Endogenous Expression of G-CSF in Rat Dorsal Root Ganglion Neurons after Nerve Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Animals
2.3. Establishment of the Neuropathic Pain Animal Model
2.4. Behavioral Testing
2.5. G-CSF Administration
2.6. Tissue Preparation and Sectioning
2.7. Immunohistochemistry
2.8. Double Immunofluorescence Staining
2.9. Image Analysis
2.10. Blood Sample Collection and Analysis
2.11. Statistical Analysis
3. Results
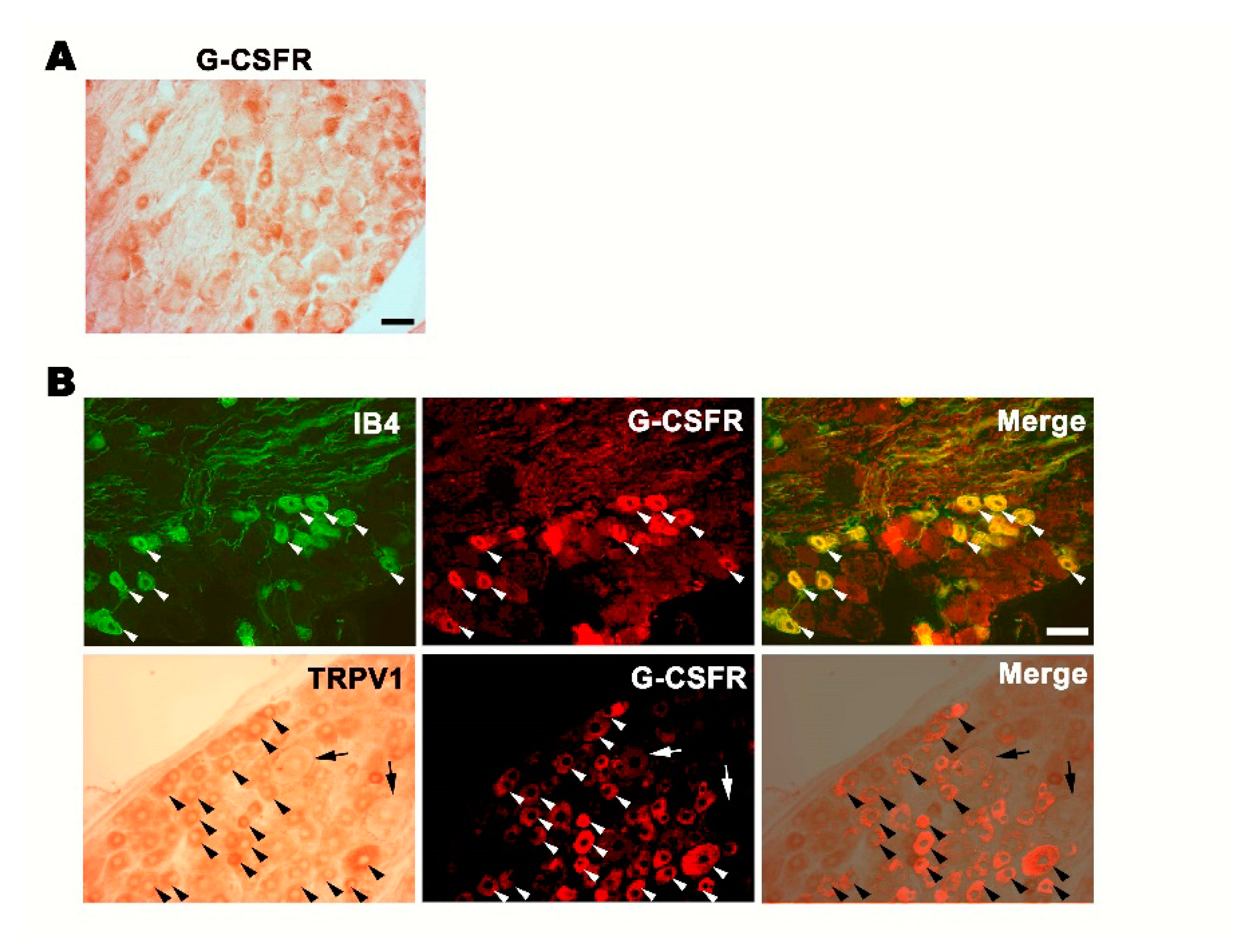
3.1. G-CSFR Is Expressed in Small- and Medium-Sized DRG Neurons
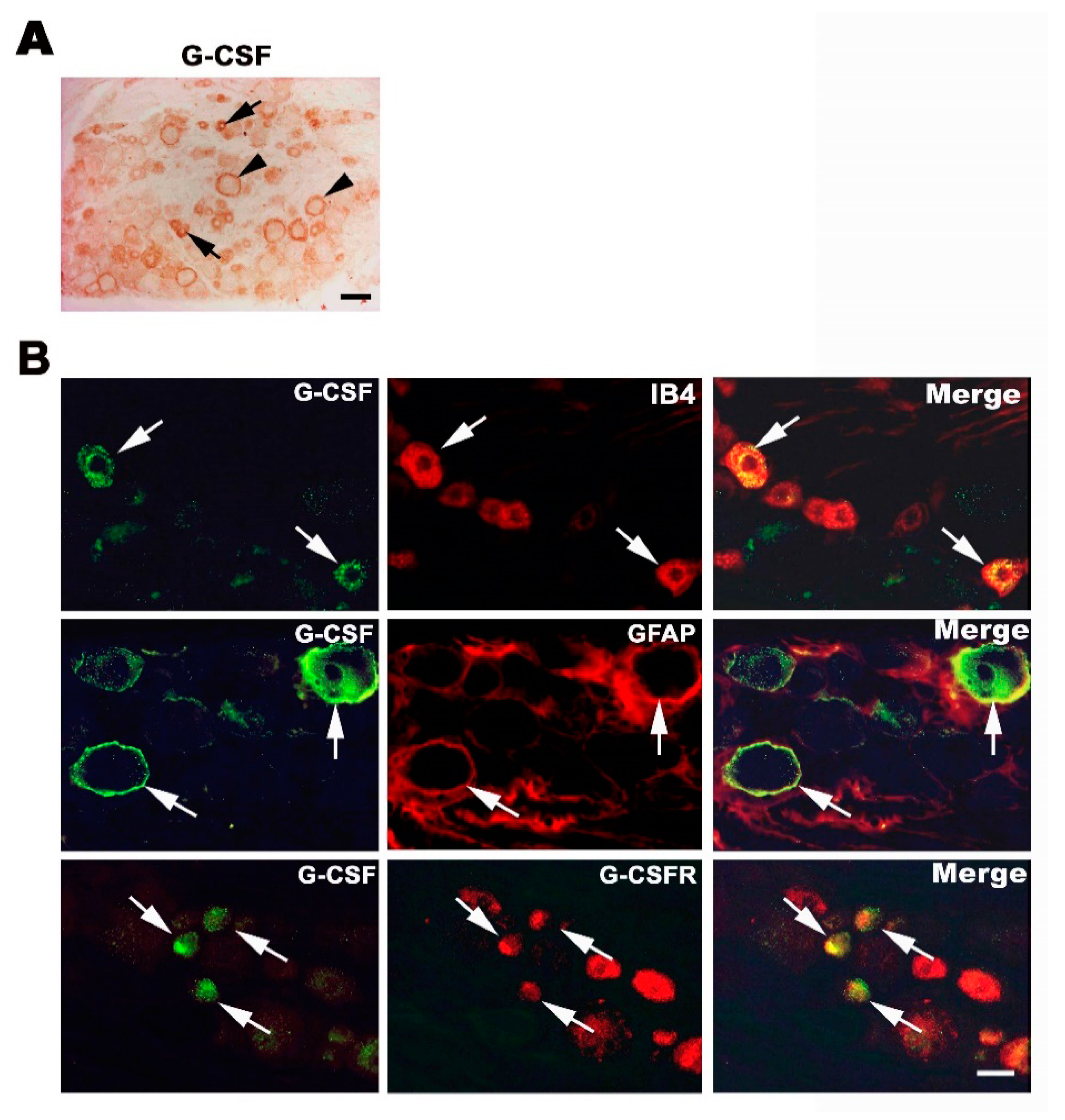
3.2. Endogenous G-CSF Is Expressed in Small and Medium-Size DRG Neurons and Satellite Cells
3.3. Changes in G-CSF Expression in the DRG in Rats with Partial Sciatic Nerve Transection (PST)
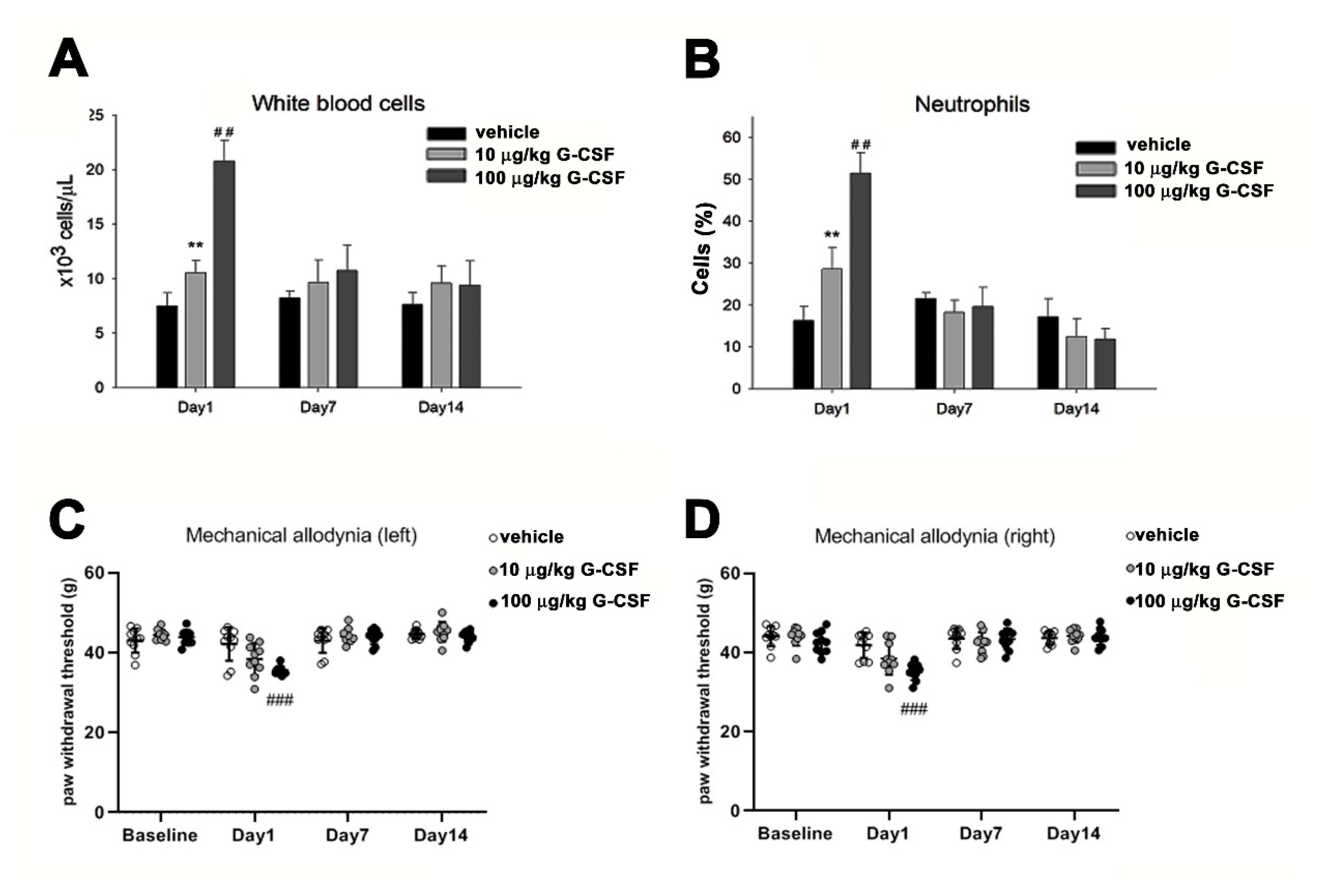
3.4. G-CSF Induces Pain Behavior
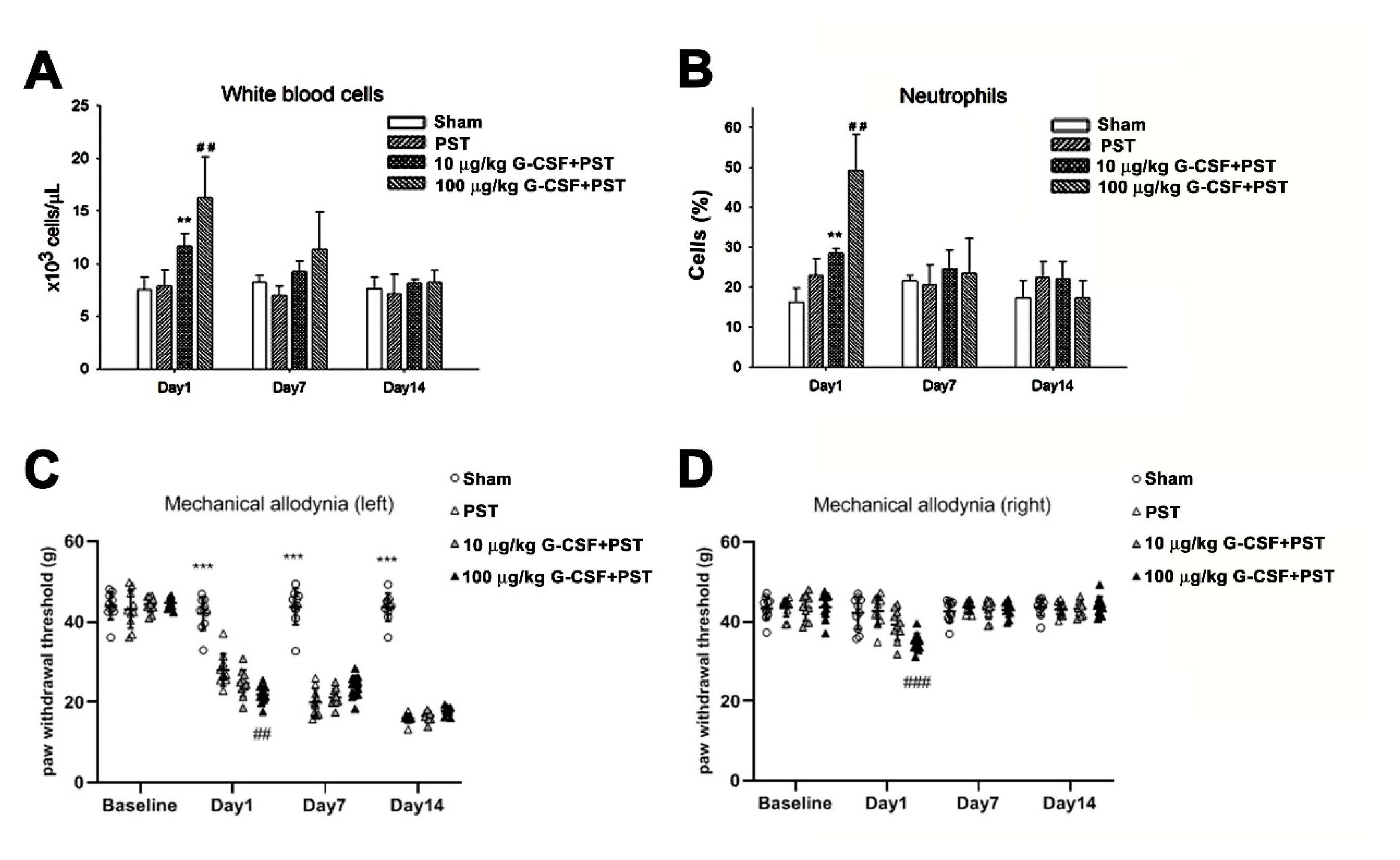
3.5. G-CSF Enhances Mechanical Allodynia Following PST
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grace, P.M.; Tawfik, V.L.; Svensson, C.I.; Burton, M.D.; Loggia, M.L.; Hutchinson, M.R. The Neuroimmunology of Chronic Pain: From Rodents to Humans. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 855–865. [Google Scholar] [CrossRef]
- Yeh, T.Y.; Luo, I.W.; Hsieh, Y.L.; Tseng, T.J.; Chiang, H.; Hsieh, S.T. Peripheral Neuropathic Pain: From Experimental Models to Potential Therapeutic Targets in Dorsal Root Ganglion Neurons. Cells 2020, 9, 2725. [Google Scholar] [CrossRef] [PubMed]
