Probable Pain on the Pain Assessment in Impaired Cognition (PAIC15) Instrument: Assessing Sensitivity and Specificity of Cut-Offs against Three Standards
Abstract
:1. Introduction
2. Materials and Methods
2.1. PAIC15
2.2. Data Collection
2.2.1. Data Source
2.2.2. Reporting and Ethics
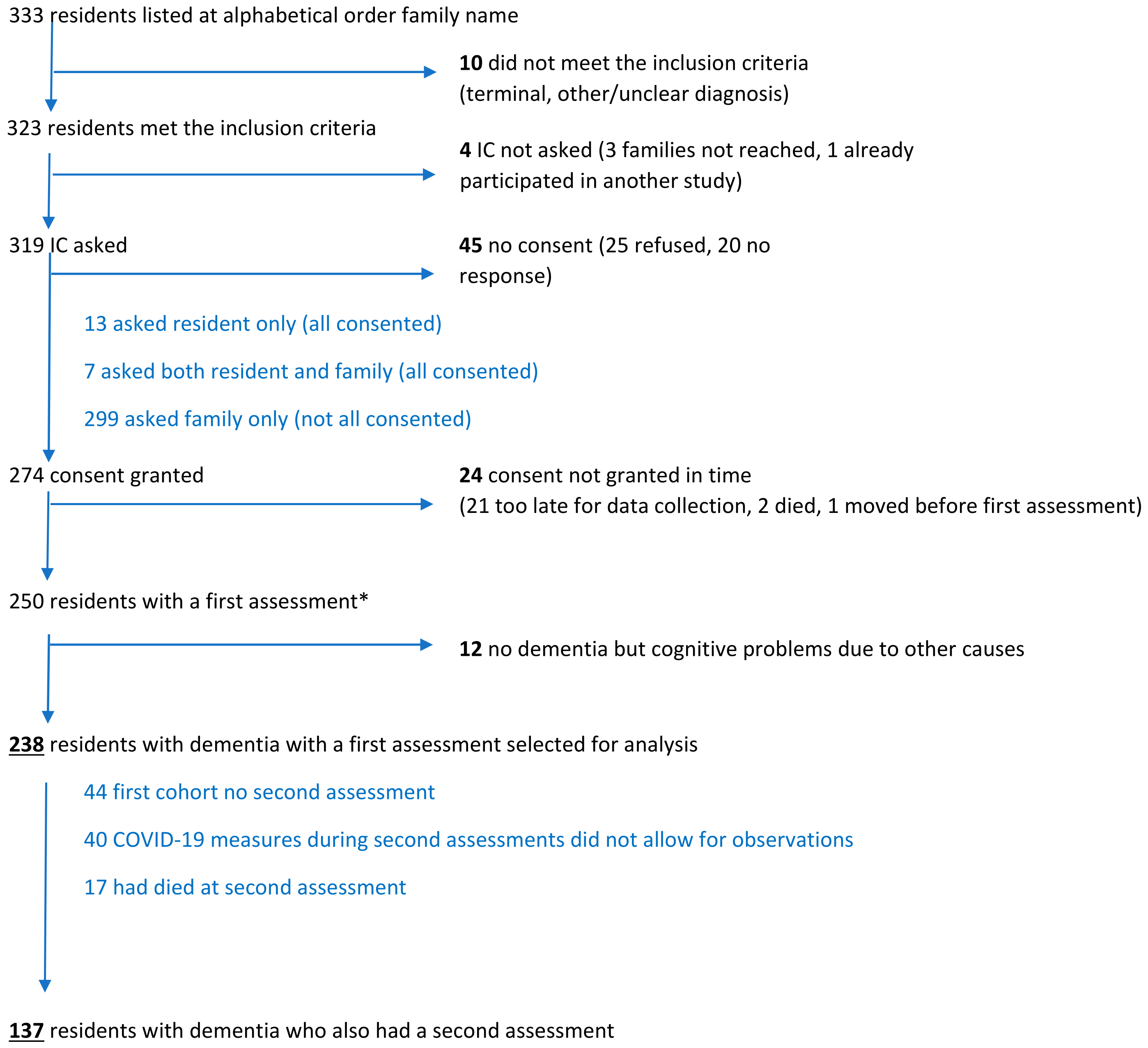
2.2.3. Subjects
2.2.4. Assessments
2.2.5. Observations and Assessment Order
2.2.6. Data Processing
2.3. Measures and Pain Standards
2.4. Analyses and Cut-Off Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Kooten, J.; Binnekade, T.T.; van der Wouden, J.C.; Stek, M.L.; Scherder, E.J.; Husebø, B.S.; Smalbrugge, M.; Hertogh, C.M. A review of pain prevalence in Alzheimer’s, vascular, frontotemporal and Lewy Body dementias. Dement. Geriatr. Cogn. Disord. 2016, 41, 220–232. [Google Scholar] [CrossRef] [PubMed]
- De Knegt, N.C.; Pieper, M.J.; Lobbezoo, F.; Schuengel, C.; Evenhuis, H.M.; Passchier, J.; Scherder, E.J. Behavioral pain indicators in people with intellectual disabilities: A systematic review. J. Pain 2013, 14, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Dillane, I.; Doody, O. Nursing people with intellectual disability and dementia experiencing pain: An integrative review. J. Clin. Nurs. 2019, 28, 2472–2485. [Google Scholar] [CrossRef]
- Herr, K.; Coyne, P.J.; Ely, E.; Gélinas, C.; Manworren, R.C.B. Pain assessment in the patient unable to self-report: Clinical practice recommendations in support of the ASPMN 2019 position statement. Pain Manag. Nurs. 2019, 20, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Hadjistavropoulos, T.; Herr, K.; Prkachin, K.M.; Craig, K.D.; Gibson, S.J.; Lukas, A.; Smith, J.H. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014, 13, 1216–1227. [Google Scholar] [CrossRef]
- Binnekade, T.T.; van Kooten, J.; Lobbezoo, F.; Rhebergen, D.; van der Wouden, J.C.; Smalbrugge, M.; Scherder, E.J.A. Pain experience in dementia subtypes: A systematic review. Curr. Alzheimer Res. 2017, 14, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Pieper, M.J.; van Dalen-Kok, A.H.; Francke, A.L.; van der Steen, J.T.; Scherder, E.J.; Husebø, B.S.; Achterberg, W.P. Interventions targeting pain or behaviour in dementia: A systematic review. Ageing Res. Rev. 2013, 12, 1042–1055. [Google Scholar] [CrossRef] [Green Version]
- Strand, L.I.; Gundrosen, K.F.; Lein, R.K.; Laekeman, M.; Lobbezoo, F.; Defrin, R.; Husebo, B.S. Body movements as pain indicators in older people with cognitive impairment: A systematic review. Eur. J. Pain 2019, 23, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, S.A.; Smalbrugge, M.; Galindo-Garre, F.; Hertogh, C.M.; van der Steen, J.T. From admission to death: Prevalence and course of pain, agitation, and shortness of breath, and treatment of these symptoms in nursing home residents with dementia. J. Am. Med. Dir. Assoc. 2015, 16, 475–481. [Google Scholar] [CrossRef]
