Volumetry of Olfactory Structures in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and a Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria of the Selected Studies
Outcome
2.2. Search Strategy and Information Source
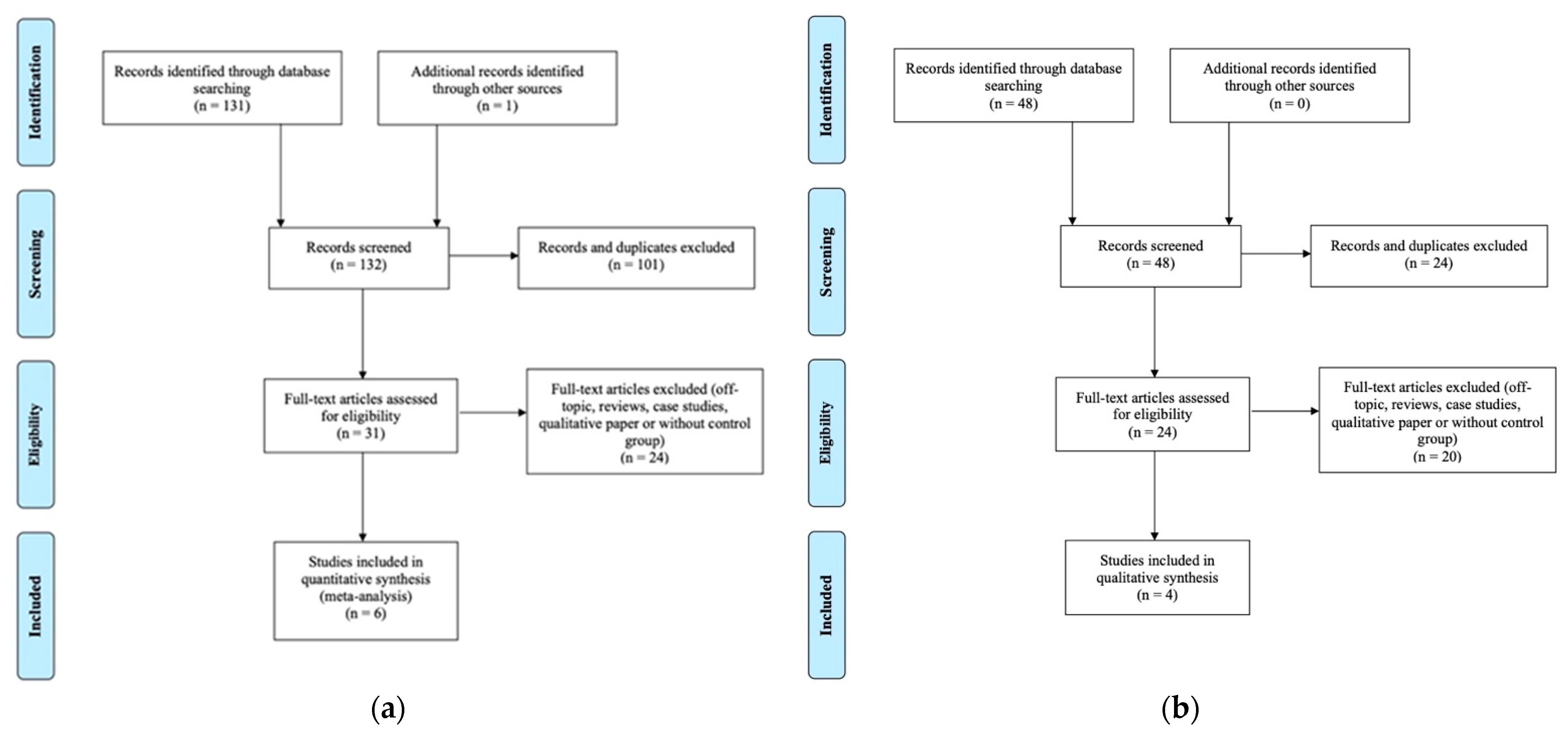
2.3. Study Selection and Risk of Bias in Individual Studies
2.4. Analysis
Risk of Bias across Studies
3. Results
3.1. Volumetry of the OB in Patients with AD
3.1.1. Study Selection and Characteristics
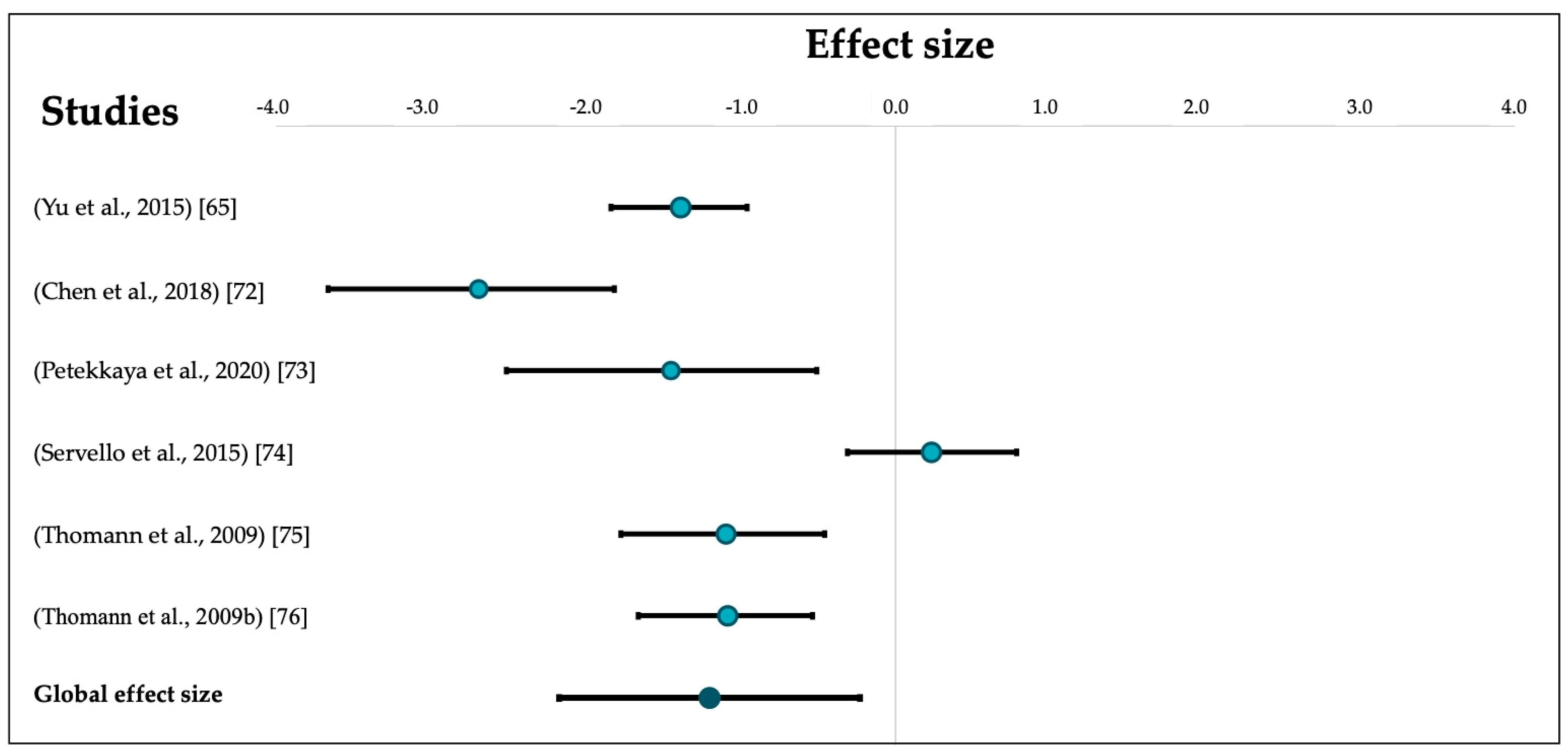
3.1.2. Main Effect
3.2. Volumetry of the OB in Patients with Mild Cognitive Impairment
3.2.1. Study Selection and Characteristics
3.2.2. Effect Sizes
3.3. Volumetry of the Primary Olfactory Cortex
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- Fiest, K.M.; Roberts, J.I.; Maxwell, C.J.; Hogan, D.B.; Smith, E.E.; Frolkis, A.; Cohen, A.; Kirk, A.; Pearson, D.; Pringsheim, T.; et al. The Prevalence and Incidence of Dementia Due to Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. 2016, 43, S51–S82. [Google Scholar] [CrossRef]
- Jack, C., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.J.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Prevalence of Cerebral Amyloid Pathology in Persons Without Dementia: A Meta-Analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; Van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Beaumont, H.; Ferguson, D.; Yadegarfar, M.; Stubbs, B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatr. Scand. 2014, 130, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yu, D.; Sun, X.; Zhang, M.; Wang, L.; Qin, H. The prevalence and progression of mild cognitive impairment among clinic and community populations: A systematic review and meta-analysis. Int. Psychogeriatr. 2017, 29, 1595–1608. [Google Scholar] [CrossRef]
- Bateman, R.J.; Goate, A.; Blazey, T.M.; Moulder, K.; Martins, R.N.; Rossor, M.N.; Morris, J.C. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N. Engl. J. Med. 2012, 9, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D. Tracking Pathophysiological Processes in Alzheimer’s Disease: An Updated Hypothetical Model of Dynamic Biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Benzinger, T.L.; Blazey, T.; Jack, C.R.; Koeppe, R.A.; Su, Y.; Xiong, C.; Raichle, M.E.; Snyder, A.Z.; Ances, B.M.; Bateman, R.J.; et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2013, 110, E4502–E4509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Ferry, B.; Ferreira, G.; Traissard, N.; Majchrzak, M. Selective involvement of the lateral entorhinal cortex in the control of the olfactory memory trace during conditioned odor aversion in the rat. Behav. Neurosci. 2006, 120, 1180–1186. [Google Scholar] [CrossRef]
- Maass, A.; Lockhart, S.N.; Harrison, T.M.; Bell, R.K.; Mellinger, T.; Swinnerton, K.; Baker, S.L.; Rabinovici, G.D.; Jagust, W.J. Entorhinal Tau Pathology, Episodic Memory Decline, and Neurodegeneration in Aging. J. Neurosci. 2018, 38, 530–543. [Google Scholar] [CrossRef] [Green Version]
- Rémy, F.; Mirrashed, F.; Campbell, B.; Richter, W. Verbal episodic memory impairment in Alzheimer’s disease: A combined structural and functional MRI study. NeuroImage 2005, 25, 253–266. [Google Scholar] [CrossRef]
- Wilson, D.A.; Chapuis, J.; Sullivan, R.M. Cortical Olfactory Anatomy and Physiology. In Handbook of Olfaction and Gustation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; Volume 3, pp. 209–225. [Google Scholar]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Feldman, H.H.; Frisoni, G.B.; Hampel, H.; Jagust, W.J.; Johnson, K.A.; Knopman, D.S.; et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016, 87, 539–547. [Google Scholar] [CrossRef]
