Brain Functional Correlates of Episodic Memory Using an Ecological Free Recall Task
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Paradigm
2.3. Behavioral Data Analysis
2.4. MRI Data Acquisition and Preprocessing
2.5. fMRI Analysis
2.5.1. Group Level Analysis
2.5.2. Weighted Dice Coefficient (wDC) Analysis
2.5.3. IPL wDC Analysis
2.5.4. Performance-Related Analysis
2.5.5. MVPA Analysis
3. Results
3.1. Behavioral Performance
3.2. Questionnaire Ratings
3.3. Neuroimaging Results
3.3.1. Group Level Analysis
3.3.2. RSNs wDC Analysis
3.3.3. IPL wDC Analysis
3.3.4. Performance-Related Analysis
4. Discussion
4.1. Free Recall of a Story during fMRI and Behavioral Results
4.2. Brain Activation during Memory Recall
4.3. Correlation of RSNs and IPL Patterns of Activation with Memory Performance
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tulving, E. What Is Episodic Memory? Curr. Dir. Psychol. Sci. 1993, 2, 67–70. [Google Scholar] [CrossRef]
- Tulving, E.; Markowitsch, H.J. Episodic and Declarative Memory: Role of the Hippocampus. Hippocampus 1998, 8, 198–204. [Google Scholar] [CrossRef]
- Mahr, J.B.; Csibra, G. Why Do We Remember? The Communicative Function of Episodic Memory. Behav. Brain Sci. 2018, 41. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Eichenbaum, H. The Episodic Memory System: Neurocircuitry and Disorders. Neuropsychopharmacology 2010, 35, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C.; Dubois, B.; Boccardi, M.; Monsch, A.U.; Demonet, J.F.; Cappa, S.F.; Geneva Task Force for the Roadmap of Alzheimer’s Biomarkers. Clinical Validity of Delayed Recall Tests as a Gateway Biomarker for Alzheimer’s Disease in the Context of a Structured 5-Phase Development Framework. Neurobiol. Aging 2017, 52, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Weber, F.D.; Zinke, K.; Noack, H.; Born, J. Effects of Sleep on Word Pair Memory in Children—Separating Item and Source Memory Aspects. Front. Psychol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Saive, A.-L.; Royet, J.-P.; Garcia, S.; Thévenet, M.; Plailly, J. “What-Where-Which” Episodic Retrieval Requires Conscious Recollection and Is Promoted by Semantic Knowledge. PLoS ONE 2015, 10, e0143767. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiller, M.S.; Voss, J.L. Targeted Enhancement of Cortical-Hippocampal Brain Networks and Associative Memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef]