- Sampson, E.L.; Candy, B.; Davis, S.; Gola, A.B.; Harrington, J.; King, M.; Kupeli, N.; Leavey, G.; Moore, K.; Nazareth, I.; et al. Living and dying with advanced dementia: A prospective cohort study of symptoms, service use and care at the end of life. Palliat. Med. 2018, 32, 668–681. [Google Scholar] [CrossRef] [Green Version]
- Lichtner, V.; Dowding, D.; Esterhuizen, P.; Closs, S.J.; Long, A.F.; Corbett, A.; Briggs, M. Pain assessment for people with dementia: A systematic review of systematic reviews of pain assessment tools. BMC Geriatr. 2014, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Flo, E.; Gulla, C.; Husebo, B.S. Effective pain management in patients with dementia: Benefits beyond pain? Drugs Aging 2014, 31, 863–871. [Google Scholar] [CrossRef]
- Ersek, M.; Herr, K.; Hilgeman, M.M.; Neradilek, M.B.; Polissar, N.; Cook, K.F.; Nash, P.; Snow, A.L.; McDarby, M.; Nelson, F.X. Developing a pain intensity measure for persons with dementia: Initial construction and testing. Pain Med. 2019, 20, 1078–1092. [Google Scholar] [CrossRef]
- Richey, S.A.; Capezuti, E.; Cron, S.G.; Reed, D.; Torres-Vigil, I.; de Oliveira Otto, M.C. Development and testing of the Pain Assessment Tool in Cognitively Impaired Elders (PATCIE): Nonverbal pain behaviors in African American and Caucasian nursing home residents with dementia. Pain Manag. Nurs. 2020, 21, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Van der Steen, J.T.; Sampson, E.L.; Van den Block, L.; Lord, K.; Vankova, H.; Pautex, S.; Vandervoort, A.; Radbruch, L.; Shvartzman, P.; Sacchi, V.; et al. Tools to assess pain or lack of comfort in dementia: A content analysis. J. Pain Symptom Manag. 2015, 50, 659–675.e3. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.; Achterberg, W.; Husebo, B.; Lobbezoo, F.; de Vet, H.; Kunz, M.; Strand, L.; Constantinou, M.; Tudose, C.; Kappesser, J.; et al. An international road map to improve pain assessment in people with impaired cognition: The development of the Pain Assessment in Impaired Cognition (PAIC) meta-tool. BMC Neurol. 2014, 14, 229. [Google Scholar] [CrossRef] [Green Version]
- Kunz, M.; de Waal, M.W.M.; Achterberg, W.P.; Gimenez-Llort, L.; Lobbezoo, F.; Sampson, E.L.; van Dalen-Kok, A.H.; Defrin, R.; Invitto, S.; Konstantinovic, L.; et al. The Pain Assessment in Impaired Cognition scale (PAIC15): A multidisciplinary and international approach to develop and test a meta-tool for pain assessment in impaired cognition, especially dementia. Eur. J. Pain 2020, 24, 192–208. [Google Scholar] [CrossRef] [Green Version]
- De Waal, M.W.M.; van Dalen-Kok, A.H.; de Vet, H.C.W.; Gimenez-Llort, L.; Konstantinovic, L.; de Tommaso, M.; Fischer, T.; Lukas, A.; Kunz, M.; Lautenbacher, S.; et al. Observational pain assessment in older persons with dementia in four countries: Observer agreement of items and factor structure of the Pain Assessment in Impaired Cognition. Eur. J. Pain 2020, 24, 279–296. [Google Scholar] [CrossRef]