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Du, A.T.; Schuff, N.; Kramer, J.H.; Ganzer, S.; Zhu, X.P.; Jagust, W.J.; Miller, B.L.; Reed, B.R.; Mungas, D.; Yaffe, K.; et al. Higher atrophy rate of entorhinal cortex than hippocampus in AD. Neurology 2004, 62, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Pennanen, C.; Kivipelto, M.; Tuomainen, S.; Hartikainen, P.; Hänninen, T.; Laakso, M.P.; Hallikainen, M.; Vanhanen, M.; Nissinen, A.; Helkala, E.-L.; et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol. Aging 2004, 25, 303–310. [Google Scholar] [CrossRef]
- Whitwell, J.L.; Josephs, K.A.; Murray, M.E.; Kantarci, K.; Przybelski, S.A.; Weigand, S.D.; Vemuri, P.; Senjem, M.L.; Parisi, J.E.; Knopman, D.S.; et al. MRI correlates of neurofibrillary tangle pathology at autopsy: A voxel-based morphometry study. Neurology 2008, 71, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Whitwell, J.L.; Przybelski, S.A.; Weigand, S.D.; Knopman, D.S.; Boeve, B.F.; Petersen, R.C.; Jack, C.R. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain 2007, 130, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Pini, L.; Pievani, M.; Bocchetta, M.; Altomare, D.; Bosco, P.; Cavedo, E.; Galluzzi, S.; Marizzoni, M.; Frisoni, G.B. Brain atrophy in Alzheimer’s Disease and aging. Ageing Res. Rev. 2016, 30, 25–48. [Google Scholar] [CrossRef]
- Detoledo-Morrell, L.; Stoub, T.; Bulgakova, M.; Wilson, R.S.; Bennett, D.A.; Leurgans, S.; Wuu, J.; Turner, D.A. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol. Aging 2004, 25, 1197–1203. [Google Scholar] [CrossRef]
- Tapiola, T.; Pennanen, C.; Tapiola, M.; Tervo, S.; Kivipelto, M.; Hänninen, T.; Pihlajamäki, M.; Laakso, M.P.; Hallikainen, M.; Hämäläinen, A.; et al. MRI of hippocampus and entorhinal cortex in mild cognitive impairment: A follow-up study. Neurobiol. Aging 2008, 29, 31–38. [Google Scholar] [CrossRef]
- Visser, P.; Verhey, F.; Hofman, P.; Scheltens, P.; Jolles, J. Medial Temporal Lobe Atrophy Predicts Alzheimer’s Disease in Pa-tients with Minor Cognitive Impairment. J. Neurol. Neurosurg. Psychiatry 2002, 72, 491–497. [Google Scholar]
- Apostolova, L.G.; Mosconi, L.; Thompson, P.M.; Green, A.E.; Hwang, K.S.; Ramirez, A.; Mistur, R.; Tsui, W.H.; de Leon, M.J. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol. Aging 2010, 31, 1077–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csernansky, J.G.; Wang, L.; Swank, J.; Miller, J.P.; Gado, M.; McKeel, D.; Miller, M.I.; Morris, J.C. Preclinical detection of Alzheimer’s disease: Hippocampal shape and volume predict dementia onset in the elderly. NeuroImage 2005, 25, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, B.C.; Stoub, T.R.; Shah, R.C.; Sperling, R.A.; Killiany, R.J.; Albert, M.S.; Hyman, B.T.; Blacker, D.; Detoledo-Morrell, L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 2011, 76, 1395–1402. [Google Scholar] [CrossRef] [Green Version]
- De Leon, M.; Golomb, J.; George, A.; Convit, A.; Tarshish, C.; McRae, T.; De Santi, S.; Smith, G.; Ferris, S.; Noz, M. The Radi-ologic Prediction of Alzheimer Disease: The Atrophic Hippocampal Formation. Am. J. Neuroradiol. 1993, 14, 897–906. [Google Scholar] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; DeKosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Frisoni, G.B.; Fox, N.C.; Galasko, D.; et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef]
- Petersen, R.C.; Jack, C.; Xu, Y.-C.; Waring, S.; O’Brien, P.; Smith, G.; Ivnik, R.; Tangalos, E.G.; Boeve, B.F.; Kokmen, E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology 2000, 54, 581. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.; Ballard, C.; McKeith, I.; Gholkar, A.; O’Brien, J. MRI Volumetric Study of Dementia with Lewy Bodies: A Com-parison with AD and Vascular Dementia. Neurology 2000, 54, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.J.; Barber, R.; Mukaetova-Ladinska, E.B.; Robson, J.; Perry, R.H.; Jaros, E.; Kalaria, R.N.; O’Brien, J.T. Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: A prospective study with pathological verification of diagnosis. Brain 2009, 132, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laakso, M.P.; Partanen, K.; Riekkinen, P.; Lehtovirta, M.; Helkala, E.