Hyperbaric Oxygen Therapy for Children and Youth with Autism Spectrum Disorder: A Review
Abstract
1. Introduction
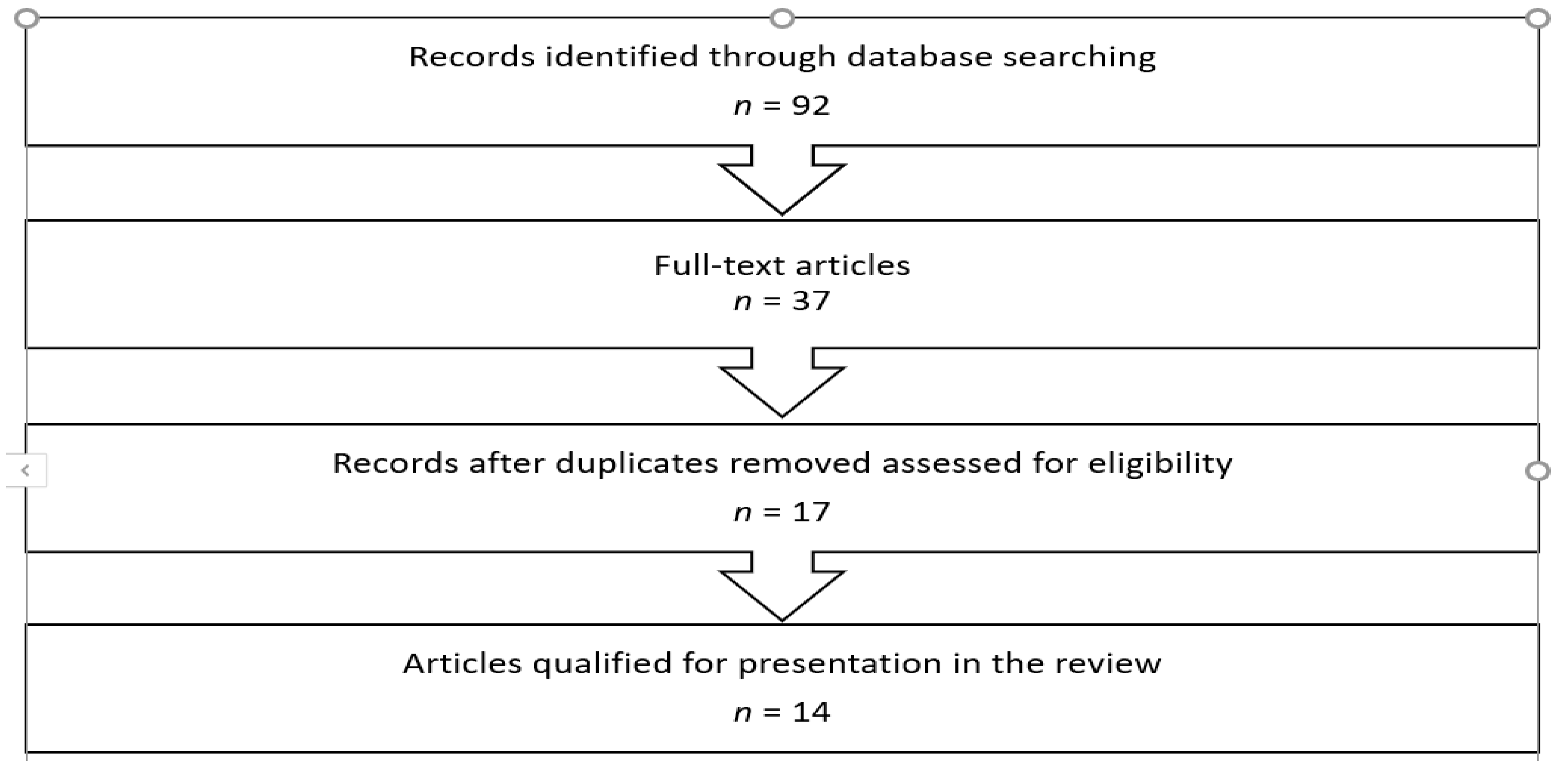
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Floris, D.L.; Wolfers, T.; Zabihi, M.; Holz, N.E.; Zwiers, M.P.; Charman, T.; Tillmann, J.; Ecker, C.; Dell’Acqua, F.; Banaschewski, T.; et al. Atypical brain asymmetry in autism—A candidate for clinically meaningful stratification. Biol. Psychiatry Cogn. Neurosci. Neuroimag. 2020. [Google Scholar] [CrossRef]
- National Institute of Mental Health. Available online: https://www.nimh.nih.gov/health/publications/autism-spectrum-disorder/19-mh-8084-autismspecdisordr_152236.pdf (accessed on 1 March 2020).