- Knopp-Sihota, J.A.; Dirk, K.L.; Rachor, G.S. Factors associated with pain assessment for nursing home residents: A systematic review and meta-synthesis. J. Am. Med. Dir. Assoc. 2019, 20, 884–892.e3. [Google Scholar] [CrossRef]
- De Witt Jansen, B.; Brazil, K.; Passmore, P.; Buchanan, H.; Maxwell, D.; McIlfatrick, S.J.; Morgan, S.M.; Watson, M.; Parsons, C. Exploring healthcare assistants’ role and experience in pain assessment and management for people with advanced dementia towards the end of life: A qualitative study. BMC Palliat. Care 2017, 16, 6. [Google Scholar] [CrossRef] [Green Version]
- De Witt Jansen, B.; Brazil, K.; Passmore, P.; Buchanan, H.; Maxwell, D.; McIlfatrick, S.; Morgan, S.M.; Watson, M.; Parsons, C. “A tool doesn’t add anything”. The importance of added value: Use of observational pain tools with patients with advanced dementia approaching the end of life-a qualitative study of physician and nurse experiences and perspectives. Int. J. Geriatr. Psychiatry 2018, 33, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Zwakhalen, S.M.; Hamers, J.P.; Berger, M.P. The psychometric quality and clinical usefulness of three pain assessment tools for elderly people with dementia. Pain 2006, 126, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Warden, V.; Hurley, A.C.; Volicer, L. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J. Am. Med. Dir. Assoc. 2003, 4, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zwakhalen, S.M.; van der Steen, J.T.; Najim, M.D. Which score most likely represents pain on the observational PAINAD pain scale for patients with dementia? J. Am. Med. Dir. Assoc. 2012, 13, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Van der Maaden, T.; van der Steen, J.T.; Koopmans, R.T.C.M.; Doncker, S.M.M.M.; Anema, J.R.; Hertogh, C.M.P.M.; de Vet, H.C.W. Symptom relief in patients with pneumonia and dementia: Implementation of a practice guideline. Int. J. Geriatr. Psychiatry 2017, 32, 829–839. [Google Scholar] [CrossRef]
- AGS. The management of persistent pain in older persons. J. Am. Geriatr. Soc. 2002, 50, S205–S224. [Google Scholar] [CrossRef] [Green Version]
- van der Steen, J.T.; Klapwijk, M.S.; Achterberg, W.P. Palliative care and impact of the COVID-19 pandemic on nursing home residents with dementia. Riv. Ital. Cure Palliat. 2020, 22, 129–139. Available online: https://www.ricp.it/archivio/3439/ (accessed on 15 May 2021). (In English) [CrossRef]
- Tong, W.H.; Ommering, B.W.C.; van der Steen, J.T. Perception and motivation of residents in elderly care medicine concerning evidence-based medicine education. Eur. Ger. Med. 2020, 11. [Google Scholar] [CrossRef]
