Management Challenges of Severe, Complex Dyskinesia. Data from a Large Cohort of Patients Treated with Levodopa-Carbidopa Intestinal Gel for Advanced Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
- Does the expected benefit of improving motor fluctuations compensate for the potential worsening of dyskinesias?
- In patients with pre-existing troublesome dyskinesias (especially if they have DID), as well as those with low compliance and/or high expectations, how long should one try to follow the 16-h LCIG regimen before starting, with all the necessary precautions, the 24-h administration?
- How do we deal with add-on medication, especially amantadine (discontinuation, maintenance and starting the drug if it was not used previously)?
- Do DIDs that occur during treatment with LCIG have the same pattern as those that occurred during treatment with oral LD?
- How do we interpret the change of the LID profile observed in clinical practice during the LCIG treatment, and does this change the imposed additional measures?
- What is the importance of individual susceptibility, and does it impose additional measures?
- In cases of patients that are eligible for both LCIG and DBS (patients with APD under 70 years, with moderate or severe motor fluctuations and dyskinesias or without depression or dementia), if a patient opted for LCIG, considering the treatment as “less invasive” compared to DBS, what is the degree and type of dyskinesia that should suggest (if available) a switch to DBS?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, J.J.; Katzenschlager, R.; Bloem, B.R.; Bonuccelli, U.; Burn, D.; Deuschl, G.; Dietrichs, E.; Fabbrini, G.; Friedman, A.; Kanovsky, P.; et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur. J. Neurol. 2013, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Pirtošek, Z.; Bajenaru, O.; Kovács, N.; Milanov, I.; Relja, M.; Skorvanek, M. Update on the Management of Parkinson’s Disease for General Neurologists. Parkinson’s Dis. 2020, 2020, 9131474. [Google Scholar] [CrossRef] [PubMed]
- Odin, P.; Ray Chaudhuri, K.; Slevin, J.T.; Volkmann, J.; Dietrichs, E.; Martinez-Martin, P.; Krauss, J.K.; Henriksen, T.; Katzenschlager, R.; Antonini, A.; et al. Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: Consensus from an international survey and discussion program. Park. Relat. Disord. 