-L.; Hallikainen, M.; Hanninen, T.; Vainio, P.; Soininen, H. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: An MRI study. Neurology 1996, 46, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Van De Pol, L.; Gertz, H.-J.; Scheltens, P.; Wolf, H. Hippocampal Atrophy in Subcortical Vascular Dementia. Neurodegener. Dis. 2011, 8, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Frisoni, G.B.; Fox, N.C.; Jack, C.R.; Scheltens, P.; Thompson, P.M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 2010, 6, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain Imaging in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006213. [Google Scholar] [CrossRef]
- Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 11–24. [Google Scholar] [CrossRef]
- Rahayel, S.; Frasnelli, J.; Joubert, S. The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: A meta-analysis. Behav. Brain Res. 2012, 231, 60–74. [Google Scholar] [CrossRef]
- Roalf, D.R.; Moberg, M.J.; Turetsky, B.I.; Brennan, L.; Kabadi, S.; Wolk, D.A.; Moberg, P.J. A quantitative meta-analysis of olfactory dysfunction in mild cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2017, 88, 226–232. [Google Scholar] [CrossRef]
- Conti, M.Z.; Vicini-Chilovi, B.; Riva, M.; Zanetti, M.; Liberini, P.; Padovani, A.; Rozzini, L. Odor Identification Deficit Predicts Clinical Conversion from Mild Cognitive Impairment to Dementia Due to Alzheimer’s Disease. Arch. Clin. Neuropsychol. 2013, 28, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devanand, D.P.; Liu, X.; Tabert, M.H.; Pradhaban, G.; Cuasay, K.; Bell, K.; de Leon, M.J.; Doty, R.L.; Stern, Y.; Pelton, G.H. Combining Early Markers Strongly Predicts Conversion from Mild Cognitive Impairment to Alzheimer’s Disease. Biol. Psychiatry 2008, 64, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Jobin, B.; Zahal, R.; Bussières, E.-L.; Frasnelli, J.; Boller, B. Olfactory Identification in Subjective Cognitive Decline: A Meta-Analysis. J. Alzheimer’s Dis. 2021, 79, 1497–1507. [Google Scholar] [CrossRef]
- Lundström, J.N.; Boesveldt, S.; Albrecht, J. Central Processing of the Chemical Senses: An Overview. ACS Chem. Neurosci. 2011, 2, 5–16. [Google Scholar] [CrossRef]
- Carmichael, S.T.; Clugnet, M.-C.; Price, J.L. Central olfactory connections in the macaque monkey. J. Comp. Neurol. 1994, 346, 403–434. [Google Scholar] [CrossRef]
- Xu, W.; Wilson, D.A. Odor-evoked activity in the mouse lateral entorhinal cortex. Neuroscience 2012, 223, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Staubli, U.; Ivy, G.; Lynch, G. Hippocampal denervation causes rapid forgetting of olfactory information in rats. Proc. Natl. Acad. Sci. USA 1984, 81, 5885–5887. [Google Scholar] [CrossRef] [Green Version]
- Attems, J.; Jellinger, K. Olfactory tau pathology in Alzheimer disease and mild cognitive impairment. Clin. Neuropathol. 2006, 25, 265. [Google Scholar] [PubMed]
- Kovács, T.; Cairns, N.J.; Lantos, P.L. beta-Amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 1999, 25, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, Y.; Wszolek, Z.K.; Graffradford, N.R.; Cookson, N.; Dickson, D.W. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein E4. Neuropathol. Appl. Neurobiol. 2003, 29, 503–510. [Google Scholar] [CrossRef]
- Vasavada, M.M.; Wang, J.; Eslinger, P.J.; Gill, D.J.; Sun, X.; Karunanayaka, P.; Yang, Q.X. Olfactory Cortex Degeneration in Alzheimer’s Disease and Mild Cognitive Impairment. J. Alzheimer’s Dis. 2015, 45, 947–958. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Goncharova, I.; Sullivan, M.P.; Forchetti, C.; Wilson, R.S.; Bennett, D.A.; Beckett, L.A. MRI-Derived Entorhinal and Hippocampal Atrophy in Incipient and Very Mild Alzheimer’s Disease. Neurobiol. Aging 2001, 22, 747–754. [Google Scholar] [CrossRef]