- Koopmans, R.T.; Lavrijsen, J.C.; Hoek, F. Concrete steps toward academic medicine in long term care. J. Am. Med. Dir. Assoc. 2013, 14, 781–783. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. Available online: https://www.equator-network.org/reporting-guidelines/stard/ (accessed on 15 May 2021). [CrossRef] [Green Version]
- Gruneir, A.; Lapane, K.L.; Miller, S.C.; Mor, V. Does the presence of a Dementia Special Care Unit improve nursing home quality? J. Aging Health 2008, 20, 837–854. [Google Scholar] [CrossRef] [Green Version]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef] [Green Version]
- Hurley, A.C.; Volicer, B.J.; Hanrahan, P.A.; Houde, S.; Volicer, L. Assessment of discomfort in advanced Alzheimer patients. Res. Nurs. Health 1992, 15, 369–377. [Google Scholar] [CrossRef]
- Van der Steen, J.T.; Pasman, H.R.; Ribbe, M.W.; van der Wal, G.; Onwuteaka-Philipsen, B.D. Discomfort in dementia patients dying from pneumonia and its relief by antibiotics. Scand. J. Infect. Dis. 2009, 41, 143–151. [Google Scholar] [CrossRef]
- Volicer, L.; Hurley, A.C.; Lathi, D.C.; Kowall, N.W. Measurement of severity in advanced Alzheimer’s disease. J. Gerontol. 1994, 49, M223–M226. [Google Scholar] [CrossRef] [PubMed]
- Van der Steen, J.T.; Volicer, L.; Gerritsen, D.L.; Kruse, R.L.; Ribbe, M.W.; Mehr, D.R. Defining severe dementia with the Minimum Data Set. Int. J. Geriatr. Psychiatry 2006, 21, 1099–1106. [Google Scholar] [CrossRef]
- Groll, D.L.; To, T.; Bombardier, C.; Wright, J.G. The development of a comorbidity index with physical function as the outcome. J. Clin. Epidemiol. 2005, 58, 595–602. [Google Scholar] [CrossRef]
- Kabboord, A.D.; Godfrey, D.; Gordon, A.L.; Gladman, J.R.F.; van Eijk, M.; van Balen, R.; Achterberg, W.P. The modified functional comorbidity index performed better than the Charlson index and original functional comorbidity index in predicting functional outcome in geriatric rehabilitation: A prospective observational study. BMC Geriatr. 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Hadjistavropoulos, T.; Herr, K.; Turk, D.C.; Fine, P.G.; Dworkin, R.H.; Helme, R.; Jackson, K.; Parmelee, P.A.; Rudy, T.E.; Lynn Beattie, B.; et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin. J. Pain 2007, 23 (Suppl. 1), S1–S43. [Google Scholar] [CrossRef] [PubMed]
- Pautex, S.; Herrmann, F.; Le Lous, P.; Fabjan, M.; Michel, J.P.; Gold, G. Feasibility and reliability of four pain self-assessment scales and correlation with an observational rating scale in hospitalized elderly demented patients. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 524–529. [Google Scholar] [CrossRef]
- CMS Centers for Medicare & Medicaid Services. MDS 3.0 RAI Manual. 2019; pp. 7–8. Available online: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/MDS30RAIManual (accessed on 2 April 2021).