2015, 21, 1133–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olanow, C.W.; Kieburtz, K.; Odin, P.; Espay, A.J.; Standaert, D.G.; Fernandez, H.H.; Vanagunas, A.; Othman, A.A.; Widnell, K.L.; Robieson, W.Z.; et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol. 2014, 13, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Antonini, A.; Poewe, W.; Chaudhuri, K.R.; Jech, R.; Pickut, B.; Pirtošek, Z.; Szasz, J.; Valldeoriola, F.; Winkler, C.; Bergmann, L.; et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: Final results of the GLORIA registry. Park. Relat. Disord. 2017, 45, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Juhász, A.; Aschermann, Z.; Ács, P.; Janszky, J.; Kovács, M.; Makkos, A.; Harmat, M.; Tényi, D.; Karádi, K.; Komoly, S.; et al. Levodopa/carbidopa intestinal gel can improve both motor and non-motor experiences of daily living in Parkinson’s disease: An open-label study. Parkinsonism Relat. Disord. 2017, 37, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Băjenaru, O.; Ene, A.; Popescu, B.O.; Szász, J.A.; Sabău, M.; Mureşan, D.F.; Perju-Dumbrava, L.; Popescu, C.D.; Constantinescu, A.; Buraga, I.; et al. The effect of levodopa-carbidopa intestinal gel infusion long-term therapy on motor complications in advanced Parkinson’s disease: A multicenter Romanian experience. J. Neural Transm. 2016, 123, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Timpka, J.; Fox, T.; Fox, K.; Honig, H.; Odin, P.; Martinez-Martin, P.; Antonini, A.; Ray Chaudhuri, K. Improvement of dyskinesias with l-dopa infusion in advanced Parkinson’s disease. Acta Neurol. Scand. 2016, 133, 451–458. [Google Scholar] [CrossRef]
- Antonini, A.; Fung, V.S.C.; Boyd, J.T.; Slevin, J.T.; Hall, C.; Chatamra, K.; Eaton, S.; Benesh, J.A. Effect of levodopa-carbidopa intestinal gel on dyskinesia in advanced Parkinson’s disease patients. Mov. Disord. 2016, 31, 530–537. [Google Scholar] [CrossRef]
- Lopiano, L.; Modugno, N.; Marano, P.; Sensi, M.; Meco, G.; Solla, P.; Gusmaroli, G.; Tamma, F.; Mancini, F.; Quatrale, R.; et al. Motor and non-motor outcomes in patients with advanced Parkinson’s disease treated with levodopa/carbidopa intestinal gel: Final results of the GREENFIELD observational study. J. Neurol. 2019, 266, 2164–2176. [Google Scholar] [CrossRef] [Green Version]