- Tulving, E. Episodic Memory: From Mind to Brain. Annu. Rev. Psychol. 2002, 53, 1–25. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Gilmore, A.W.; Nelson, S.M.; McDermott, K.B. Are There Multiple Kinds of Episodic Memory? An FMRI Investigation Comparing Autobiographical and Recognition Memory Tasks. J. Neurosci. 2017, 37, 2764–2775. [Google Scholar] [CrossRef]
- Smith, S.A. Virtual Reality in Episodic Memory Research: A Review. Psychon. Bull. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Memory Scale; Psychological Corporation: San Antonio, TX, USA, 1945. [Google Scholar]
- Babcock, H.; Levy, L. Test and Manual of Directions; the Revised Examination for the Measurement of Efficiency of Mental Functioning; Stoelting: Wood Dale, IL, USA, 1940; p. 41. [Google Scholar]
- Tremont, G.; Halpert, S.; Javorsky, D.J.; Stern, R.A. Differential Impact of Executive Dysfunction on Verbal List Learning and Story Recall. Clin. Neuropsychol. 2000, 14, 295–302. [Google Scholar] [CrossRef]
- Kopelman, M.D.; Stanhope, N. Recall and Recognition Memory in Patients with Focal Frontal, Temporal Lobe and Diencephalic Lesions. Neuropsychologia 1998, 36, 785–796. [Google Scholar] [CrossRef]
- Brooks, B.L.; Weaver, L.E.; Scialfa, C.T. Does Impaired Executive Functioning Differentially Impact Verbal Memory Measures in Older Adults with Suspected Dementia? Clin. Neuropsychol. 2006, 20, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Hasson, U.; Furman, O.; Clark, D.; Dudai, Y.; Davachi, L. Enhanced Intersubject Correlations during Movie Viewing Correlate with Successful Episodic Encoding. Neuron 2008, 57, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Leong, Y.C.; Honey, C.J.; Yong, C.H.; Norman, K.A.; Hasson, U. Shared Memories Reveal Shared Structure in Neural Activity across Individuals. Nat. Neurosci. 2017, 20, 115–125. [Google Scholar] [CrossRef]
- Bellana, B.; Liu, Z.; Anderson, J.A.E.; Moscovitch, M.; Grady, C.L. Laterality Effects in Functional Connectivity of the Angular Gyrus during Rest and Episodic Retrieval. Neuropsychologia 2016, 80, 24–34. [Google Scholar] [CrossRef]