- Kwon, S.-H.; Cho, Y.-S.; Kim, H. Reliability and feasibility of the Pain Assessment in Advanced Dementia Scale-Korean version (PAINAD-K). Pain Manag. Nurs. 2021. [Google Scholar] [CrossRef]
- Herr, K.; Bursch, H.; Ersek, M.; Miller, L.L.; Swafford, K. Use of pain-behavioral assessment tools in the nursing home: Expert consensus recommendations for practice. J. Gerontol. Nurs. 2010, 36, 18–29. [Google Scholar] [CrossRef]
- Natavio, T.; McQuillen, E.; Dietrich, M.S.; Wells, N.; Rhoten, B.A.; Vallerand, A.H.; Monroe, T.B. A comparison of the Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC) and Pain Assessment in Advanced Dementia Scale (PAINAD). Pain Manag. Nurs. 2020, 21, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Hadjistavropoulos, T.; Fitzgerald, T.D.; Marchildon, G.P. Practice guidelines for assessing pain in older persons with dementia residing in long-term care facilities. Physiother. Can. 2010, 62, 104–113. [Google Scholar] [CrossRef]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; de Vet, H.C.W.; Terwee, C.B. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2000; Chapter 5; p. 162. [Google Scholar] [CrossRef]
- Van Soest-Poortvliet, M.C.; van der Steen, J.T.; Zimmerman, S.; Cohen, L.W.; Reed, D.; Achterberg, W.P.; Ribbe, M.W.; de Vet, H.C.W. Selecting the best instruments to measure quality of end-of-life care and quality of dying in long term care. J. Am. Med. Dir. Assoc. 2013, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pautex, S.; Michon, A.; Guedira, M.; Emond, H.; Le Lous, P.; Samaras, D.; Michel, J.P.; Herrmann, F.; Giannakopoulos, P.; Gold, G. Pain in severe dementia: Self-assessment or observational scales? J. Am. Geriatr. Soc. 2006, 54, 1040–1045. [Google Scholar] [CrossRef]
- Lukas, A.; Niederecker, T.; Günther, I.; Mayer, B.; Nikolaus, T. Self- and proxy report for the assessment of pain in patients with and without cognitive impairment: Experiences gained in a geriatric hospital. Z. Gerontol. Geriatr. 2013, 46, 214–221. [Google Scholar] [CrossRef]
- Bullock, L.; Bedson, J.; Jordan, J.L.; Bartlam, B.; Chew-Graham, C.A.; Campbell, P. Pain assessment and pain treatment for community-dwelling people with dementia: A systematic review and narrative synthesis. Int. J. Geriatr. Psychiatry 2019, 34, 807–821. [Google Scholar] [CrossRef]
- Lukas, A.; Hagg-Grün, U.; Mayer, B.; Fischer, T.; Schuler, M. Pain assessment in advanced dementia. Validity of the German PAINAD-a prospective double-blind randomised placebo-controlled trial. Pain 2019, 160, 742–753. [Google Scholar] [CrossRef]