- Szász, J.A.; Constantin, V.A.; Orbán-Kis, K.; Rácz, A.; Bancu, L.A.; Georgescu, D.; Szederjesi, J.; Mihály, I.; Fárr, A.-M.; Kelemen, K.; et al. Profile of Patients with Advanced Parkinson’s disease Suitable for Device-Aided Therapies: Restrospective Data of a Large Cohort Of Romanian Patients. Neuropsychiatr. Dis. Treat. 2019, 15, 3187–3195. [Google Scholar] [CrossRef] [Green Version]
- Szász, J.A.; Szatmári, S.; Constantin, V.; Mihály, I.; Rácz, A.; Domokos, L.C.; Vajda, T.; Orbán-Kis, K. Characteristics of levodopa treatment in advanced Parkinson’s disease in the experiences of the neurology clinics of Târgu Mures, Romania. Orv. Hetil. 2019, 160, 662–669. [Google Scholar] [CrossRef]
- Szász, J.A.; Constantin, V.A.; Orbán-Kis, K.; Bancu, L.A.; Georgescu, D.; Szederjesi, J.; Rácz, A.; Mihály, I.; Szatmári, S. Characteristics of dopaminergic treatments in advanced Parkinson’s before levodopa-carbidopa intestinal gel infusion: Data from 107 tested patients. Mov. Disord. 2018, 33, 171. [Google Scholar]
- Szasz, J.; Constantin, V.; Orban-Kis, K.; Bancu, L.; Georgescu, D.; Szederjesi, J.; Racz, A.; Mihaly, I.; Szederjesi, J. Spectrum of motor complications in advanced Parkinson’s disease: Data from a large Romanian case series evaluated for suitability for device aided therapy. Mov. Disord. 2019, 34, 210. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Szasz, J.A.; Jianu, D.C.; Simu, M.A.; Constantin, V.A.; Dulamea, A.O.; Onuk, K.; Popescu, D.; Vasile, M.T.; Popescu, B.O.; Fasano, A.; et al. Characterizing Advanced Parkinson’s Disease: Romanian Subanalysis from the OBSERVE-PD Study. Parkinson’s Dis. 2021, 2021, 6635618. [Google Scholar] [CrossRef]
- Constantin, V.A.; Szász, J.A.; Orbán-Kis, K.; Rosca, E.C.; Popovici, M.; Cornea, A.; Bancu, L.A.; Ciorba, M.; Mihály, I.; Nagy, E.; et al. Levodopa-carbidopa intestinal gel infusion therapy discontinuation: A ten-year retrospective analysis of 204 treated patients. Neuropsychiatr. Dis. Treat. 2020, 16, 1835–1844. [Google Scholar] [CrossRef]
- Krüger, R.; Hilker, R.; Winkler, C.; Lorrain, M.; Hahne, M.; Redecker, C.; Lingor, P.; Jost, W.H. Advanced stages of PD: Interventional therapies and related patient-centered care. J. Neural Transm. 2016, 123, 31–43. [Google Scholar] [CrossRef]
- Oertel, W.; Schulz, J.B. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J. Neurochem. 2016, 139, 325–337. [Google Scholar] [CrossRef]
- Luquin, M.R.; Kulisevsky, J.; Martinez-Martin, P.; Mir, P.; Tolosa, E.S. Consensus on the Definition of Advanced Parkinson’s Disease: A Neurologists-Based Delphi Study (CEPA Study). Parkinson’s Dis. 2017, 2017, 4047392. [Google Scholar] [CrossRef] [Green Version]