- Szok, D.; Tajti, J.; Nyari, A.; Vecsei, L. Therapeutic Approaches for Peripheral and Central Neuropathic Pain. Behav. Neurol. 2019, 2019, 8685954. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, A.; Marsch, L.A.; Joseph, H.; Portenoy, R.K. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp. Clin. Psychopharmacol. 2008, 16, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Oelhaf, R.C.; Azadfard, M. Opioid Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- O'Brien, J.B.; Roman, D.L. Novel treatments for chronic pain: Moving beyond opioids. Transl. Res. J. Lab. Clin. Med. 2021, 234, 1–19. [Google Scholar]
- Jahandideh, B.; Derakhshani, M.; Abbaszadeh, H.; Akbar Movassaghpour, A.; Mehdizadeh, A.; Talebi, M.; Yousefi, M. The pro-Inflammatory cytokines effects on mobilization, self-renewal and differentiation of hematopoietic stem cells. Hum. Immunol. 2020, 81, 206–217. [Google Scholar] [CrossRef]
- Dwivedi, P.; Greis, K.D. Granulocyte colony-stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies. Exp. Hematol. 2017, 46, 9–20. [Google Scholar] [CrossRef]
- Chen, S.H.; Wang, T.F.; Yang, K.L. Hematopoietic stem cell donation. Int. J. Hematol. 2013, 97, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.C.; Hoffmann, B.W.; Csar, X.F.; Hamilton, J.A. Granulocyte colony-stimulating factor-stimulated proliferation of myeloid cells: Mode of cell cycle control by a range of inhibitors. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 1996, 16, 869–877. [Google Scholar] [CrossRef]
- Lejnieks, D.V.; Han, S.W.; Ramesh, N.; Lau, S.; Osborne, W.R. Granulocyte colony-stimulating factor expression from transduced vascular smooth muscle cells provides sustained neutrophil increases in rats. Hum. Gene Ther. 1996, 7, 1431–1436. [Google Scholar] [CrossRef]