- Squire, L.R.; Stark, C.E.L.; Clark, R.E. The Medial Temporal Lobe. Annu. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Corkin, S. Lasting Consequences of Bilateral Medial Temporal Lobectomy: Clinical Course and Experimental Findings in H. Semin. Neurol. 1984, 4, 249–259. [Google Scholar] [CrossRef]
- Rossi, S.; Cappa, S.F.; Babiloni, C.; Pasqualetti, P.; Miniussi, C.; Carducci, F.; Babiloni, F.; Rossini, P.M. Prefontal Cortex in Long-Term Memory: An “Interference” Approach Using Magnetic Stimulation. Nat. Neurosci. 2001, 4, 948–952. [Google Scholar] [CrossRef]
- Rossi, S. Age-Related Functional Changes of Prefrontal Cortex in Long-Term Memory: A Repetitive Transcranial Magnetic Stimulation Study. J. Neurosci. 2004, 24, 7939–7944. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Innocenti, I.; Polizzotto, N.R.; Feurra, M.; De Capua, A.; Ulivelli, M.; Bartalini, S.; Cappa, S.F. Temporal Dynamics of Memory Trace Formation in the Human Prefrontal Cortex. Cereb. Cortex 2011, 21, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Pasqualetti, P.; Zito, G.; Vecchio, F.; Cappa, S.F.; Miniussi, C.; Babiloni, C.; Rossini, P.M. Prefrontal and Parietal Cortex in Human Episodic Memory: An Interference Study by Repetitive Transcranial Magnetic Stimulation. Eur. J. Neurosci. 2006, 23, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Rugg, M.D.; King, D.R. Ventral Lateral Parietal Cortex and Episodic Memory Retrieval. Cortex 2018, 107, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.; Petersen, S. The Attention System of the Human Brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Wagner, A.D.; Shannon, B.J.; Kahn, I.; Buckner, R.L. Parietal Lobe Contributions to Episodic Memory Retrieval. Trends Cogn. Sci. 2005, 9, 445–453. [Google Scholar] [CrossRef]
- Cabeza, R.; Ciaramelli, E.; Olson, I.R.; Moscovitch, M. The Parietal Cortex and Episodic Memory: An Attentional Account. Nat. Rev. Neurosci. 2008, 9, 613–625. [Google Scholar] [CrossRef]
- Sestieri, C.; Shulman, G.L.; Corbetta, M. The Contribution of the Human Posterior Parietal Cortex to Episodic Memory. Nat. Rev. Neurosci. 2017, 18, 183–192. [Google Scholar] [CrossRef]