- Husebo, B.S.; Achterberg, W.; Flo, E. identifying and managing pain in people with Alzheimer’s disease and other types of dementia: A systematic review. CNS Drugs 2016, 30, 481–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosele, M.; Inelmen, E.M.; Toffanello, E.D.; Girardi, A.; Coin, A.; Sergi, G.; Manzato, E. Psychometric properties of the pain assessment in advanced dementia scale compared to self assessment of pain in elderly patients. Dement. Geriatr. Cogn. Disord. 2012, 34, 38–43. [Google Scholar] [CrossRef] [PubMed]
| Assessment 1 (n = 238) | Assessment 2 (n = 137) | |
|---|---|---|
| Women, % (n) | 63.4% (151) | 67.9% (93) |
| Age, mean (SD) | 85.7 (7.5) | 86.3 (7.8) |
| Residence, type of ward, % (n) psychogeriatric unit (almost all dementia) unit for mostly physical disability or combined physical and cognitive impairment | 96.2 (229) 3.8 (9) | 94.9 (130) 5.1 (7) |
| Type of dementia (more possible), % (n) | ||
| Alzheimer’s | 68.5 (163) | 67.9 (93) |
| vascular | 35.3 (84) | 32.1 (44) |
| Lewy body or Parkinson | 5.0 (12) | 4.4 (6) |
| other types/mixtures and unknown | 10.9 (26) | 11.7 (16) |
| Stage of dementia | ||
| BANS-S, mean (SD) | 14.4 (4.5) | 14.4 (4.9) |
| BANS-S 17 and higher, % (n) | 33.6% (80) | 33.6% (46) |
| GDS, mean (SD) | 5.7 (0.86) | 5.8 (0.84) 1 |
| GDS 7, % (n) | 16.0%(38) | 19.1% (26) 1 |
| ADL dependency, % (n) | ||
| full dressing dependency | 42.0 (100) | 43.8 (60) |
| full mobility dependency | 25.2 (60) | 26.3 (36) |
| full eating dependency | 6.3 (15) | 7.3 (10) |
| dependency 0–6 scale, mean (SD) | 2.7 (1.7) | 2.7 (1.8) |
| Acute disease at time of assessment, % (n) | 9.7% (23) | 11.7% (16) |
| Weighted Functional Comorbidity Index, mean (SD) | 8.5 (4.0) | 8.8 (4.2) |
| Co-morbidity potentially related to pain, % (n) | ||
| degenerative disc disease (e.g., back disease, spinal stenosis, or severe chronic back pain) | 31.5 (75) | 29.9 (41) |
| arthritis | 25.2 (60) | 27.8 (38) |
| cerebrovascular accident (CVA) | 24.4 (58) | 23.4 (32) |
| diabetes mellitus type I or II | 22.3 (53) | 28.5 (39) |
| depression | 18.9 (45) | 23.4 (32) |
| peripheral vascular disease | 12.2 (29) | 13.1 (18) |
| angina pectoris | 11.3 (27) | 10.9 (15) |
| DS-DAT (discomfort score mean (SD)/median (IQR)) | 5.6 (5.2)/4.5 (1–9) 1 | 5.3 (5.0)/4 (1–8) |
| Pain Assessment with Theoretical Range | Assessment 1 | Assessment 2 |
|---|---|---|
| PAIC15 (0–45) | 4.6 (5.2)/3 (1–6) (238) | 4.0 (5.2)/2.1 (0–5.5) (137) |
| Self-report intensity (0–10) | 0.75 (1.94)/0 (0–0) (177) | 0.46 (1.47)/0 (0-0) (99) |
| PAINAD (0–10) | 1.1 (1.6)/0 (0–2) (238) | 1.1 (1.6)/0 (0–2) (137) |
| Observer’s overall estimate (0–10) | 0.81 (1.70)/0 (0–0) (237) | 0.69 (1.56)/0 (0–0) (136) |
| Score 1 | Assessment 1 (n = 238) | Assessment 2 (n = 137) |
|---|---|---|
| 0 | 16% (39) | 28% (38) |
| 1 | 12% (29) | 12% (16) |
| 2 | 13% (32) | 14% (19) |
| 3 | 13% (30) | 7% (10) |
| 4 | 10% (24) | 12% (16) |
| 5 | 6% (15) | 3% (4) |
| 6 | 6% (14) | 4% (5) |
| 7 | 1% (3) | 3% (4) |