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Rosenberg, C.R.; White, T.; et al. Dowling prevalence of autism spectrum disorder among children aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chaste, P.; Leboyer, M. Autism risk factors: Genes, environment, and gene-environment interactions. Dialogues Clin. Neurosci. 2012, 14, 281–292. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/ncbddd/autism/data.html (accessed on 4 June 2021).
- Shrestha, R.; Dissanayake, C.; Barbaro, J. Caregivers’ knowledge of autism in a local peri-urban community of Nepal: A cross-sectional study in Kirtipur, Kathmandu. Res. Autism Spectr. Disord. 2020, 80, 101696. [Google Scholar] [CrossRef]
- Lindsey, R.A.; Saltness, S.R.; Lau, A.F.; Barry, T.D. A longitudinal examination of interactions between autism symptom severity and parenting behaviors in predicting change in child behavior problems. Res. Autism Spectr. Disord. 2019, 70, 101469. [Google Scholar] [CrossRef]
- Volkmar, F.; Siegel, M.; Woodbury-Smith, M.; King, B.; McCracken, J.; State, M.; American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 237–257. [Google Scholar] [CrossRef]
- LeClerc, S.; Easley, D. Pharmacological therapies for autism spectrum disorder: A review. Pharm. Therapeutics 2015, 40, 389–397. [Google Scholar]
- Fusar-Poli, L.; Brondino, N.; Rocchetti, M.; Petrosino, B.; Arillotta, D.; Damiani, S.; Provenzani, U.; Petrosino, C.; Aguglia, E.; Politi, P. Prevalence and predictors of psychotropic medication use in adolescents and adults with autism spectrum disorder in Italy: A cross-sectional study. Psychiatry Res. 2019, 276, 203–209. [Google Scholar] [CrossRef]
- Beamish, W.; Taylor, A.; Macdonald, L.; Hay, S.; Tucker, M.; Paynter, J. Field testing an Australian model of practice for teaching young school-age students on the autism spectrum. Res. Dev. Disabil. 2021, 113, 103942. [Google Scholar] [CrossRef]
- Didden, R.; Sturmey, P.; Sigafoos, J.; Lang, R.; O’Reilly, M.F.; Lancioni, G.E. Nature, prevalence, and characteristics of challenging behavior. In Functional Assessment for Challenging Behaviors; Springer: New York, NY, USA, 2012; pp. 25–44. [Google Scholar]
- Levy, S.E.; Hyman, S.L. Complementary and alternative medicine treatments for children with autism spectrum disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2015, 24, 117–143. [Google Scholar] [CrossRef]
- Marín, L.; Fioravanti, G.; Portas, M. Hyperbaric oxygen therapy for a pediatric electrical burn. A case report. Burns Open 2020, 4, 137–139. [Google Scholar] [CrossRef]
- Ciarlone, G.E.; Hinojo, C.M.; Stavitzski, N.M.; Dean, J.B. CNS function and dysfunction during exposure to hyperbaric oxygen in operational and clinical settings. Redox Biol. 2019, 27, 101159. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A.; Frye, R.E. A review of research trends in physiological abnormalities in autism spectrum disorders: Immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol. Psychiatry 2012, 17, 389–401. [Google Scholar] [CrossRef]
- Boddaert, N.; Zilbovicius, M. Functional neuroimaging and childhood autism. Pediatr. Radiol. 2002, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Saad, K.; Chirumbolo, S.; Kern, J.K.; Geier, D.A.; Geier, M.R.; Urbina, M.A. Immune dysfunction and neuroinflammation in autism spectrum disorder. Acta Neurobiol. Exp. 2016, 76, 257–268. [Google Scholar] [CrossRef]
- Gut, D.C. Microbiota, inflammation, and probiotics on neural development in autism spectrum disorder. Neuroscience 2018, 15, 271–286. [Google Scholar]
- Rose, S.; Niyazov, D.M.; Rossignol, D.A.; Goldenthal, M.; Kahler, S.G.; Frye, R.E. Clinical and molecular characteristics of mitochondrial dysfunction in autism spectrum disorder. Mol. Diagn. Ther. 2018, 22, 571–593. [Google Scholar] [CrossRef]
- Gutsaeva, D.R.; Suliman, H.B.; Carraway, M.S.; Demchenko, I.T.; Piantadosi, C.A. Oxygen-induced mitochondrial biogenesis in the rat hippocampus. Neuroscience 2006, 137, 493–504. [Google Scholar] [CrossRef]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative stress in autism spectrum disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, D.A. Hyperbaric oxygen therapy might improve certain pathophysiological findings in autism. Med. Hypotheses 2007, 68, 1208–1227. [Google Scholar] [CrossRef]
- Oxford Recovery Center. Available online: https://oxfordrecoverycenter.com/conditions/autism/why-treat-autism-with-hbot/r (accessed on 1 March 2020).