- Rugg, M.D. ERP studies of memory. In Electrophysiology of Mind: Event-Related Brain Potentials and Cognition; Oxford Psychology Series, No. 25; Oxford University Press: New York, NY, USA, 1995; pp. 133–170. ISBN 978-0-19-852135-8. [Google Scholar]
- Rugg, M.; Fletcher, P.; Frith, C.; Frackowiak, R.; Dolan, R. Brain Regions Supporting Intentional and Incidental Memory: A PET Study. Neuroreport 1997, 8, 1283–1287. [Google Scholar] [CrossRef]
- Vincent, J.L.; Snyder, A.Z.; Fox, M.D.; Shannon, B.J.; Andrews, J.R.; Raichle, M.E.; Buckner, R.L. Coherent Spontaneous Activity Identifies a Hippocampal-Parietal Memory Network. J. Neurophysiol. 2006, 96, 3517–3531. [Google Scholar] [CrossRef]
- Plaut, D.C. Graded Modality-Specific Specialisation in Semantics: A Computational Account of Optic Aphasia. Cogn. Neuropsychol. 2002, 19, 603–639. [Google Scholar] [CrossRef] [PubMed]
- Thomas Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The Organization of the Human Cerebral Cortex Estimated by Intrinsic Functional Connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.B.; Szpunar, K.K.; Christ, S.E. Laboratory-Based and Autobiographical Retrieval Tasks Differ Substantially in Their Neural Substrates. Neuropsychologia 2009, 47, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Branzi, F.M.; Pobric, G.; Jung, J.; Lambon Ralph, M.A. The Left Angular Gyrus Is Causally Involved in Information Buffering and Context Formation: Evidence from a Narrative Reading Task. Neuroscience 2019. [Google Scholar] [CrossRef]
- Buckner, R.L. Memory and Executive Function in Aging and AD: Multiple Factors That Cause Decline and Reserve Factors That Compensate. Neuron 2004, 44, 195–208. [Google Scholar] [CrossRef]
- Ben-Zvi, S.; Soroker, N.; Levy, D.A. Parietal Lesion Effects on Cued Recall Following Pair Associate Learnin g. Neuropsychologia 2015, 73, 176–194. [Google Scholar] [CrossRef]
- Ciaramelli, E.; Faggi, G.; Scarpazza, C.; Mattioli, F.; Spaniol, J.; Ghetti, S.; Moscovitch, M. Subjective Recollection Independent from Multifeatural Context Retrieval Following Damage to the Posterior Parietal Cortex. Cortex 2017, 91, 114–125. [Google Scholar] [CrossRef]