- Stoub, T.R.; Rogalski, E.J.; Leurgans, S.; Bennett, D.A.; Detoledo-Morrell, L. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: Relation to memory function. Neurobiol. Aging 2010, 31, 1089–1098. [Google Scholar] [CrossRef] [Green Version]
- Stoub, T.R.; Bulgakova, M.; Leurgans, S.; Bennett, D.A.; Fleischman, D.; Turner, D.A.; Detoledo-Morrell, L. MRI predictors of risk of incident Alzheimer disease: A longitudinal study. Neurology 2005, 64, 1520–1524. [Google Scholar] [CrossRef]
- Traschütz, A.; Enkirch, S.J.; Polomac, N.; Widmann, C.N.; Schild, H.H.; Heneka, M.T.; Hattingen, E. The Entorhinal Cortex Atrophy Score Is Diagnostic and Prognostic in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2020, 75, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild Cognitive Impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303. [Google Scholar] [CrossRef]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011. [Google Scholar]
- Zeng, X.-T.; Zhang, Y.; Kwong, J.S.W.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review: Methodological Quality Assessment Tools. J. Evid. Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef]
- Hang, W.; Liu, G.; Han, T.; Zhou, Y.; Zhang, J.; Zhang, Q. Olfactory Function in Patients with Mild Cognitive Impairment. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi Chin. J. Otorhinolaryngol. Head Neck Surg. 2014, 49, 738–742. [Google Scholar]
- Yu, H.; Hang, W.; Zhang, J.; Liu, G. Olfactory Function in Patients with Alzheimer’disease. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi J. Clin. Otorhinolaryngol. Head Neck Surg. 2015, 29, 444–447. [Google Scholar]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, Comparison, and Validation of Meta-Essentials: A Free and Simple Tool for Meta-analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borenstein, M. (Ed.) Introduction to Meta-Analysis; John Wiley & Sons: Chichester, UK, 2009; ISBN 9780470057247. [Google Scholar]
- Brydges, C.R. Effect Size Guidelines, Sample Size Calculations, and Statistical Power in Gerontology. Innov. Aging 2019, 3, igz036. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: Cambridge, MA, USA, 2014; ISBN 0080570658. [Google Scholar]
- Fu, R.; Gartlehner, G.; Grant, M.; Shamliyan, T.; Sedrakyan, A.; Wilt, T.J.; Griffith, L.; Oremus, M.; Raina, P.; Ismaila, A.; et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J. Clin. Epidemiol. 2011, 64, 1187–1197. [Google Scholar] [CrossRef]
- Rosenthal, R. The File Drawer Problem and Tolerance for Null Results. Psychol. Bull. 1979, 86, 638. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, X.; Mai, N.; Peng, Q.; Wu, Z.; Ouyang, C.; Zhang, W.; Liang, W.; Wu, Y.; Liu, S.; et al. Cognitive Impairment and Structural Abnormalities in Late Life Depression with Olfactory Identification Impairment: An Alzheimer’s Disease-Like Pattern. Int. J. Neuropsychopharmacol. 2018, 21, 640–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petekkaya, E.; Kaptan, Z.; Unalmis, D.; Burakgazi, G.; Kus, B.; Melek, I.; Arpaci, A. An investigation of olfactory bulb and entorhinal cortex volumes in both patients with Alzheimer’s disease and healthy individuals, and a comparative analysis of neuropeptides. Med. Sci. 2020, 9, 866. [Google Scholar] [CrossRef]
- Servello, A.; Fioretti, A.; Gualdi, G.; Di Biasi, C.; Pittalis, A.; Sollaku, S.; Pavaci, S.; Tortorella, F.; Fusetti, M.; Valenti, M.; et al. Olfactory Dysfunction, Olfactory Bulb Volume and Alzheimer’s Disease: Is There a Correlation? A Pilot Study1. J. Alzheimer’s Dis. 2015, 48, 395–402. [Google Scholar] [CrossRef]
- Thomann, P.A.; Dos Santos, V.; Toro, P.; Schönknecht, P.; Essig, M.; Schröder, J. Reduced olfactory bulb and tract volume in early Alzheimer’s disease—A MRI study. Neurobiol. Aging 2009, 30, 838–841. [Google Scholar] [CrossRef]