- Camporesi, E.M. Side effects of hyperbaric oxygen therapy. Undersea Hyperb. Med. 2014, 41, 253–257. [Google Scholar] [PubMed]
- Zhukova, M.A.; Talantseva, O.I.; Logvinenko, T.I.; Titova, O.S.; Grigorenko, E.L. Complementary and alternative treatments for autism spectrum disorders: A review for parents and clinicians. Clin. Psychol. Spec. Educ. 2020, 9, 142–173. [Google Scholar] [CrossRef]
- Sakulchit, T.; Ladish, C.; Goldman, R.D. Hyperbaric oxygen therapy for children with autism spectrum disorder. Can. Fam. Physician 2017, 63, 446–448. [Google Scholar]
- Lyra, L.; Rizzo, L.E.; Sunahara, C.S.; Pachito, D.V.; de Oliveira Cruz Latorraca, C.; Martimbianco, A.L.C.; Riera, R. What do Cochrane systematic reviews say about interventions for autism spectrum disorders? Sao Paulo Med. J. 2017, 135, 192–201. [Google Scholar] [CrossRef][Green Version]
- Xiong, T.; Chen, H.; Luo, R.; Mu, D. Hyperbaric oxygen therapy for people with autism spectrum disorder (ASD). Cochrane Database Syst. Rev. 2016, 10, CD010922. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Kemper, K.J. Integrative Approaches to Caring for Children with Autism. Curr. Probl. Pediatr. Adolesc. Health Care 2016, 46, 195–201. [Google Scholar] [CrossRef]
- Goldfarb, C.; Genore, L.; Hunt, C.; Flanagan, J.; Handley-Derry, M.; Jethwa, A.; Jones-Stokreef, N.; Kirkpatrick, S.M.L.; Richards, A.; Rojnica, L.; et al. Hyperbaric oxygen therapy for the treatment of children and youth with Autism Spectrum Disorders: An evidence-based systematic review. Res. Autism Spectr. Disord. 2016, 29–30, 1–7. [Google Scholar] [CrossRef]
- Martin, R.; Srivastava, T.; Lee, J.; Raj, N.; Koth, K.A.; Whelan, H.T. Using hyperbaric oxygen for autism treatment: A review and discussion of literature. Undersea Hyperb. Med. 2015, 42, 353–359. [Google Scholar]
- Li, H.H.; Shan, L.; Du, L.; Jia, F.Y. Research advances in the management of autism spectrum disorders in children. Zhongguo Dang Dai Er Ke Za Zhi 2015, 17, 886–892. (In Chinese) [Google Scholar]
- Brondino, N.; Fusar-Poli, L.; Rocchetti, M.; Provenzani, U.; Barale, F.; Politi, P. Complementary and alternative therapies for autism spectrum disorder. Evid. Based Complement. Alternat. Med. 2015, 2015, 258589. [Google Scholar] [CrossRef] [PubMed]
- Politte, L.C.; Yamini Howe, Y.; Nowinski, L.; Palumbo, M.; McDougle, C.J. Current evidence-based treatments for autism spectrum disorder. Curr. Treat. Options Psychiatry 2015, 2, 38–56. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.A.; Zaky, E.A.; Aboulsaoud, M.; Elhossiny, R.M.; Youssef, W.Y.; Mahmoud, A.M.; Ali, S.S. Autism spectrum disorder (ASD)-associated mitochondrial deficits are revealed in children’s platelets but unimproved by hyperbaric oxygen therapy. Free Radic. Res. 2021, 55, 26–40. [Google Scholar] [CrossRef]
- Kostiukow, A.; Samborski, W. The effectiveness of hyperbaric oxygen therapy (HBOT) in children with autism spectrum disorders. Pol. Merkur. Lekarski. 2020, 48, 15–18. [Google Scholar]
- Lasheen, R.H.; Abu-Zaid, M.H.; AlHamed Tabra, S.A. Evaluation of auditory attention and memory skills in autistic children after hyperbaric O2 treatment. EJENTAS 2019, 20, 60–66. [Google Scholar] [CrossRef][Green Version]