- Antonini, A.; Stoessl, A.J.; Kleinman, L.S.; Skalicky, A.M.; Marshall, T.S.; Sail, K.R.; Onuk, K.; Odin, P.L.A. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: A multi-country Delphi-panel approach. Curr. Med. Res. Opin. 2018, 34, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E.; Muenter, M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001, 16, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Ferreira, J.J.; Lees, A.; Stocchi, F.; Poewe, W.; Tolosa, E.; Rascol, O. Opicapone for the treatment of Parkinson’s disease: A review of a new licensed medicine. Mov. Disord. 2018, 33, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Borgohain, R.; Szasz, J.; Stanzione, P.; Meshram, C.; Bhatt, M.; Chirilineau, D.; Stocchi, F.; Lucini, V.; Giuliani, R.; Forrest, E.; et al. Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov. Disord. 2014, 29, 229–237. [Google Scholar] [CrossRef]
- Borgohain, R.; Szasz, J.; Stanzione, P.; Meshram, C.; Bhatt, M.H.; Chirilineau, D.; Stocchi, F.; Lucini, V.; Giuliani, R.; Forrest, E.; et al. Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov. Disord. 2014, 29, 1273–1280. [Google Scholar] [CrossRef]
- Martí-Andrés, G.; Jiménez-Bolaños, R.; Arbelo-González, J.M.; Pagonabarraga, J.; Duran-Herrera, C.; Valenti-Azcarate, R.; Luquin, M.R. Safinamide in clinical practice: A Spanish multicenter cohort study. Brain Sci. 2019, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Szasz, J.A.; Szatmari, S.; Constantin, V.; Mihaly, I.; Racz, A.; Torok, I.; Nagy, E.; Kelemen, K.; Forro, T.; Baroti, B.; et al. The importance of evaluation of gastrointestinal features in advanced Parkinson’s disease. Orv. Hetil. 2020, 161, 161. [Google Scholar] [CrossRef]
- Patel, A.B.; Jimenez-Shahed, J. Profile of inhaled levodopa and its potential in the treatment of Parkinson’s disease: Evidence to date. Neuropsychiatr. Dis. Treat. 2018, 14, 2955–2964. [Google Scholar] [CrossRef] [Green Version]
- Szász, J.; Constantin, V.; Fazakas, P.; Blényesi, E.; Grieb, L.; Balla, A.; Sárig, M.; Szegedi, K.; Bartha, E.; Sz, S. The role of selective monoamine oxidase B inhibitors in the therapeutic strategy of Parkinson’s disease in the neurology clinics of Tirgu Mures County Emergency Clinical Hospital. Orv. Hetil. 2017, 158, 2023–2028. [Google Scholar] [CrossRef]