- Leizer, T.; Cebon, J.; Layton, J.E.; Hamilton, J.A. Cytokine regulation of colony-stimulating factor production in cultured human synovial fibroblasts: I. Induction of GM-CSF and G-CSF production by interleukin-1 and tumor necrosis factor. Blood 1990, 76, 1989–1996. [Google Scholar] [CrossRef]
- Sieff, C.A.; Niemeyer, C.M.; Mentzer, S.J.; Faller, D.V. Interleukin-1, tumor necrosis factor, and the production of colony-stimulating factors by cultured mesenchymal cells. Blood 1988, 72, 1316–1323. [Google Scholar] [CrossRef]
- Horio, H.; Nomori, H.; Morinaga, S.; Kikuchi, T.; Tomonari, H.; Kuriyama, S.; Suemasu, K. Granulocyte colony-stimulating factor-producing primary pericardial mesothelioma. Hum. Pathol. 1999, 30, 718–720. [Google Scholar] [CrossRef]
- Shukla, A.; MacPherson, M.B.; Hillegass, J.; Ramos-Nino, M.E.; Alexeeva, V.; Vacek, P.M.; Bond, J.P.; Pass, H.I.; Steele, C.; Mossman, B.T. Alterations in gene expression in human mesothelial cells correlate with mineral pathogenicity. Am. J. Respir. Cell Mol. Biol. 2009, 41, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Tweardy, D.J.; Mott, P.L.; Glazer, E.W. Monokine modulation of human astroglial cell production of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. I. Effects of IL-1 alpha and IL-beta. J. Immunol. 1990, 144, 2233–2241. [Google Scholar] [PubMed]
- Rahi, V.; Jamwal, S.; Kumar, P. Neuroprotection through G-CSF: Recent advances and future viewpoints. Pharmacol. Rep. PR 2021. [Google Scholar] [CrossRef] [PubMed]