| 8 | 5% (13) | 3% (4) |
| 9 | 3% (7) | 5% (7) |
| 10 to 20 | 11% (25) | 9% (12) |
| 20 and up | 3% (7) (max score 34) | 1% (2) (max score 33) |
| Measure (Scale or Assessment) | Score 1 | Assessment 1 (n = 238) | Assessment 2 (n = 137) |
|---|---|---|---|
| Self-report, if able | any pain (standard A) | 23.9% (n = 47/197) | 19.1% (n = 21/110) |
| Self-report, intensity | 0 no pain | (150) | (89) |
| pain, but no score | (20) | (11) | |
| 1 mild | (0) | (0) | |
| 2 mild | (2) | (0) | |
| 3 mild | (8) | (3) | |
| 4 mild | (3) | (2) | |
| 5 moderate to severe | (5) | (3) | |
| 6 moderate to severe | (1) | (1) | |
| 7 moderate to severe | (5) | (0) | |
| 8 moderate to severe | (2) | (1) | |
| 9 moderate to severe | (0) | (0) | |
| 10 very severe/horrible pain | (1) | (0) | |
| PAINAD | 0 | 51% (121) | 52% (71) |
| 1 | 21% (50) | 21% (29) | |
| 2 and up (standard B) | 28.2% (67) (max score 8) | 27.0% (37) (max score 8) | |
| Observer’s overall estimate | any pain (standard C) | 23.2% (55; 1 missing) | 19.7% (27/137) 2 |
| Observer’s overall estimate, intensity | 0 | (182) | (110) 2 |
| 1 | (2) | (2) | |
| 2 | (17) | (4) | |
| 3 | (15) | (5) | |
| 4 | (10) | (10) | |
| 5 | (5) | (2) | |
| 6 | (2) | (2) | |
| 7 | (1) | (1) | |
| 8 | (2) | (0) | |
| 9 | (0) | (0) | |
| 10 | (1) | (0) |
| Pain | Discomfort | ||||
|---|---|---|---|---|---|
| PAIC15 | Self-Report (Standard 1) | PAINAD (Standard 2) | Observer’s Overall Estimate (Standard 3) | DS-DAT | |
| PAIC with other instruments: assessment 1 | 0.09 | 0.72 * | 0.28 * | 0.69 * | |
| PAIC with other instruments: assessment 2 | 0.06 | 0.72 * | 0.41 * | 0.63 * | |
| Same instrument: assessment 1 withassessment 2 | 0.57 * | 0.47 * | 0.55 * | 0.32 * | 0.52 * |
| Standard | Assessment 1 Area (CI) | n | Assessment 2 Area (CI) | n |
|---|---|---|---|---|
| Against self-report | 0.58 (0.49–0.67) | 197 | 0.69 (0.56–0.81) | 110 |
| Against PAINAD cut-off 2 | 0.87 (0.82–0.92) | 238 | 0.88 (0.83–0.94) | 137 |
| Against observer’s overall estimate | 0.69 (0.61–0.76) | 237 | 0.78 (0.68–0.89) | 137 |
| Assessment 1 | Assessment 2 | ||||
|---|---|---|---|---|---|
| PAIC15 Score 1 | Self-Report 2 | PAIC15 Score 1 | Self-Report 2 | ||
| Sensitivity | Specificity | Sensitivity | Specificity | ||
| 0.5 | 0.896 | 0.188 | 0.5 | 0.857 | 0.326 |
| 1.0 | 0.813 | 0.309 | 1.0 | 0.810 | 0.416 |
| 1.5 | 0.771 | 0.315 | 1.5 | 0.810 | 0.438 |
| 2.1 | 0.688 | 0.456 | 2.1 | 0.714 | 0.551 |
| 2.2 | 0.688 | 0.470 | 2.6 | 0.714 | 0.607 |
| 2.7 | 0.688 | 0.490 | 3.1 | 0.619 | 0.674 |
| 3.1 | 0.521 | 0.577 | 3.6 | 0.619 | 0.685 |
| 3.3 | 0.521 | 0.611 | 4.1 | 0.476 | 0.787 |
| 3.7 | 0.521 | 0.617 | 4.6 | 0.429 | 0.809 |
| 4.1 | 0.375 | 0.678 | 5.5 | 0.381 | 0.843 |
| 4.5 | 0.375 | 0.698 | 6.5 | 0.333 | 0.865 |
| 4.8 | 0.375 | 0.705 | 7.3 | 0.286 | 0.888 |