- Thomann, P.A.; Dos Santos, V.; Seidl, U.; Toro, P.; Essig, M.; Schröder, J. MRI-Derived Atrophy of the Olfactory Bulb and Tract in Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 17, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Al-Otaibi, M.; Lessard-Beaudoin, M.; Castellano, C.-A.; Gris, D.; Cunnane, S.C.; Graham, R.K. Volumetric MRI demonstrates atrophy of the olfactory cortex in AD. Curr. Alzheimer Res. 2021, 17, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Testa, N.; Jordan, R.; Elyan, R.; Kanekar, S.; Wang, J.; Eslinger, P.; Yang, Q.X.; Zhang, B.; Karunanayaka, P.R. Functional Connectivity between the Resting-State Olfactory Network and the Hippocampus in Alzheimer’s Disease. Brain Sci. 2019, 9, 338. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Yang, Q.X.; Zhang, H.; Eslinger, P.J.; Zhang, X.; Wu, S.; Zhang, B.; Zhu, B.; Karunanayaka, P.R. Disruptions of the olfactory and default mode networks in Alzheimer’s disease. Brain Behav. 2019, 9, e01296. [Google Scholar] [CrossRef]
- Kovács, T.; Cairns, N.J.; Lantos, P.L. Olfactory centres in Alzheimer’s disease: Olfactory bulb is involved in early Braak’s stages. NeuroReport 2001, 12, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Jernigan, T.L.; Fennema-Notestine, C. Left hippocampal volume loss in Alzheimer’s disease is reflected in performance on odor identification: A structural MRI study. J. Int. Neuropsychol. Soc. 2003, 9, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Buschhüter, D.; Smitka, M.; Puschmann, S.; Gerber, J.C.; Witt, M.; Abolmaali, N.D.; Hummel, T. Correlation between olfactory bulb volume and olfactory function. NeuroImage 2008, 42, 498–502. [Google Scholar] [CrossRef]
- Gudziol, V.; Buschhüter, D.; Abolmaali, N.; Gerber, J.; Rombaux, P.; Hummel, T. Increasing olfactory bulb volume due to treatment of chronic rhinosinusitis—A longitudinal study. Brain 2009, 132, 3096–3101. [Google Scholar] [CrossRef]
- Haehner, A.; Rodewald, A.; Gerber, J.C.; Hummel, T. Correlation of Olfactory Function With Changes in the Volume of the Human Olfactory Bulb. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 621–624. [Google Scholar] [CrossRef] [Green Version]
- Gottfried, J.A. Central mechanisms of odour object perception. Nat. Rev. Neurosci. 2010, 11, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Howard, J.D.; Gottfried, J.A. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer’s disease. Brain 2010, 133, 2714–2726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macknin, J.B.; Higuchi, M.; Lee, V.M.-Y.; Trojanowski, J.Q.; Doty, R.L. Olfactory dysfunction occurs in transgenic mice overexpressing human τ protein. Brain Res. 2004, 1000, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.W.; Levy, E.; Nixon, R.A.; Wilson, D.A. Olfactory Dysfunction Correlates with Amyloid- Burden in an Alzheimer’s Disease Mouse Model. J. Neurosci. 2010, 30, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Devanand, D.P.; Tabert, M.H.; Cuasay, K.; Manly, J.J.; Schupf, N.; Brickman, A.M.; Andrews, H.; Brown, T.R.; DeCarli, C.; Mayeux, R. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol. Aging 2010, 31, 1593–1600. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; You, H.; Liu, J.-F.; Ni, D.-F.; Zhang, Z.-X.; Guan, J. Association of Olfactory Bulb Volume and Olfactory Sulcus Depth with Olfactory Function in Patients with Parkinson Disease. AJNR Am. J. Neuroradiol. 2011, 32, 677–681. [Google Scholar] [CrossRef] [Green Version]
- Potvin, O.; Khademi, A.; Chouinard, I.; Farokhian, F.; Dieumegarde, L.; Leppert, I.; Hoge, R.; Rajah, M.N.; Bellec, P.; Duchesne, S.; et al. Measurement Variability Following MRI System Upgrade. Front. Neurol. 2019, 10, 726. [Google Scholar] [CrossRef] [Green Version]