- Hasselblatt, M.; Jeibmann, A.; Riesmeier, B.; Maintz, D.; Schabitz, W.R. Granulocyte-colony stimulating factor (G-CSF) and G-CSF receptor expression in human ischemic stroke. Acta Neuropathol. 2007, 113, 45–51. [Google Scholar] [CrossRef]
- Taichman, R.S.; Emerson, S.G. Human osteosarcoma cell lines MG-63 and SaOS-2 produce G-CSF and GM-CSF: Identification and partial characterization of cell-associated isoforms. Exp. Hematol. 1996, 24, 509–517. [Google Scholar]
- Menzie-Suderam, J.M.; Modi, J.; Xu, H.; Bent, A.; Trujillo, P.; Medley, K.; Jimenez, E.; Shen, J.; Marshall, M.; Tao, R.; et al. Granulocyte-colony stimulating factor gene therapy as a novel therapeutics for stroke in a mouse model. J. Biomed. Sci. 2020, 27, 99. [Google Scholar] [CrossRef]
- Lu, F.; Nakamura, T.; Toyoshima, T.; Liu, Y.; Shinomiya, A.; Hirooka, K.; Okabe, N.; Miyamoto, O.; Tamiya, T.; Keep, R.F.; et al. Neuroprotection of granulocyte colony-stimulating factor during the acute phase of transient forebrain ischemia in gerbils. Brain Res. 2014, 1548, 49–55. [Google Scholar] [CrossRef]
- Schabitz, W.R.; Kollmar, R.; Schwaninger, M.; Juettler, E.; Bardutzky, J.; Scholzke, M.N.; Sommer, C.; Schwab, S. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 2003, 34, 745–751. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, S.; Wang, P.; Zhang, H.; Wang, F.; Bing, L.; Gao, J.; Yang, J.; Hao, A. Granulocyte colony-stimulating factor improves neuron survival in experimental spinal cord injury by regulating nucleophosmin-1 expression. J. Neurosci. Res. 2014, 92, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Khorasanizadeh, M.; Eskian, M.; Vaccaro, A.R.; Rahimi-Movaghar, V. Granulocyte Colony-Stimulating Factor (G-CSF) for the Treatment of Spinal Cord Injury. CNS Drugs 2017, 31, 911–937. [Google Scholar] [CrossRef]