| 5.2 | 0.354 | 0.765 | 7.8 | 0.286 | 0.899 |
| 5.6 | 0.313 | 0.765 | 8.5 | 0.238 | 0.910 |
| 5.9 | 0.313 | 0.772 | 9.3 | 0.143 | 0.955 |
| 6.2 | 0.188 | 0.792 | 10.8 | 0.143 | 0.966 |
| 6.7 | 0.167 | 0.799 | |||
| 7.3 | 0.167 | 0.819 | |||
| 7.8 | 0.167 | 0.826 | |||
| 8.0 | 0.146 | 0.879 | |||
| 8.5 | 0.146 | 0.886 | |||
| 9.1 | 0.146 | 0.906 | |||
| 9.6 | 0.125 | 0.906 | |||
| 10.4 | 0.125 | 0.933 | |||
| a. Against self-report | |||||
| Assessment 1 | Reported no pain | Reported pain | Assessment 2 | Reported no pain | Reported pain |
| PAIC15 < 3 | 73 | 15 | PAIC15 < 3 | 54 | 6 |
| PAIC15 ≥ 3 | 76 | 33 | PAIC15 ≥ 3 | 35 | 15 |
| PAIC15 < 4 | 92 | 23 | PAIC15 < 4 | 61 | 8 |
| PAIC15 ≥ 4 | 57 | 25 | PAIC15 ≥ 4 | 28 | 13 |
| b. Against PAINAD | |||||
| Assessment 1 | No pain observed | Pain observed | Assessment 2 | No pain observed | Pain observed |
| PAIC15 < 3 | 94 | 6 | PAIC15 < 3 | 72 | 1 |
| PAIC15 ≥ 3 | 77 | 61 | PAIC15 ≥ 3 | 28 | 36 |
| PAIC15 < 4 | 121 | 9 | PAIC15 < 4 | 77 | 6 |
| PAIC15 ≥ 4 | 50 | 58 | PAIC15 ≥ 4 | 23 | 31 |
| c. Against observer’s estimate | |||||
| Assessment 1 | No pain observed | Pain observed | Assessment 2 | No pain observed | Pain observed |
| PAIC15 < 3 | 91 | 8 | PAIC15 < 3 | 67 | 6 |
| PAIC15 ≥ 3 | 91 | 47 | PAIC15 ≥ 3 | 43 | 21 |
| PAIC15 < 4 | 111 | 18 | PAIC15 < 4 | 77 | 6 |
| PAIC15 ≥ 4 | 71 | 37 | PAIC15 ≥ 4 | 33 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Steen, J.T.; Westzaan, A.; Hanemaayer, K.; Muhamad, M.; de Waal, M.W.M.; Achterberg, W.P. Probable Pain on the Pain Assessment in Impaired Cognition (PAIC15) Instrument: Assessing Sensitivity and Specificity of Cut-Offs against Three Standards. Brain Sci. 2021, 11, 869. https://doi.org/10.3390/brainsci11070869
van der Steen JT, Westzaan A, Hanemaayer K, Muhamad M, de Waal MWM, Achterberg WP. Probable Pain on the Pain Assessment in Impaired Cognition (PAIC15) Instrument: Assessing Sensitivity and Specificity of Cut-Offs against Three Standards. Brain Sciences. 2021; 11(7):869. https://doi.org/10.3390/brainsci11070869
Chicago/Turabian Stylevan der Steen, Jenny T., Andrew Westzaan, Kimberley Hanemaayer, Muhamad Muhamad, Margot W. M. de Waal, and Wilco P. Achterberg. 2021. "Probable Pain on the Pain Assessment in Impaired Cognition (PAIC15) Instrument: Assessing Sensitivity and Specificity of Cut-Offs against Three Standards" Brain Sciences 11, no. 7: 869. https://doi.org/10.3390/brainsci11070869
APA Stylevan der Steen, J. T., Westzaan, A., Hanemaayer, K., Muhamad, M., de Waal, M. W. M., & Achterberg, W. P. (2021). Probable Pain on the Pain Assessment in Impaired Cognition (PAIC15) Instrument: Assessing Sensitivity and Specificity of Cut-Offs against Three Standards. Brain Sciences, 11(7), 869. https://doi.org/10.3390/brainsci11070869