- Bonnici, H.M.; Richter, F.R.; Yazar, Y.; Simons, J.S. Multimodal Feature Integration in the Angular Gyrus during Episodic and Semantic Retrieval. J. Neurosci. 2016, 36, 5462–5471. [Google Scholar] [CrossRef]
- Bonnici, H.M.; Cheke, L.G.; Green, D.A.E.; FitzGerald, T.H.B.; Simons, J.S. Specifying a Causal Role for Angular Gyrus in Autobiographical Memory. Neuroscience 2018, 38, 10438–10443. [Google Scholar] [CrossRef]
- Ciaramelli, E.; Neri, F.; Marini, L.; Braghittoni, D. Improving Memory Following Prefrontal Cortex Damage with the PQRST Method. Front. Behav. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, B.A.; Vasanawala, S.S.; Pauly, J.M.; Nishimura, D.G. Characterization and Reduction of the Transient Response in Steady-State MR Imaging. Magn. Reson. Med. 2001, 46, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.L.R.; Hutton, C.; Ashburner, J.; Turner, R.; Friston, K. Modeling Geometric Deformations in EPI Time Series. NeuroImage 2001, 13, 903–919. [Google Scholar] [CrossRef]
- Dice, L.R. Measures of the Amount of Ecologic Association Between Species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Shirer, W.R.; Ryali, S.; Rykhlevskaia, E.; Menon, V.; Greicius, M.D. Decoding Subject-Driven Cognitive States with Whole-Brain Connectivity Patterns. Cereb. Cortex 2012, 22, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Caspers, S.; Geyer, S.; Schleicher, A.; Mohlberg, H.; Amunts, K.; Zilles, K. The Human Inferior Parietal Cortex: Cytoarchitectonic Parcellation and Interindividual Variability. NeuroImage 2006, 33, 430–448. [Google Scholar] [CrossRef]
- Caspers, S.; Eickhoff, S.B.; Geyer, S.; Scheperjans, F.; Mohlberg, H.; Zilles, K.; Amunts, K. The Human Inferior Parietal Lobule in Stereotaxic Space. Brain Struct. Funct. 2008, 212, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Caspers, S.; Schleicher, A.; Bacha-Trams, M.; Palomero-Gallagher, N.; Amunts, K.; Zilles, K. Organization of the Human Inferior Parietal Lobule Based on Receptor Architectonics. Cereb. Cortex 2013, 23, 615–628. [Google Scholar] [CrossRef]
- Triarhou, L.C. The Cytoarchitectonic Map of Constantin von Economo and Georg N. Koskinas. In Microstructural Parcellation of the Human Cerebral Cortex; Geyer, S., Turner, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Barigazzi, R.; Sala, S.D.; Laiacona, M.; Spinnler, H.; Valenti, V. La Memoria di Prosa: Taratura di un test. Ric. Psicol. 1987, 1, 49–80. [Google Scholar]