- Liao, M.F.; Hsu, J.L.; Lu, K.T.; Chao, P.K.; Cheng, M.Y.; Hsu, H.C.; Lo, A.L.; Lee, Y.L.; Hung, Y.H.; Lyu, R.K.; et al. Granulocyte Colony Stimulating Factor (GCSF) Can Attenuate Neuropathic Pain by Suppressing Monocyte Chemoattractant Protein-1 (MCP-1) Expression, through Upregulating the Early MicroRNA-122 Expression in the Dorsal Root Ganglia. Cells 2020, 9, 1669. [Google Scholar] [CrossRef] [PubMed]
- Ro, L.S.; Chen, S.R.; Chao, P.K.; Lee, Y.L.; Lu, K.T. The potential application of granulocyte colony stimulating factor therapy on neuropathic pain. Chang Gung Med. J. 2009, 32, 235–246. [Google Scholar]
- Liao, M.F.; Yeh, S.R.; Lo, A.L.; Chao, P.K.; Lee, Y.L.; Hung, Y.H.; Lu, K.T.; Ro, L.S. An early granulocyte colony-stimulating factor treatment attenuates neuropathic pain through activation of mu opioid receptors on the injured nerve. Sci. Rep. 2016, 6, 25490. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Koda, M.; Takahashi, H.; Sakuma, T.; Inada, T.; Kamiya, K.; Ota, M.; Maki, S.; Okawa, A.; Takahashi, K.; et al. Granulocyte colony-stimulating factor attenuates spinal cord injury-induced mechanical allodynia in adult rats. J. Neurol. Sci. 2015, 355, 79–83. [Google Scholar] [CrossRef]
- Liou, J.T.; Lui, P.W.; Liu, F.C.; Lai, Y.S.; Day, Y.J. Exogenous granulocyte colony-stimulating factor exacerbate pain-related behaviors after peripheral nerve injury. J. Neuroimmunol. 2011, 232, 83–93. [Google Scholar] [CrossRef]
- Lindenlaub, T.; Sommer, C. Partial sciatic nerve transection as a model of neuropathic pain: A qualitative and quantitative neuropathological study. Pain 2000, 89, 97–106. [Google Scholar] [CrossRef]