- Nyholm, D.; Odin, P.; Johansson, A.; Chatamra, K.; Locke, C.; Dutta, S.; Othman, A.A. Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced parkinson’s disease patients. AAPS J. 2013, 15, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Othman, A.A.; Dutta, S. Population pharmacokinetics of levodopa in subjects with advanced Parkinson’s disease: Levodopa-carbidopa intestinal gel infusion vs. oral tablets. Br. J. Clin. Pharmacol. 2014, 78, 94–105. [Google Scholar] [CrossRef]
- Szász, J.; Simu, M.; Perju-Dumbrava, L.; Antonini, A.; Bergmann, L.; Popescu, D.; Bajenaru, O.A. Efficacy, safety and patient’s quality of life of long-term treatment with levodopa-carbidopa intestinal gel in advanced parkinson’s disease in romania: Results from gloria observational study. Rom. J. Neurol. Rev. Rom. Neurol. 2020, 19, 27–35. [Google Scholar] [CrossRef]
- Müller, T.; Laar, T. van; Cornblath, D.R.; Odin, P.; Klostermann, F.; Grandas, F.J.; Ebersbach, G.; Urban, P.P.; Valldeoriola, F.; Antonini, A. Peripheral neuropathy in Parkinson’s disease: Levodopa exposure and implications for duodenal delivery. Park. Relat. Disord. 2013, 19, 501–507. [Google Scholar] [CrossRef]
- Liu, X.D.; Bao, Y.; Liu, G.J. Comparison between levodopa-carbidopa intestinal gel infusion and subthalamic nucleus deep-brain stimulation for advanced Parkinson’s disease: A systematic review and meta-analysis. Front. Neurol. 2019, 10, 934. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, L.; Bove, F.; Espay, K.J.; Lena, F.; Modugno, N.; Poon, Y.Y.; Krikorian, R.; Espay, A.J.; Fasano, A. 24-Hour infusion of levodopa/carbidopa intestinal gel for nocturnal akinesia in advanced Parkinson’s disease. Mov. Disord. 2016, 31, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Fung, V.S.C.; Merola, A.; Rollins, M.; Soileau, M.J.; Kovács, N. 24-Hour Levodopa-Carbidopa Intestinal Gel: Clinical Experience and Practical Recommendations. CNS Drugs 2021, 35, 137–149. [Google Scholar] [CrossRef]
- Cruse, B.; Morales-Briceño, H.; Chang, F.C.F.; Mahant, N.; Ha, A.D.; Kim, S.D.; Wolfe, N.; Kwan, V.; Tsui, D.S.; Griffith, J.M.; et al. 24-hour levodopa-carbidopa intestinal gel may reduce troublesome dyskinesia in advanced Parkinson’s disease. NPJ Park. Dis. 2018, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Morales-Briceño, H.; Mahant, N.; Ha, A.D.; Chang, F.C.F.; Kim, S.D.; Griffith, J.; Tsui, D.; Galea, D.; Fung, V.S.C. Long-term safety and efficacy of 24-hour levodopa-carbidopa intestinal gel in Parkinson’s disease. Mov. Disord. 