- Gates, A.I. Correlations of Immediate and Delayed Recall. J. Educ. Psychol. 1918, 9, 489–496. [Google Scholar] [CrossRef][Green Version]
- Muth, K.D.; Britton, B.K.; Glynn, S.M.; Graves, M.F. Thinking Out Loud While Studying Text: Rehearsing Key Ideas. J. Educ. Psychol. 1988, 80, 315–318. [Google Scholar] [CrossRef]
- Yarets, M.Y.; Sharova, E.V.; Smirnov, A.S.; Pogozbekyan, A.L.; Boldyreva, G.N.; Zaytsev, O.S.; Enikolopova, E.V. Analysis of the Structural-Functional Organization of a Counting Task in the Context of a Study of Executive Functions. Neurosci. Behav. Physiol. 2019, 49, 694–703. [Google Scholar] [CrossRef]
- Cabeza, R.; Dolcos, F.; Prince, S.E.; Rice, H.J.; Weissman, D.H.; Nyberg, L. Attention-Related Activity during Episodic Memory Retrieval: A Cross-Function FMRI Study. Neuropsychologia 2003, 41, 390–399. [Google Scholar] [CrossRef]
- Ciaramelli, E.; Grady, C.L.; Moscovitch, M. Top-down and Bottom-up Attention to Memory: A Hypothesis (AtoM) on the Role of the Posterior Parietal Cortex in Memory Retrieval. Neuropsychologia 2008, 46, 1828–1851. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, G.F.; Lambon Ralph, M.A. Fusion and Fission of Cognitive Functions in the Human Parietal Cortex. Cereb. Cortex 2015, 25, 3547–3560. [Google Scholar] [CrossRef]
- Humphreys, G.F.; Jackson, R.L.; Lambon Ralph, M.A. Overarching Principles and Dimensions of the Functional Organisation in the Inferior Parietal Cortex. Cereb. Cortex 2019, 30, 5639–5653. [Google Scholar] [CrossRef] [PubMed]
- Vilberg, K.L.; Rugg, M.D. Memory Retrieval and the Parietal Cortex: A Review of Evidence from a Dual-Process Perspective. Neuropsychologia 2008, 46, 1787–1799. [Google Scholar] [CrossRef]
- Postle, B.R.; Berger, J.S.; D’Esposito, M. Functional Neuroanatomical Double Dissociation of Mnemonic and Executive Control Processes Contributing to Working Memory Performance. Proc. Natl. Acad. Sci. USA 1999, 96, 12959–12964. [Google Scholar] [CrossRef]