- Kalmar, B.; Greensmith, L.; Malcangio, M.; McMahon, S.B.; Csermely, P.; Burnstock, G. The effect of treatment with BRX-220, a co-inducer of heat shock proteins, on sensory fibers of the rat following peripheral nerve injury. Exp. Neurol. 2003, 184, 636–647. [Google Scholar] [CrossRef]
- Lever, I.; Cunningham, J.; Grist, J.; Yip, P.K.; Malcangio, M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur. J. Neurosci. 2003, 18, 1169–1174. [Google Scholar] [CrossRef]
- Loeser, J.D.; Treede, R.D. The Kyoto protocol of IASP Basic Pain Terminology. Pain 2008, 137, 473–477. [Google Scholar] [CrossRef]
- Huh, Y.; Ji, R.R.; Chen, G. Neuroinflammation, Bone Marrow Stem Cells, and Chronic Pain. Front. Immunol. 2017, 8, 1014. [Google Scholar] [CrossRef]
- Chao, P.K.; Lu, K.T.; Lee, Y.L.; Chen, J.C.; Wang, H.L.; Yang, Y.L.; Cheng, M.Y.; Liao, M.F.; Ro, L.S. Early systemic granulocyte-colony stimulating factor treatment attenuates neuropathic pain after peripheral nerve injury. PLoS ONE 2012, 7, e43680. [Google Scholar] [CrossRef]
- Ha, S.O.; Kim, J.K.; Hong, H.S.; Kim, D.S.; Cho, H.J. Expression of brain-derived neurotrophic factor in rat dorsal root ganglia, spinal cord and gracile nuclei in experimental models of neuropathic pain. Neuroscience 2001, 107, 301–309. [Google Scholar] [CrossRef]
- Terada, Y.; Morita-Takemura, S.; Isonishi, A.; Tanaka, T.; Okuda, H.; Tatsumi, K.; Shinjo, T.; Kawaguchi, M.; Wanaka, A. NGF and BDNF expression in mouse DRG after spared nerve injury. Neurosci. Lett. 2018, 686, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Schroeter, M.; Jander, S.; Stoll, G. Induction of granulocyte colony-stimulating factor mRNA by focal cerebral ischemia and cortical spreading depression. Brain Res. Mol. Brain Res. 2004, 131, 73–78. [Google Scholar] [CrossRef]
- Schneider, A.; Kruger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.H.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Kuhn, H.G.; Schabitz, W.R. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle 2005, 4, 1753–1757. [Google Scholar] [PubMed]
- Liongue, C.; Wright, C.; Russell, A.P.; Ward, A.C. Granulocyte colony-stimulating factor receptor: Stimulating granulopoiesis and much more. Int. J. Biochem. Cell Biol. 2009, 41, 2372–2375. [Google Scholar] [CrossRef] [PubMed]
- Villa, G.; Fumagalli, M.; Verderio, C.; Abbracchio, M.P.; Ceruti, S. Expression and contribution of satellite glial cells purinoceptors to pain transmission in sensory ganglia: An update. Neuron Glia Biol. 2010, 6, 31–42. [Google Scholar] [CrossRef]
- Goldschmidt, E.; Fellows-Mayle, W.; Wolfe, R.; Niranjan, A.; Flickinger, J.C.; Lunsford, L.D.; Gerszten, P.C. Radiosurgery to the spinal dorsal root ganglion induces fibrosis and inhibits satellite glial cell activation while preserving axonal neurotransmission. J. Neurosurg. Spine 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schweizerhof, M.; Stosser, S.; Kurejova, M.; Njoo, C.; Gangadharan, V.; Agarwal, N.; Schmelz, M.; Bali, K.K.; Michalski, C.W.; Brugger, S.; et al. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat. Med. 2009, 15, 802–807. [Google Scholar] [CrossRef]