2019, 34, 1747–1748. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Morgante, F.; Merola, A.; Fasano, A.; Marsili, L.; Fox, S.H.; Bezard, E.; Picconi, B.; Calabresi, P.; Lang, A.E. Levodopa-induced dyskinesia in Parkinson disease: Current and evolving concepts. Ann. Neurol. 2018, 84, 797–811. [Google Scholar] [CrossRef] [Green Version]
- Guridi, J.; González-Redondo, R.; Obeso, J.A. Clinical features, pathophysiology, and treatment of levodopa-induced dyskinesias in Parkinson’s disease. Parkinson’s Dis. 2012, 2012, 943159. [Google Scholar] [CrossRef] [Green Version]
- Szász, J.A.; Szatmári, S.; Constantin, V.; Mihály, I.; Rácz, A.; Frigy, A.; Nagy, E.; Kelemen, K.; Forró, T.; Almásy, E.; et al. Decision-making and duration to accept device-aided therapy in advanced Parkinson’s disease. Retrospective data from an Eastern European center with high patient turnover. Orv. Hetil. 2021, 162, 839–847. [Google Scholar] [CrossRef]
- Poewe, W.; Chaudhuri, K.R.; Bergmann, L.; Antonini, A. Levodopa-carbidopa intestinal gel in a subgroup of patients with dyskinesia at baseline from the GLORIA Registry. Neurodegener. Dis. Manag. 2019, 9, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Solla, P.; Mascia, M.M.; Marrosu, F.; Cannas, A. Diphasic dyskinesias during levodopa-carbidopa intestinal gel (LCIG) infusion in Parkinson’s disease. Park. Relat. Disord. 2017, 37, 92–96. [Google Scholar] [CrossRef]
- Marano, M.; Naranian, T.; di Biase, L.; Di Santo, A.; Poon, Y.Y.; Arca, R.; Cossu, G.; Marano, P.; Di Lazzaro, V.; Fasano, A. Complex dyskinesias in Parkinson patients on levodopa/carbidopa intestinal gel. Park. Relat. Disord. 2019, 69, 140–146. [Google Scholar] [CrossRef]
- Catalán, M.J.; Escribano, P.M.; Alonso-Frech, F. Dyskinesias in levodopa-carbidopa intestinal gel infusion era: New challenges, new features. Mov. Disord. 2017, 32, 624–625. [Google Scholar] [CrossRef]
- Fasano, A.; Gurevich, T.; Jech, R.; Kovács, N.; Svenningsson, P.; Szász, J.; Parra, J.C.; Bergmann, L.; Johnson, A.; Sanchez-Soliño, O.; et al. Concomitant Medication Usage with Levodopa-Carbidopa Intestinal Gel: Results from the COSMOS Study. Mov. Disord. 2021. [Google Scholar] [CrossRef]
- Zadikoff, C.; Poewe, W.; Boyd, J.T.; Bergmann, L.; Ijacu, H.; Kukreja, P.; Robieson, W.Z.; Benesh, J.; Antonini, A. Safety of Levodopa-Carbidopa Intestinal Gel Treatment in Patients with Advanced Parkinson’s Disease Receiving ≥2000 mg Daily Dose of Levodopa. Parkinson’s Dis. 2020, 2020, 9716317. [Google Scholar] [CrossRef]