- Spreng, R.N.; Gerlach, K.D.; Turner, G.R.; Schacter, D.L. Autobiographical Planning and the Brain: Activation and Its Modulation by Qualitative Features. J. Cogn. Neurosci. 2015, 27, 2147–2157. [Google Scholar] [CrossRef]
- Lundstrom, B.N.; Ingvar, M.; Petersson, K.M. The Role of Precuneus and Left Inferior Frontal Cortex during Source Memory Episodic Retrieval. NeuroImage 2005, 27, 824–834. [Google Scholar] [CrossRef]
- Henson, R.N.A.; Rugg, M.D.; Shallice, T.; Josephs, O.; Dolan, R.J. Recollection and Familiarity in Recognition Memory: An Event-Related Functional Magnetic Resonance Imaging Study. J. Neurosci. 1999, 19, 3962–3972. [Google Scholar] [CrossRef] [PubMed]
- Konishi, S.; Wheeler, M.E.; Donaldson, D.I.; Buckner, R.L. Neural Correlates of Episodic Retrieval Success. NeuroImage 2000, 12, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Supekar, K.; Amin, H.; Rykhlevskaia, E.; Nguyen, D.A.; Greicius, M.D.; Menon, V. Dissociable Connectivity within Human Angular Gyrus and Intraparietal Sulcus: Evidence from Functional and Structural Connectivity. Cereb. Cortex 2010, 20, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
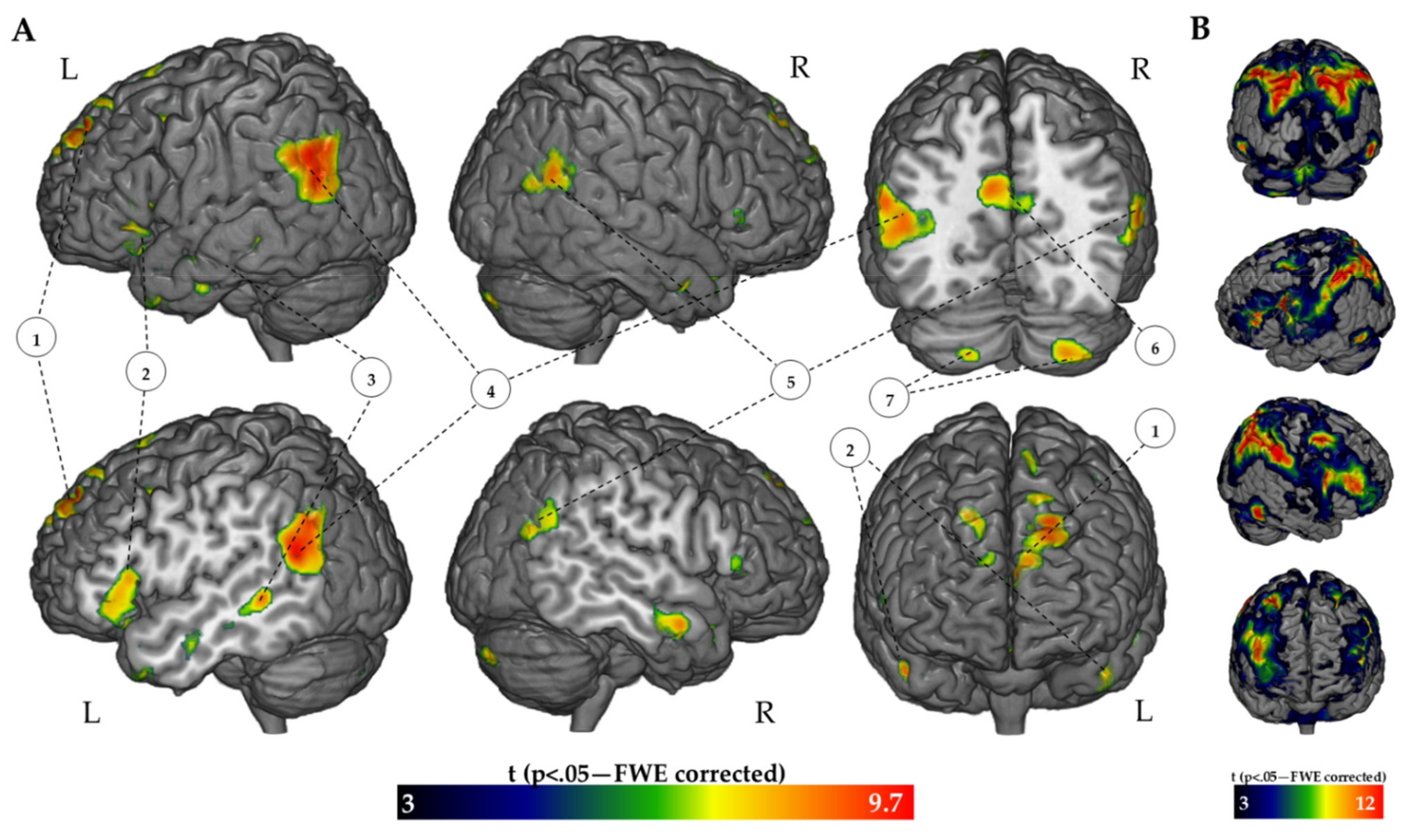
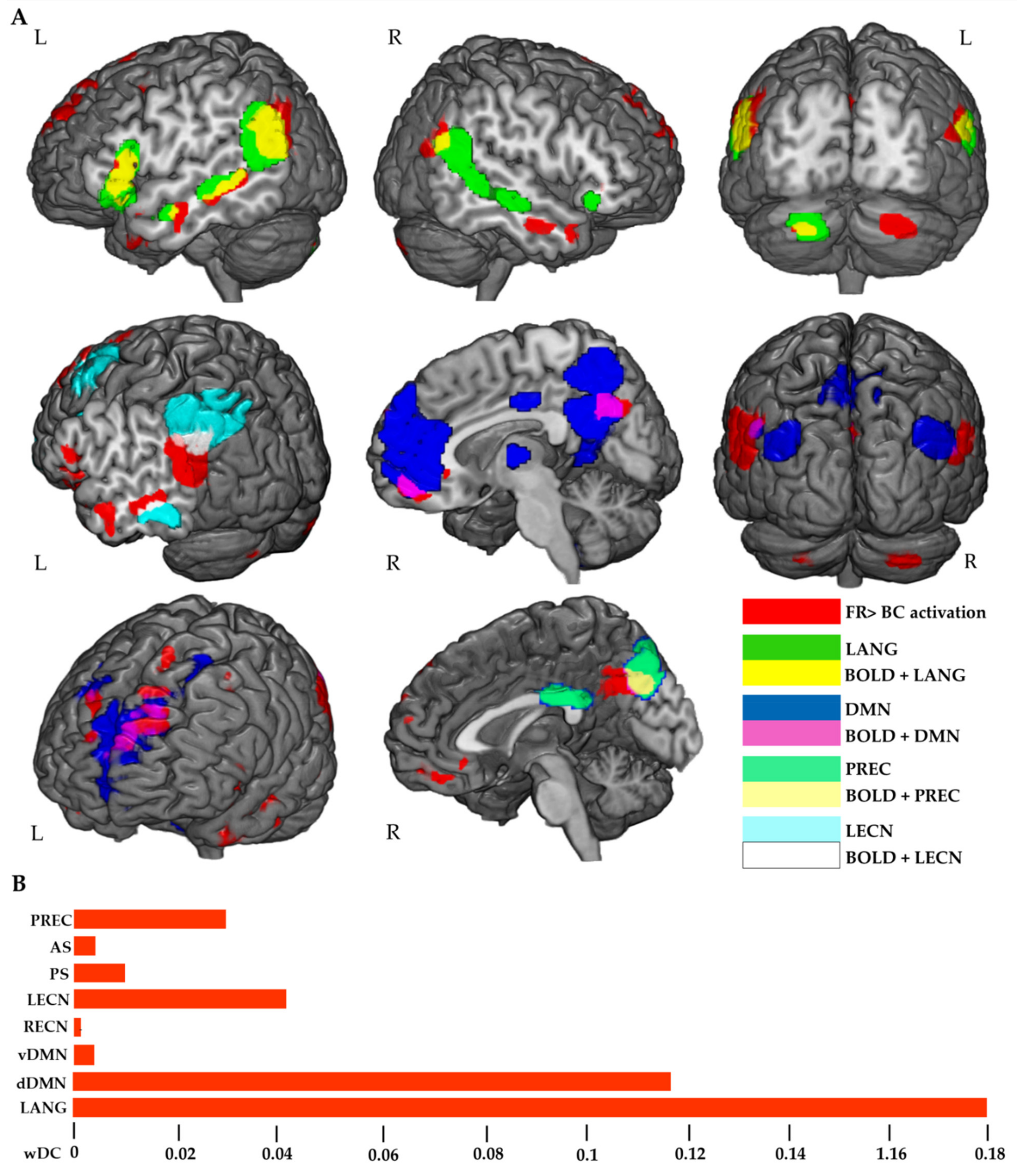
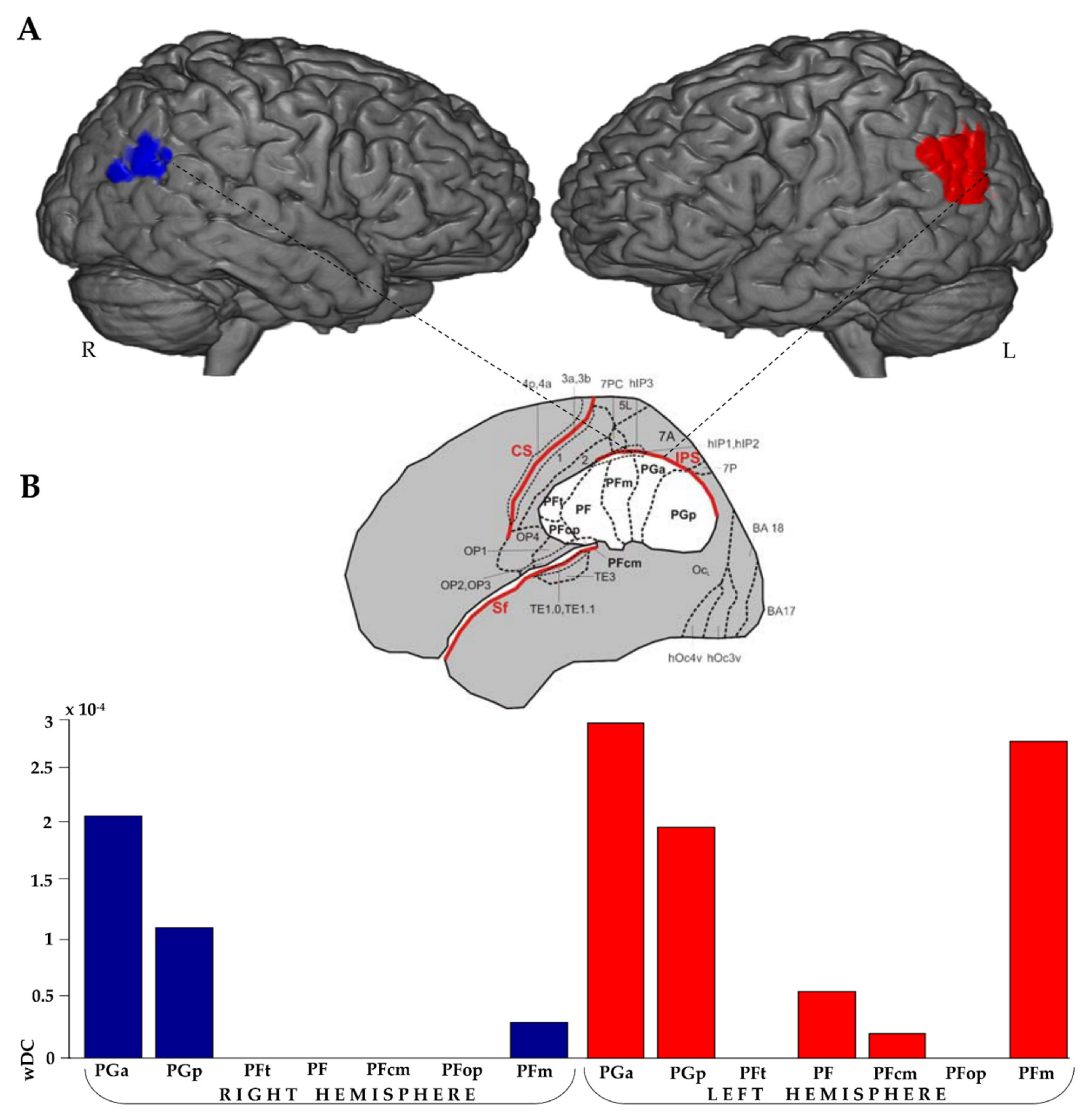

| Activation Loci (FR > BC Contrast) | Cluester Size (Voxels) | t | Coordinates (MNI) | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Left Parietal-Occipital cortex | 1386 | ||||
| Angular Gyrus | 9.70 | −52 | −58 | 22 | |
| Lateral Occipital Cortex-Sup. Division | 9.19 | −46 | −62 | 26 | |
| Left Parietal-Cingulate Cortex | 957 | ||||
| Precuneus | 8.41 | −2 | −68 | 34 | |
| Posterior Cingulate Cortex | 8.35 | −10 | −50 | 38 | |
| Left Frontal Cortex | 518 | ||||
| Frontal Pole | 9.71 | −18 | 50 | 44 | |
| Left Temporal Cortex | 492 | ||||
| Planum Temporale | 8.09 | −50 | −42 | 0 | |
| Middle Temporal Gyrus-Posterior | 7.38 | −62 | −40 | −2 | |
| Right Parietal Cortex | 477 | ||||
| Angular Gyrus | 9.37 | 62 | −58 | 24 | |
| Left Frontal Cortex | 473 | ||||
| Frontorbital Cortex | 8.20 | −46 | 30 | −8 | |
| Inferior Frontal Gyrus-Pars Triangularis | 7.61 | −52 | 20 | 8 | |
| Left & Right Frontal Cortex | 305 | ||||
| Frontal Pole | 7.03 | 10 | 44 | −22 | |
| Frontal Medial Cortex | 6.91 | 8 | 54 | −12 | |