- Rizzato, A.; D’Alessandro, N.; Berenci, E.; Rinchi, A.; Enten, G.; Vezzani, G.; Proietti, M.; Fiorito, A.; Camporesi, E.; Bosco, G. Effect of mild hyperbaric oxygen therapy on children diagnosed with autism. Undersea Hyperb. Med. 2018, 45, 639–645. [Google Scholar]
- Heyboer, M.; Sharma, D.; Santiago, W.; McCulloch, N. Hyperbaric oxygen therapy: Side effects defined and quantified. Adv. Wound Care (New Rochelle) 2017, 6, 210–224. [Google Scholar] [CrossRef]
- Plafki, C.; Peters, P.; Almeling, M.; Welslau, W.; Busch, R. Complications and side effects of hyperbaric oxygen therapy. Aviat. Space Environ. Med. 2000, 71, 119–124. [Google Scholar]
- Karahatay, S.; Yilmaz, Y.F.; Birkent, H.; Ay, H.; Satar, B. Middle ear barotrauma with hyperbaric oxygen therapy: Incidence and the predictive value of the nine-step inflation/deflation test and otoscopy. Ear Nose Throat J. 2008, 87, 684–688. [Google Scholar] [CrossRef]
- Shupak, A.; Gilbey, P. Effects of pressure. In Physiology and Medicine of Hyperbaric Oxygen Therapy; Saunders Elsevier: Philadelphia, PA, USA, 2008; pp. 513–526. [Google Scholar]
- Manning, E.P. Central nervous system oxygen toxicity and hyperbaric oxygen seizures. Aerosp. Med. Hum. Perform. 2016, 87, 477–486. [Google Scholar] [CrossRef]
- MedicalNewsToday. Available online: www.medicalnewstoday.com/articles/37062 (accessed on 3 June 2021).
- Treatments for Autism Spectrum Disorder. Available online: https://www.cdc.gov/ncbddd/actearly/autism/curriculum/documents/treatments-autism_508.pdf (accessed on 15 February 2020).
| (1a) Reviews | Number of Patients/ Participants/ Cases | Methodology | Main Outcome | Measures |
| Zhukova, M.A. et al. (2020) [26] | N/A | N/A | “We advise against the use of (…) hyperbaric oxygen therapy, (…) due their documented harmful psychological and physical effects.” | N/A |
| Sakulchit, T. et al. (2017) [27] | N/A | N/A | “Currently, there is insufficient evidence to support use of HBOT to treat children with ASD (…)” | N/A |
| Lyra, L. et al. (2017) [28] | N/A | N/A | “No benefits were found for (…) hyperbaric oxygen therapy (…)” | N/A |
| Xiong, T. et al. (2016) [29] | N/A | N/A | “To date, there is no evidence that hyperbaric oxygen therapy improves core symptoms and associated symptoms of ASD.” | N/A |
| Klein, N. et al. (2016) [30] | N/A | N/A | “Given their risks, costs, and limited evidence of efficacy, chelation, secretin, and hyperbaric oxygen should be avoided.” | N/A |
| Goldfarb, C. et al. (2016) [31] | N/A | N/A | “Current evidence does not support HBOT as an effective treatment for children and youth with ASD.” | N/A |
| Martin, R. et al. (2015) [32] | N/A | N/A | “The evidence is weak for the use of HBO2 in ASD, with only one, likely flawed, randomized control study showing treatment benefit.” | N/A |
| Li, HH. et al. (2015) [33] | N/A | N/A | “With the in-depth study of the pathogenesis of ASD, bumetanide, oxytocin, vitamin D and hyperbaric oxygen therapy have been found to be promising for the improvement of core symptoms of ASD.” | N/A |
| Brondino, N. et al. (2015) [34] | N/A | N/A | “In conclusion, there are still few data on the potential efficacy of CAM in autism, and no evidence-based recommendation could be done so far for the use of such therapies.” | N/A |
| Politte L.C. et al. (2015) [35] | N/A | N/A | “At this time, the use of HBOT for the treatment of ASD is not recommended.” | N/A |