- Szász, J.A.; Orbán-Kis, K.; Constantin, V.A.; Péter, C.; Bíró, I.; Mihály, I.; Szegedi, K.; Balla, A.; Szatmári, S. Therapeutic strategies in the early stages of Parkinson’s disease: A cross-sectional evaluation of 15 years’ experience with a large cohort of Romanian patients. Neuropsychiatr. Dis. Treat. 2019, 15, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Szász, J.A.; Constantin, V.; Mihály, I.; Biró, I.; Péter, C.; Orbán-Kis, K.; Szatmári, S. Dopamine agonists in Parkinson’s disease therapy—15 years of experience of the neurological clinics from Tirgu Mures. A cross-sectional study. Ideggyogy. Szle. 2019, 72, 187–193. [Google Scholar] [CrossRef] [PubMed]
| DID (n = 40) | ||
|---|---|---|
| Gender (n, %) | ||
| Male | 12, 30% | |
| Female | 28, 70% | |
| Age (years, mean ± SD) | p = 0.92 | |
| All patients | 64.13 ± 7.21 | |
| Male | 63.25 ± 8.79 | |
| Female | 64.50 ± 6.56 | |
| Disease duration until LCIG infusion | ||
| Years, mean ± SD | 12.63 ± 5.01 | |
| Years, median | 11.50 | |
| MMSE (mean ± SD) | 25.83 ± 1.62 | |
| Hoehn and Yahr score (Mean ± SD) | <0.0001 | |
| on state | 3.25 ± 0.44 | |
| off state | 4.5 ± 0.50 | |
| Off duration (hours, mean ± SD) | 4.80 ± 1.19 | |
| Peak dose dyskinesia (hours, mean ± SD) | ||
| Mild/moderate | 1.98 ± 0.71 | |
| Severe | 1.56 ± 0.58 | |
| Diphasic dyskinesia (hours, mean ± SD) | ||
| Early deficient on | 2.84 ± 0.55 | |
| Late deficient on | 1.21 ± 0.32 | |
| Total | 4.05 ± 0.69 | |
| Dystonia (hours, mean ± SD) | 1.83 ± 0.88 | |
| Early morning akinesia (hours, mean ± SD) | 0.98 ± 0.35 | |
| Delayed on (n, %) | 35, 87.5% | |
| No on (n, %) | 16, 40% | |
| Sudden off (n, %) | 20, 50% | |
| Freezing (n, %) | 23, 57.5% | |
| Levodopa until LCIG infusion | ||
| Frequency (x/day, mean ± SD) | 5.38 ± 1.23 | |
| Dose (mg/day, mean ± SD) | 756.3 ± 273.0 | |
| DA (n,%) | 34, 85% | |
| Pramipexole (n; mean ± SD mg) | 19; 2.23 ± 0.76 | |
| Ropinirole (n; mean ± SD mg) | 16; 14 ± 4.9 | |
| Rotigotine (n; mean ± SD mg) | 10; 7.6 ± 2.1 | |
| COMT-I (n,%) | 26, 65% | |
| Amantadine (n,%) | 16, 40% | |
| MAO-I (n,%) | 33, 82.5% | |
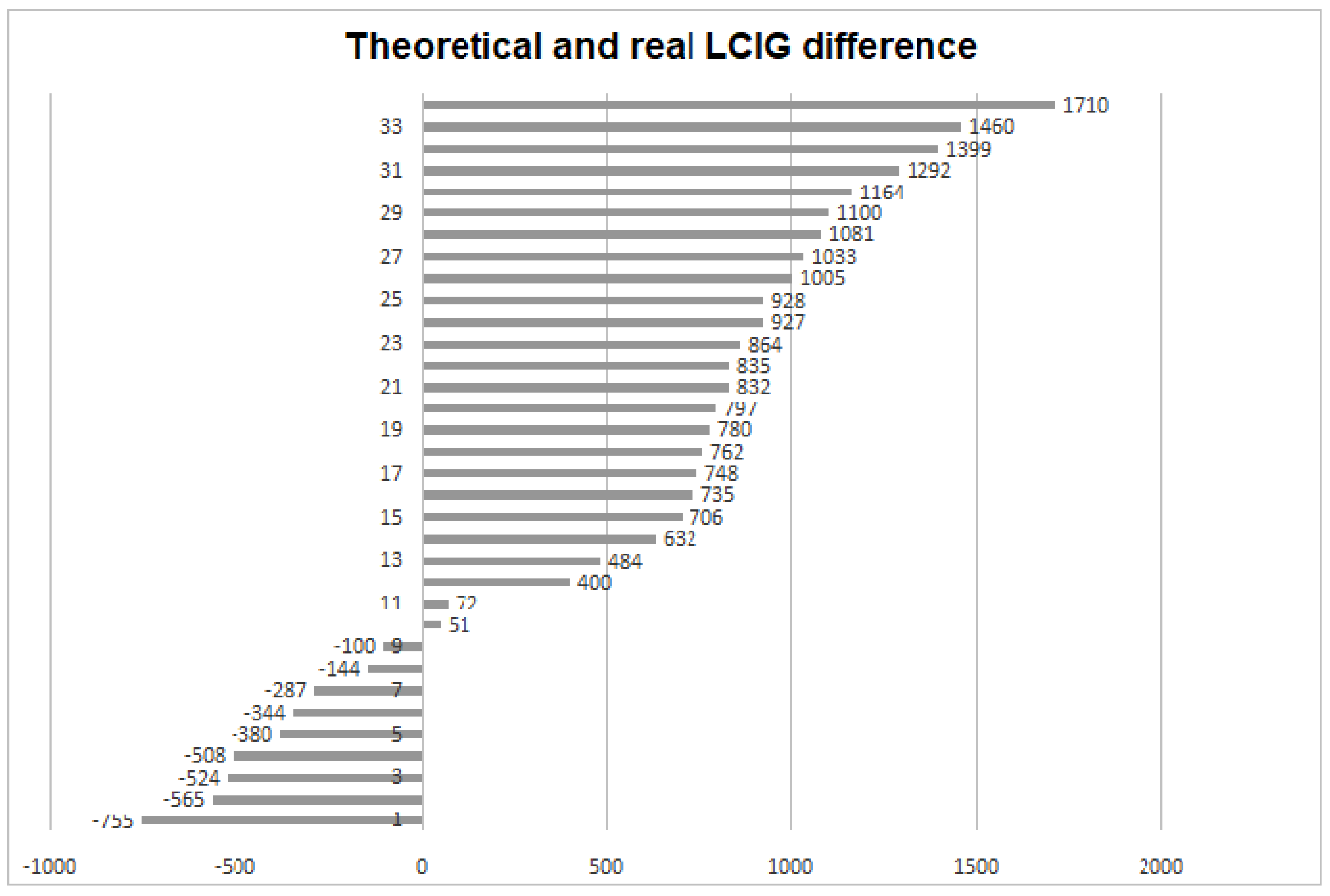
| LCIG calculated | 1232 ± 337.6 | |
| LCIG real | 1823 ± 728.4 |
| n = 34 | Before PEG | After PEG | 6 Months | 12 Months | 18 Months | p |
