculate that the women with higher motor coordination might have participated in more sports, thus enhancing their action imitation ability. During imitation, the efficiency of neural resources related to the MNS was high, and general nervous system activation was low. Thus, it appears that the degree of MNS activation cannot be used to predict the action imitation ability. However, women with low motor coordination may need to recruit more MNS-related resources in order to efficiently complete an imitation task. Therefore, in this population, higher MNS activation may be related to better imitation.
Several studies have pointed out that many regions in the MNS are activated during imitation; specifically, the SMG, PMC, SMA, S1, SSAC, and ANG [11,27]. Our study confirmed this with respect to the PMC, ANG, SMG, and other MNS ROIs. Thus, this study once again verified that brain regions associated with the MNS are activated during imitation via the use of fNIRS. When obtaining fNIRS data, the changes in HbO and HbR concentrations were measured simultaneously. However, there is controversy about which signal to choose in order to analyze the brain hemodynamic response. In this study, we mainly focus on the HbO signal, because it is usually observed that its amplitude is higher than that of the HbR and HbT signal [31,32]. In other words, the signal-to-noise ratio of HbO is better, and the signal is more sensitive to the task response [33]. Similar to the results of previous studies, it was found that HbO is a more effective measurement [30,34].
There are also some other important findings in this study. In this study, there is no correlation between the imitation and motor coordination of men. This study speculates that the possible reason is that men participate in more sports activities, and the coordination ability is more affected by heredity or congenital. This view is only the assertion of this study. In the future, we will further study the relationship between action imitation and coordination ability.
5. Conclusions
We found a significantly positive correlation between motor coordination and the average imitation time, such that when motor coordination was low, a higher degree of activation in the MNS was associated with a better imitation ability. However, when the motor coordination is high, the degree of MNS activation has no relation to imitation. This study reveals the relationship between action imitation and coordination ability from two aspects of behavior and brain hemodynamics, and supplements the related research on the relationship between the two in the adult population.
Author Contributions
W.Z.—study design, behavioral and fNIRS data processing, statistical analysis, drafting the article, and final approval; M.H.—study design and conducting experiments; X.Z.—drafting the article; L.L.—study design, drafting the article, and final approval. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the University Committee on Human Research Protection of EAST CHINA NORMAL UNIVERSITY (protocol code HR 125-2019 and date of approval is 28 May 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We extend our sincere thanks to all participants for their cooperation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jones, S.S. The Development of Imitation in Infancy. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 2325–2335. [Google Scholar] [CrossRef]
- Brass, M.; Heyes, C. Imitation: Is Cognitive Neuroscience Solving the Correspondence Problem? Trends Cogn. Sci. 2005, 9, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.; Kopp, M.; Federolf, P. Partly Randomised, Controlled Study in Children Aged 6–10 Years to Investigate Motor and Cognitive Effects of a 9-Week Coordination Training Intervention with Concurrent Mental Tasks. BMJ Open 2018, 8, e021026. [Google Scholar] [CrossRef] [PubMed]
- Piek, J.P.; Dyck, M.J.; Nieman, A.; Anderson, M.; Hay, D.; Smith, L.M.; McCoy, M.; Hallmayer, J. The Relationship between Motor Coordination, Executive Functioning and Attention in School Aged Children. Arch. Clin. Neuropsychol. 2004, 19, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Festante, F.; Vanderwert, R.E.; Sclafani, V.; Paukner, A.; Simpson, E.A.; Suomi, S.J.; Fox, N.A.; Ferrari, P.F. Eeg Beta Desynchronization During Hand Goal-Directed Action Observation in Newborn Monkeys and Its Relation to the Emergence of Hand Motor Skills. Dev. Cogn. Neurosci. 2018, 30, 142–149. [Google Scholar] [CrossRef]
- Rizzolatti, G.; Luppino, G. The Cortical Motor System. Neuron 2001, 31, 889–901. [Google Scholar] [CrossRef]
- Reynolds, J.E.; Billington, J.; Kerrigan, S.; Williams, J.; Elliott, C.; Winsor, A.M.; Codd, L.; Bynevelt, M.; Licari, M.K. Mirror Neuron System Activation in Children with Developmental Coordination Disorder: A Replication Functional Mri Study. Res. Dev. Disabil. 2019, 84, 16–27. [Google Scholar] [CrossRef]
- Aziz-Zadeh, L.; Koski, L.; Zaidel, E.; Mazziotta, J.; Iacoboni, M. Lateralization of the Human Mirror Neuron System. J. Neurosci. 2006, 26, 2964–2970. [Google Scholar] [CrossRef]
- Decety, J. The Neurophysiological Basis of Motor Imagery. Behav. Brain Res. 1996, 77, 45–52. [Google Scholar] [CrossRef]
- Grafton, S.T.; Arbib, M.A.; Fadiga, L.; Rizzolatti, G. Localization of Grasp Representations in Humans by Positron Emission Tomography. 2. Observation Compared with Imagination. Exp. Brain Res. 1996, 112, 103–111. [Google Scholar] [CrossRef]