| (1b) Intervention Studies | Number of Patients/ Participants/ Cases/Age Range | Methodology | Main Outcome/Side Effects—if Any | Measures |
| Abdel-Rahman, E.A. et al. (2021) [36] | Study group: n = 10 children with ASD, 5.47 ± 0.87 years; I Control group: n = 10 neurotypical children, 5.28 ± 0.75 years II Control group: n = 10 children with autism + no HBOT treatment, 4.75 ± 0.72 years | HBOT sessions number = 10—75; 37.6 ± 12.2 | “We also found no evidence that HBOT confers any significant improvement of ASD-associated physiological or behavioural phenotypes.” “Similarly, no detectable improvement in ASD-associated behavioral deficits in HBOT group relative to untreated autistic group.”/not reported | Childhood Autism Rating Scale (CARS), Autism Treatment Evaluation Checklist (ATEC), Vineland Adaptive Behavior Scales (VABS), high resolution respirometry, activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) in isolated neutrophils |
| Kostiukow, A. et al. (2020) [37] | Study group: n = 35 boys mean age of 6.9 years, min/max = 2.0/15.8 ± 3.0; 4 girls with ASD mean age 10.2 years, min/max = 4.7/16.0 ± 4 | HBOT sessions number = 40; 1.5 atm; 60 min/daily/8 weeks or 4 weeks when 2 sessions a day | “(…) ATEC Speech/language/communication—“Can follow some commands” revealed a decline (…)” “Eight components of the ATEC and CARS scales as well as the CARS total score revealed statistically significant improvements.”/not reported | Clinical Global Impression Scale (CGIS), Autism Treatment Evaluation Checklist (ATEC) and Childhood Autism Rating Scale (CARS) |
| Lasheen, R.H. et al. (2019) [38] | Study group: n = 20 children with ASD, Age =10.6 ± 2.4; Control group: n = 20 children (neurotypical) chronologically age-matched | HBOT sessions number = 40; 1.5 ATM; 45 min/day/total of 40 sessions | “The children with autism showed improvement in both auditory attention and auditory memory after hyperbaric oxygen therapy.”/not reported | Auditory P300 and MMN (Mismatch Negativity) |
| Rizzato, A. et al. (2018) [39] | Study group: n = 7 boys: 1 girl with ASDF = 1, mean age: 7 ± 2.33; years; Control group: n = 5 boys, 2 girls (with ASD), 6.6 ± 2.7 years | HBOT sessions number = 40; 8 weeks | “Despite the improvements reported in both groups, our results do not support the utility of HBO2 in children diagnosed with autism.”/not reported | Aberrant Behavior Checklist-Community (ABC), Childhood Autism Rating Scale at T0 and T2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podgórska-Bednarz, J.; Perenc, L. Hyperbaric Oxygen Therapy for Children and Youth with Autism Spectrum Disorder: A Review. Brain Sci. 2021, 11, 916. https://doi.org/10.3390/brainsci11070916
Podgórska-Bednarz J, Perenc L. Hyperbaric Oxygen Therapy for Children and Youth with Autism Spectrum Disorder: A Review. Brain Sciences. 2021; 11(7):916. https://doi.org/10.3390/brainsci11070916
Chicago/Turabian StylePodgórska-Bednarz, Justyna, and Lidia Perenc. 2021. "Hyperbaric Oxygen Therapy for Children and Youth with Autism Spectrum Disorder: A Review" Brain Sciences 11, no. 7: 916. https://doi.org/10.3390/brainsci11070916
APA StylePodgórska-Bednarz, J., & Perenc, L. (2021). Hyperbaric Oxygen Therapy for Children and Youth with Autism Spectrum Disorder: A Review. Brain Sciences, 11(7), 916. https://doi.org/10.3390/brainsci11070916