- Reig, S.; Sánchez-González, J.; Arango, C.; Castro, J.; González-Pinto, A.; Ortuno, F.; Crespo-Facorro, B.; Bargallo, N.; Desco, M. Assessment of the Increase in Variability When Combining Volumetric Data from Different Scanners. Hum. Brain Mapp. 2009, 30, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Noothout, J.M.; Postma, E.M.; Boesveldt, S.; De Vos, B.D.; Smeets, P.A.; Išgum, I. Automatic Segmentation of the Olfactory Bulbs in MRI. Int. Soc. Opt. Photonics 2021, 11596, 115961J. [Google Scholar]
- Jackson, D.; Turner, R. Power analysis for random-effects meta-analysis: Power Analysis for Meta-Analysis. Res. Synth. Methods 2017, 8, 290–302. [Google Scholar] [CrossRef] [Green Version]
- Fjaeldstad, A.; Fernandes, H.M.; Van Hartevelt, T.; Gleesborg, C.; Møller, A.; Ovesen, T.; Kringelbach, M. Brain Fingerprints of Olfaction: A Novel Structural Method for Assessing Olfactory Cortical Networks in Health and Disease. Sci. Rep. 2017, 7, 42534. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Eslinger, P.J.; Doty, R.L.; Zimmerman, E.K.; Grunfeld, R.; Sun, X.; Meadowcroft, M.D.; Connor, J.R.; Price, J.L.; Smith, M.B.; et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain Res. 2010, 1357, 184–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seubert, J.; Freiherr, J.; Djordjevic, J.; Lundström, J.N. Statistical Localization of Human Olfactory Cortex. Neuroimage 2013, 66, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Lee, J.-E.; Lee, K.-S.; Kim, J.-S. Comparison of Odor Identification among Amnestic and Non-Amnestic Mild Cog-nitive Impairment, Subjective Cognitive Decline, and Early Alzheimer’s Dementia. Neurol. Sci. 2018, 39, 557–564. [Google Scholar] [CrossRef] [PubMed]
| Authors | Participants’ Selection | Group Comparability | OB Measurement | Sample Size | Mean Age (SD) | OB Volume (SD) |
|---|---|---|---|---|---|---|
| Yu et al., 2015 [65] | N/A. | N/A. | N/A. | AD: 50 Controls: 50 | N/A. | AD: 30.05 (5.08) Controls: 36.46 (4.11) |
| Chen et al., 2018 [72] | + NINCDS-ADRDA criteria used by two trained neurologists. + Controls were from the same community. + Consecutive recruitment. − No description of controls’ health history. − No measurement of AD-pathology biomarker (PET/CSF tau and amyloid-β) | + Control for age, sex, education, and total intracranial volume. | + Philips 3.0T MR scanner. + Sagittal 3D gradient-echo T1-weighted sequence. − Planimetric manual contouring. + Same method for both groups. | AD: 20 Controls: 25 | AD: N/A. Controls: 55+ | AD: 27.39 (3.22) Controls: 37.35 (4.04) |
| Petekkaya et al., 2020 [73] | + NINCDS-ADRDA criteria. + Random recruitment of controls with equivalent for age and education level. + Controls without history of brain pathology or disease equivalent to AD or brain trauma, brain tumor, attacks, or clinical history with other accompanying psychological symptoms. − No measurement of AD-pathology biomarker (PET/CSF tau and amyloid-β). | + Control for age and education level. | + Philips 1.5T MR scanner. + 3D axial T1-weighted sequence. + Automatic parcellation of OB volumes using the IBASPM toolbox. + Same method for both groups. | AD: 9 Controls: 12 | AD: 73.13 (4.73) Controls: 72.47 (3.35) | Left OB: AD: 0.84 (0.18) Controls: 1.04 (0.14) Right OB: AD: 0.85 (0.32) Controls: 1.21 (0.10) |
| Servello et al., 2015 [74] | + NINCDS-ADRDA criteria. + Neuropsychological, radiological, and olfactory evaluation. + Controls were from the same community. + Recruitment between January and October 2013. − No random recruitment. − No description of controls’ health history. − No measurement of AD-pathology biomarker (PET/CSF tau and amyloid-β). | − No control for sex, age, or other factors. | + Siemens 3.0T MR scanner. +T1-weighted TSE coronal plane, T2-weighted TSE coronal plane, and T2 space 3d axial plane sequences + Manual segmentation of T1 and T2-weighted coronal sections. + Same method for both groups. | AD: 25 Controls: 28 | AD: 73.7 (6.8) Controls: 69.4 (9.2) | AD: 35.91 (8.90) Controls: 33.49 (11.60) |