| Right Cerebellum | 278 | ||||
| Crus II | 8.04 | 24 | −82 | −38 | |
| Right Temporal Cortex | 210 | ||||
| Middle Temporal Gyrus-Ant. Division | 9.07 | 54 | 4 | −26 | |
| Middle Temporal Gyrus-Post. Division | 5.04 | 58 | −8 | −18 | |
| Left Temporal Cortex | 202 | ||||
| Superior Temporal Gyrus-Posterior | 6.94 | −60 | −2 | −22 | |
| Medial Temporal Gyrus-Ant. | 6.93 | −54 | −8 | −22 | |
| Right Frontal Cortex | 139 | ||||
| Frontal Pole | 8.55 | 18 | 48 | 48 | |
| Left Frontal Cortex | 139 | ||||
| Superior Frontal Gyrus | 7.26 | −6 | 16 | 64 | |
| Left Temporal Cortex | 127 | ||||
| Temporal Pole | 7.42 | −44 | 18 | −34 | |
| Left Frontal Cortex | 109 | ||||
| Frontal Pole | 6.87 | −12 | 44 | 52 | |
| Left Frontal Cortex | 107 | ||||
| Middle Frontal Gyrus | 6.67 | −38 | 10 | 46 | |
| Left Cerebellum | 80 | ||||
| Crus II | 7.16 | −24 | −82 | −38 | |
| Right Frontal Cortex | 72 | ||||
| Frontal Pole | 6.14 | 50 | 36 | −4 | |
| Inferior Frontal Gyrus-Pars Triangularis | 5.78 | 60 | 30 | 6 | |
| Right Temporal Cortex | 71 | ||||
| Temporal Pole | 8.61 | 48 | 18 | −32 | |
| Right Frontal Cortex | 63 | ||||
| Frontal Pole | 6.02 | 12 | 62 | 26 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neri, F.; Cappa, S.F.; Mencarelli, L.; Momi, D.; Santarnecchi, E.; Rossi, S. Brain Functional Correlates of Episodic Memory Using an Ecological Free Recall Task. Brain Sci. 2021, 11, 911. https://doi.org/10.3390/brainsci11070911
Neri F, Cappa SF, Mencarelli L, Momi D, Santarnecchi E, Rossi S. Brain Functional Correlates of Episodic Memory Using an Ecological Free Recall Task. Brain Sciences. 2021; 11(7):911. https://doi.org/10.3390/brainsci11070911
Chicago/Turabian StyleNeri, Francesco, Stefano F. Cappa, Lucia Mencarelli, Davide Momi, Emiliano Santarnecchi, and Simone Rossi. 2021. "Brain Functional Correlates of Episodic Memory Using an Ecological Free Recall Task" Brain Sciences 11, no. 7: 911. https://doi.org/10.3390/brainsci11070911
APA StyleNeri, F., Cappa, S. F., Mencarelli, L., Momi, D., Santarnecchi, E., & Rossi, S. (2021). Brain Functional Correlates of Episodic Memory Using an Ecological Free Recall Task. Brain Sciences, 11(7), 911. https://doi.org/10.3390/brainsci11070911