- Zhang, E.; Lee, S.; Yi, M.H.; Nan, Y.; Xu, Y.; Shin, N.; Ko, Y.; Lee, Y.H.; Lee, W.; Kim, D.W. Expression of granulocyte colony-stimulating factor 3 receptor in the spinal dorsal horn following spinal nerve ligation-induced neuropathic pain. Mol. Med. Rep. 2017, 16, 2009–2015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diederich, K.; Sevimli, S.; Dorr, H.; Kosters, E.; Hoppen, M.; Lewejohann, L.; Klocke, R.; Minnerup, J.; Knecht, S.; Nikol, S.; et al. The role of granulocyte-colony stimulating factor (G-CSF) in the healthy brain: A characterization of G-CSF-deficient mice. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 11572–11581. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-C.; Yang, C.-P.; Ma, K.-H.; Shih, J.-H.; Tseng, C.-S.; Huang, Y.-S. Endogenous Expression of G-CSF in Rat Dorsal Root Ganglion Neurons after Nerve Injury. Brain Sci. 2021, 11, 956. https://doi.org/10.3390/brainsci11070956
Yeh C-C, Yang C-P, Ma K-H, Shih J-H, Tseng C-S, Huang Y-S. Endogenous Expression of G-CSF in Rat Dorsal Root Ganglion Neurons after Nerve Injury. Brain Sciences. 2021; 11(7):956. https://doi.org/10.3390/brainsci11070956
Chicago/Turabian StyleYeh, Chun-Chang, Chih-Ping Yang, Kuo-Hsing Ma, Jui-Hu Shih, Ching-San Tseng, and Yuahn-Sieh Huang. 2021. "Endogenous Expression of G-CSF in Rat Dorsal Root Ganglion Neurons after Nerve Injury" Brain Sciences 11, no. 7: 956. https://doi.org/10.3390/brainsci11070956
APA StyleYeh, C.-C., Yang, C.-P., Ma, K.-H., Shih, J.-H., Tseng, C.-S., & Huang, Y.-S. (2021). Endogenous Expression of G-CSF in Rat Dorsal Root Ganglion Neurons after Nerve Injury. Brain Sciences, 11(7), 956. https://doi.org/10.3390/brainsci11070956