| Thomann et al., 2009 [75] | + Ascertainment of personal/family history, physical, neurological, and neuropsychological examination. + NINCDS-ADRDA criteria. + Controls from the same community. + Recruitment between 2003 and 2004. − No consecutive/random recruitment. −No measurement of AD-pathology biomarker (PET/CSF tau and amyloid-β). | + Control for age, gender, education, and total intracranial volume. | + Siemens 1.5-T MR scanner. + T1-weighted 3D MPRAGE sequence. + Manual segmentation. + Same method for both groups. | AD: 21 Controls: 21 | AD: 71.76 (4.94) Controls: 70.38 (7.14) | AD: 83.36 (9.01) Controls: 94.52 (11.26) |
| Thomann et al., 2009 [76] | + Ascertainment of personal/family history, physical, neurological, and neuropsychological examination. + NINCDS-ADRDA criteria for AD. + Controls from the same community and without cognitive complaints. + All participants were recruited between 2003 and 2004. + Controls were from the same community and without cognitive deficits. − No random recruitment. − No measurement of AD-pathology biomarker. (PET/CSF tau and amyloid-β). | + Control for age, gender, education, and total intracranial volume. | + Siemens 1.5-T MR scanner. + T1-weighted 3D MPRAGE sequence. + Manual segmentation. + Same method for both groups. | AD: 27 Controls: 30 | AD: 71.44 (3.94) Controls: 70.50 (5.48) | AD: 85.92 (8.18) Controls: 95.73 (9.77) |
| Authors | Participants’ Selection | Group Comparability | OB Measurement | Sample Size | Mean Age (SD) | OB Volume (SD) |
|---|---|---|---|---|---|---|
| Hang et al., 2014 [64] | N/A. | N/A. | N/A. | MCI: 50 Controls: 50 | N/A. | MCI: 36.47 (4.12) Controls: 46.71 (6.25) |
| Servello et al., 2015 [73] | + Petersen criteria. + Neuropsychological, radiological, and olfactory evaluation. + Controls from the same community. + Recruitment between January and October 2013. − No random recruitment. − No description of controls’ health history. − No distinction between amnesic and non-amnesic MCI. −No measurement of AD-pathology biomarker (PET/CSF tau and amyloid-β). | − No control for sex, age, or other factors. | + Siemens 3.0T MRI scanner. +T1-weighted TSE coronal plane, T2-weighted TSE coronal plane, and T2 space 3d axial plane sequences. + Manual segmentation of T1 and T2-weighted coronal sections. + Same method for both groups. | MCI: 25 Controls: 28 | MCI: 74.5 (7.5) Controls: 69.4 (9.2) | MCI: 34.87 (6.60) Controls: 33.49 (11.60) |
| Thomann et al., 2009 [75] | + Ascertainment of personal and family history, physical, neurological, and neuropsychological examination. + Controls from the same community. + Recruitment between 2003 and 2004. −No measurement of AD-pathology biomarker (PET/CSF tau and amyloid-β). − The aging associated cognitive decline was considered as a conceptual equivalent for MCI. Criteria were: (1) Performance of at least one standard deviation below the age-adjusted norm on a standardized test of cognition, (2) Exclusion of any medical, neurological, or psychiatric disorder that could lead to cognitive deterioration, (3) normal activities of daily living, (4) no dementia. − No random recruitment. − No distinction between amnesic and non-amnesic MCI. | + Control for age, gender, education, and total intracranial volume. | + Siemens 1.5-T MR scanner. + T1-weighted 3D MPRAGE sequence. + Manual segmentation. + Same method for both groups. | MCI: 29 Controls: 30 | MCI: 71.38 (6.14) Controls: 70.50 (5.48) | MCI: 90.81 (9.27) Controls: 95.73 (9.77) |
| Authors | Participants’ Selection | Group Comparability | POC Measurement | Sample Size | Mean Age (SD) | Outcome |
|---|---|---|---|---|---|---|