- de C Hamilton, A.F. The Neurocognitive Mechanisms of Imitation. Curr. Opin. Behav. Sci. 2015, 3, 63–67. [Google Scholar] [CrossRef]
- Reynolds, J.E.; Thornton, A.L.; Elliott, C.; Williams, J.; Lay, B.S.; Licari, M.K. A Systematic Review of Mirror Neuron System Function in Developmental Coordination Disorder: Imitation, Motor Imagery, and Neuroimaging Evidence. Res. Dev. Disabil. 2015, 47, 234–283. [Google Scholar] [CrossRef] [PubMed]
- Perrey, S. Non-Invasive Nir Spectroscopy of Human Brain Function During Exercise. Methods 2008, 45, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Pavia, J.M.; Wolf, U.; Wolf, M. A Review on Continuous Wave Functional near-Infrared Spectroscopy and Imaging Instrumentation and Methodology. Neuroimage 2014, 85 Pt 1, 6–27. [Google Scholar] [CrossRef]
- Agbangla, N.F.; Audiffren, M.; Albinet, C.T. Use of near-Infrared Spectroscopy in the Investigation of Brain Activation During Cognitive Aging: A Systematic Review of an Emerging Area of Research. Ageing Res. Rev. 2017, 38, 52–66. [Google Scholar] [CrossRef]
- Makuuchi, M. Is Broca’s Area Crucial for Imitation? Cereb. Cortex 2005, 15, 563–570. [Google Scholar] [CrossRef]
- Pizzamiglio, G.; Zhang, Z.; Kolasinski, J.; Riddoch, J.M.; Passingham, R.E.; Mantini, D.; Rounis, E. A Role for the Action Observation Network in Apraxia after Stroke. Front. Hum. Neurosci. 2019, 13, 422. [Google Scholar] [CrossRef]
- Cohen, N.R.; Pomplun, M.; Gold, B.J.; Sekuler, R. Sex Differences in the Acquisition of Complex Skilled Movements. Exp. Brain Res. 2010, 205, 183–193. [Google Scholar] [CrossRef]
- Cracco, E.; De Coster, L.; Andres, M.; Brass, M. Mirroring Multiple Agents: Motor Resonance During Action Observation Is Modulated by the Number of Agents. Soc. Cogn. Affect. Neurosci. 2016, 11, 1422–1427. [Google Scholar] [CrossRef]
- Halsband, U.; Schmitt, J.; Weyers, M.; Binkofski, F.; Grutzner, G.; Freund, H.J. Recognition and Imitation of Pantomimed Motor Acts after Unilateral Parietal and Premotor Lesions: A Perspective on Apraxia. Neuropsychologia 2001, 39, 200–216. [Google Scholar] [CrossRef]
- Kruger, B.; Bischoff, M.; Blecker, C.; Langhanns, C.; Kindermann, S.; Sauerbier, I.; Reiser, M.; Stark, R.; Munzert, J.; Pilgramm, S. Parietal and Premotor Cortices: Activation Reflects Imitation Accuracy During Observation, Delayed Imitation and Concurrent Imitation. Neuroimage 2014, 100, 39–50. [Google Scholar] [CrossRef]
- Alesi, M.; Bianco, A.; Padulo, J.; Vella, F.P.; Petrucci, M.; Paoli, A.; Palma, A.; Pepi, A. Motor and Cognitive Development: The Role of Karate. Muscles Ligaments Tendons J. 2014, 4, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Alesi, M.; Bianco, A.; Padulo, J.; Luppina, G.; Petrucci, M.; Paoli, A.; Palma, A.; Pepi, A. Motor and Cognitive Growth Following a Football Training Program. Front. Psychol. 2015, 6, 1627. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.C.; Tak, S.; Jang, K.E.; Jung, J.; Jang, J. Nirs-Spm: Statistical Parametric Mapping for near-Infrared Spectroscopy. Neuroimage 2009, 44, 428–447. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity: Preventing and Managing the Global Epidemic; Report of a Who Consultation on Obesity; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Kamandulis, S.; Venckunas, T.; Masiulis, N.; Matulaitis, K.; Balciunas, M.; Peters, D.; Skurvydas, A. Relationship between General and Specific Coordination in 8- to 17-Year-Old Male Basketball Players. Percept. Mot. Skills 2013, 117, 821–836. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Z.R.; Li, J.; Hu, M.; Xiang, M.Q. Effect of Acute Moderate-Intensity Exercise on the Mirror Neuron System: Role of Cardiovascular Fitness Level. Front. Psychol. 2020, 11, 312. [Google Scholar] [CrossRef]
- Kiyama, S.; Kunimi, M.; Iidaka, T.; Nakai, T. Distant Functional Connectivity for Bimanual Finger Coordination Declines with Aging: An Fmri and Sem Exploration. Front. Hum. Neurosci. 2014, 8, 251. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Kashyap, R.; Abualait, T.; Annabel Chen, S.H.; Yoo, W.K.; Bashir, S. The Role of Primary Motor Cortex: More Than Movement Execution. J. Mot. Behav. 2021, 53, 258–274. [Google Scholar] [CrossRef]
- Sun, P.P.; Tan, F.L.; Zhang, Z.; Jiang, Y.H.; Zhao, Y.; Zhu, C.Z. Feasibility of Functional near-Infrared Spectroscopy (Fnirs) to Investigate the Mirror Neuron System: An Experimental Study in a Real-Life Situation. Front. Hum. Neurosci. 2018, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Strangman, G.; Boas, D.A.; Sutton, J.P. Non-invasive neuroimaging using near-infrared light. Biol. Psychiatry 2002, 52, 679–693. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Dan, I.; Tsuzuki, D.; Kato, M.; Okamoto, M.; Kyutoku, Y.; Soya, H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage 2010, 50, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, X.; Hu, Y. Synchronous brain activity during cooperative exchange depends on gender of partner: A fNIRS-based hyperscanning study. Hum. Brain Mapp. 2015, 36, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Koehler, S.; Egetemeir, J.; Stenneken, P.; Koch, S.P.; Pauli, P.; Fallgatter, A.J.; Herrmann, M.J. The human execution/observation matching system investigated with a complex everyday task: A functional near-infrared spectroscopy (fNIRS) study. Neurosci. Lett. 2012, 508, 73–77. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).