| Al-Otaibi et al., 2020 [77] | + Diagnostic according to the National Institute on Aging—Alzheimer’s Association (NIA-AA) criteria. + MMSE to qualify controls as cognitively normal. + Participants underwent a pre-screening visit including medical history questionnaire and blood analysis. − No random recruitment. − Poor description of control’s recruitment. − No description of controls’ health history. −No measurement of AD-pathology biomarker (PET/CSF tau and amyloid-β). | + Control for sex, age, and education. | + Siemens 1.5 T MR scanner. + T1-weighted sequence. + Automatic segmentation using the Automatic Anatomical Labelling atlas. Targeted structures: the olfactory tract, amygdala, piriform cortex, anterior perforated substance, the subcallosal area (including the subcallosal cingulate gyrus), and the anterior cingulate cortex. − Olfactory tract is included in the definition of the olfactory cortex although it is constituted of white matter. + Same method for both groups. | AD: 14 Controls: 25 | AD: 75.06 (4.60) Controls: 71.1 (5.22) | Olfactory cortex volume is significantly smaller in patients with AD compared to healthy older controls. The decrease was more apparent in the left olfactory cortex. |
| Lu et al., 2019 * [78,79] | + Use of the Clinical Dementia Rating, the MMSE, the CVLT-II, the Dementia Rating Scale and a reviewed of the medical records of AD and MCI patients. + Controls were from the same community and without cognitive deficits. − No random recruitment. − Poor description of control’s recruitment. − No description of controls’ health history. − No distinction between amnesic and non-amnesic MCI. −No measurement of AD-pathology biomarker (PET/CSF tau and amyloid-β). | + Control for age. | + Siemens Trio 3.0 T scanner. + T1-weighted MPRAGE sequence. − Manual segmentation. Targeted structures: the anterior olfactory nucleus, olfactory tubercle, piriform cortex, anterior portion of the periamygdaloid cortex, amygdala, and anterior perforated substance. + Same method for both groups. | AD: 26 EMCI: 36 LMCI: 31 Controls: 44 | AD: 71.55 (7.3) EMCI: 71.69 (7.3) LMCI: 72.41 (7.4) Controls: 74.18 (6.1) | There was a decreasing trend for a smaller POC volume dependent on AD disease state, but no difference reach significance (Controls > LMCI > EMCI > AD). |
| Vasavada et al., 2015 [53] | + Diagnostics were made by a certified neurologist using NINCDS-ADRDA criteria (AD) and Peterson criteria (MCI). − Poor description of recruitment procedures. − No distinction between amnesic and non-amnesic MCI. − No measurement of AD-pathology biomarker (PET/CSF tau and amyloid-β). | + Correction for intracranial volume and age. | + Siemens 3.0 T MRI system. + T1-weighted MPRAGE images. − Manual segmentation. Targeted structures: the anterior olfactory nucleus, olfactory tubercle, piriform cortex, anterior portion of the periamygdaloid cortex and amygdala, and anterior perforated substance. + Same method for both groups. | AD: 15 MCI: 21 Controls: 27 | AD; 71.9 (11.9) MCI: 73.2 (9) Controls: 69.5 (10.4) | MCI and AD patients had a significantly lower POC volume than controls. The difference between AD and MCI patients did not reach significance. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jobin, B.; Boller, B.; Frasnelli, J. Volumetry of Olfactory Structures in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and a Meta-Analysis. Brain Sci. 2021, 11, 1010. https://doi.org/10.3390/brainsci11081010
Jobin B, Boller B, Frasnelli J. Volumetry of Olfactory Structures in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and a Meta-Analysis. Brain Sciences. 2021; 11(8):1010. https://doi.org/10.3390/brainsci11081010
Chicago/Turabian StyleJobin, Benoît, Benjamin Boller, and Johannes Frasnelli. 2021. "Volumetry of Olfactory Structures in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and a Meta-Analysis" Brain Sciences 11, no. 8: 1010. https://doi.org/10.3390/brainsci11081010
APA StyleJobin, B., Boller, B., & Frasnelli, J. (2021). Volumetry of Olfactory Structures in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and a Meta-Analysis. Brain Sciences, 11(8), 1010. https://doi.org/10.3390/brainsci11081010