|---|---|---|---|---|---|---|
| LD (LCIG) dose (mean ± SD) | -- | 1763 ± 671 | 1700 ± 607 | 1712 ± 603 | 1720 ± 595 | ns |
| LD (LCIG) infusion | -- | |||||
| administration hours | 19.64 ± 3.57 | 19.65 ± 3.57 | 19.88 ± 3.58 | 20.24 ± 3.47 | 0.8105 | |
| Mean ± SD | 18 | 18 | 18 | 18 | ||
| Median | 11 | 11 | 10 | 7 | ||
| 16 h (n) | 10 | 10 | 10 | 12 | ||
| 18 h (n) | 13 | 13 | 14 | 15 | ||
| 24 h (n) | ||||||
| Hoehn and Yahr score (Mean ± SD) | ||||||
| on state | 3.26 ± 0.45 | 3 | 3 | 3 | 3.03 ± 0.17 | <0.0001 1 |
| off state | 4.5 ± 0.51 | 3.82 ± 0.46 | 3.82 ± 0.46 | 3.82 ± 0.46 | 3.91 ± 0.51 | <0.0001 2 |
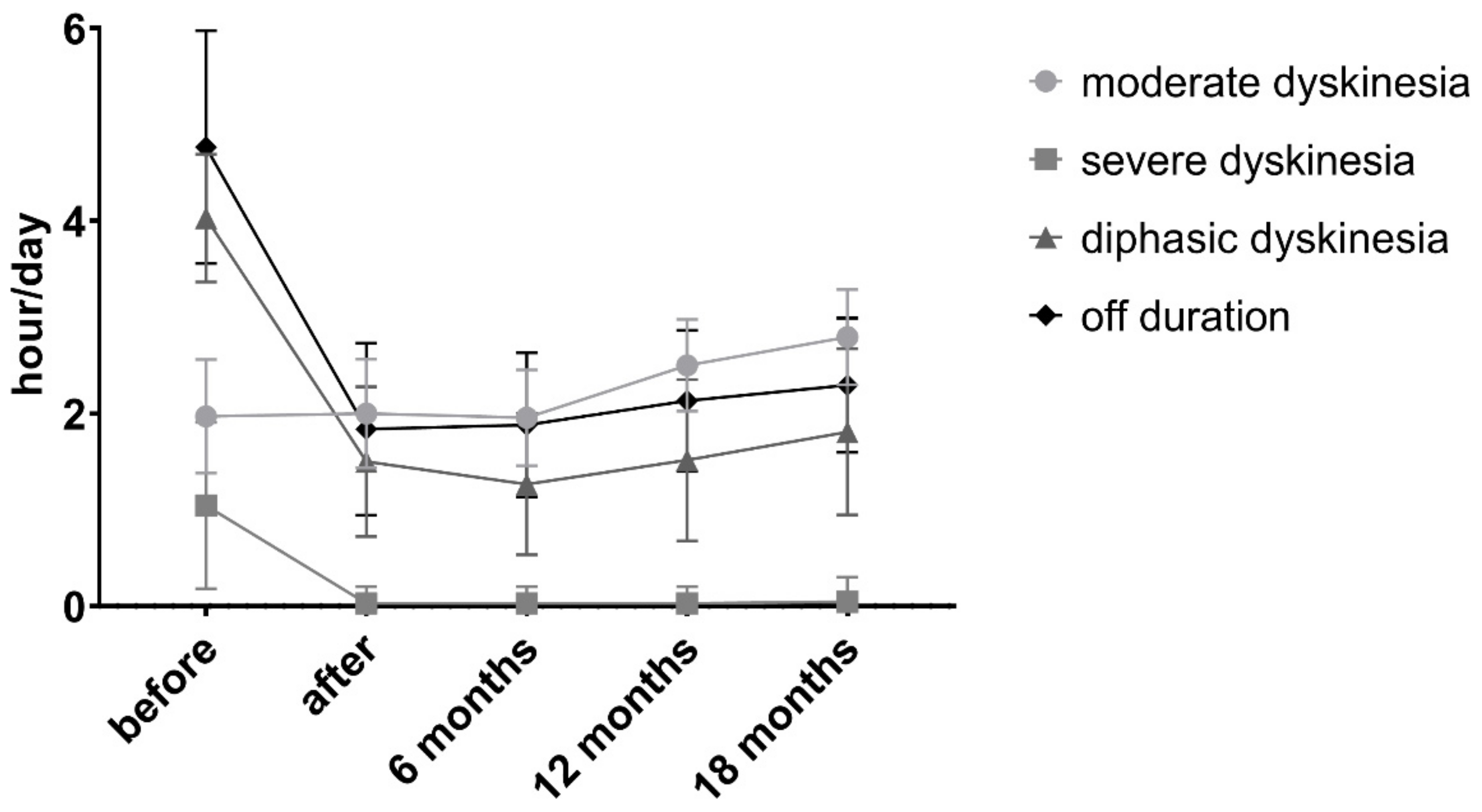
| Off duration | 4.76 ± 1.21 | 1.84 ± 0.89 | 1.88 ± 0.75 | 2.13 ± 0.73 | 2.29 ± 0.7 | <0.0001 3 |
| (n, hours, mean ± SD) | ||||||
| Peak dose dyskinesia (hours, mean ± SD) | ||||||
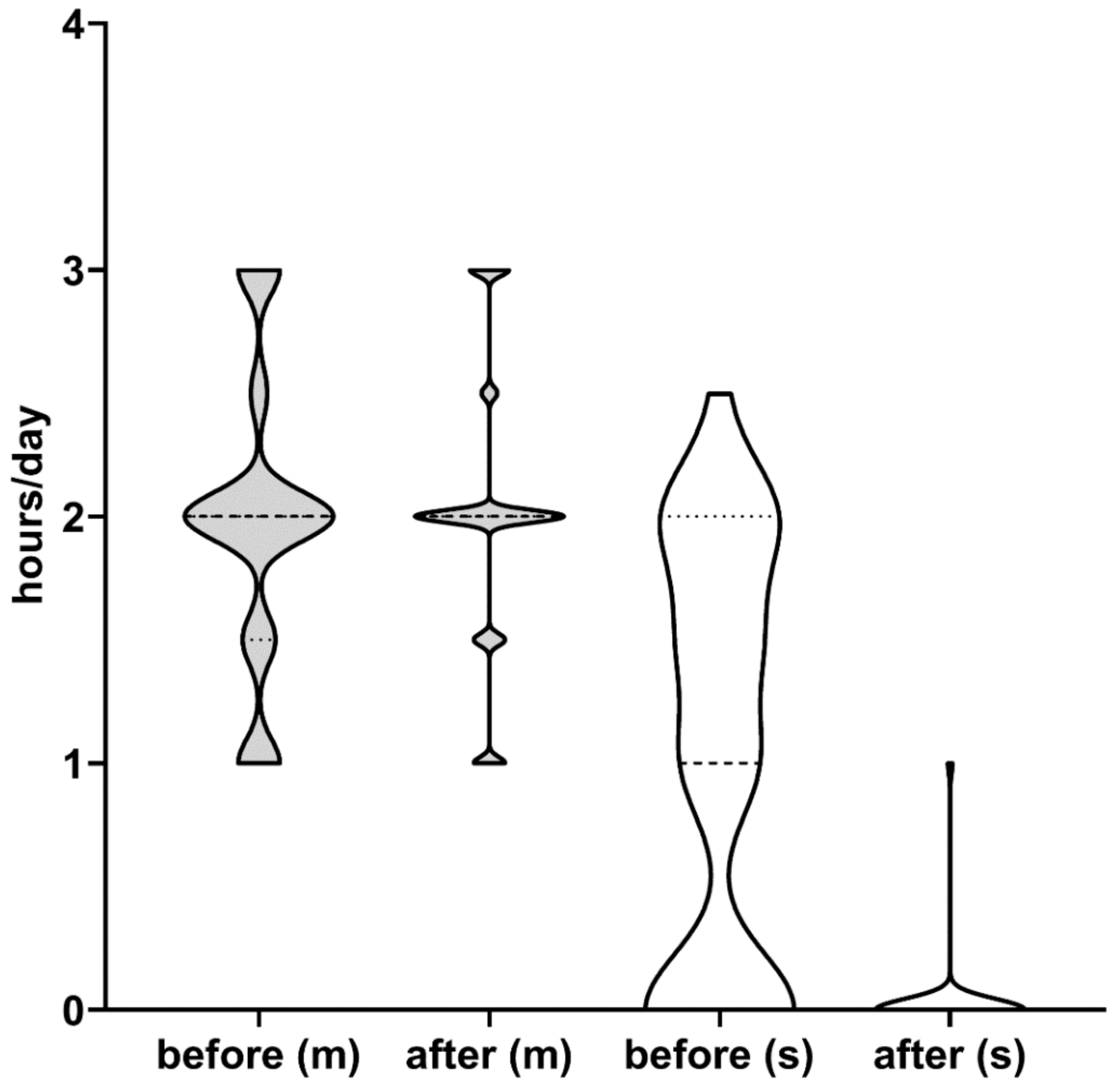
| Mild/moderate (n = 34) | 1.97 ± 0.6 | 2 ± 0.56 | 1.96 ± 0.5 | 2.5 ± 0.48 | 2.79 ± 0.49 | <0.0001 4 |
| Severe (n = 22) | 1.61 ± 0.46 | 0.03 ± 0.17 | 0.03 ± 0.17 | 0.03 ± 0.17 | 0.04 ± 0.26 | <0.0001 5 |
| Diphasic dyskinesia (hours, mean ± SD) | 4.03 ± 0.66 | 1.5 ± 0.78 | 1.27 ± 0.73 | 1.51 ± 0.84 | 1.81 ± 0.86 | <0.0001 6 |
| Dystonia | 12, 1.83 ± 0.94 | 9, 1.06 ± 0.39 | 9, 0.94 ± 0.46 | 9, 1.22 ± 0.44 | 9, 1.22 ± 0.36 | 0.0310 7 |
| (hours, mean ± SD) | ||||||
| Early morning akinesia (hours, mean ± SD) | 31, 1.06 ± 0.21 | 0, 0 | 6, 0.58 ± 0.2 | 11, 0.59 ± 0.204 | 12, 0.88 ± 0.23 | <0.0001 8 |
| Freezing (n) | 20 | 9 | 7 | 7 | 8 | 0.0018 # |
| DA (n,%) | See Table 1. | 10, 29% | 11, 32% | 11, 32% | 11, 32% | 0.9916 # |
| Amantadine (n,%) | See Table 1. | 6, 18% | 12, 35% | 18, 53% | 19, 56% | 0.0041 # |
| MAO-I (n,%) | See Table 1. | 13, 38% | 12, 35% | 15, 44% | 15, 44% | 0.8620 # |
| PGI-I (mean ± SD) | -- | 1.61 ± 0.55 | 1.71 ± 0.58 | 1.71 ± 0.58 | 2.29 ± 1.00 | 0.0040 9 |
| Very much improved (n) | 14 | 12 | 12 | 7 | ||
| Much improved (n) | 19 | 20 | 20 | 14 | ||
| Minimally improved (n) | 1 | 2 | 2 | 11 | ||
| No change (n) | 0 | 0 | 0 | |||
| Minimally worse (n) | 0 | 0 | 2 | |||
| Much worse (n) | 0 | 0 | 0 | |||
| Very much worse (n) | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szász, J.A.; Constantin, V.A.; Orbán-Kis, K.; Bancu, L.A.; Ciorba, M.; Mihály, I.; Nagy, E.E.; Szász, R.M.; Kelemen, K.; Simu, M.A.; et al. Management Challenges of Severe, Complex Dyskinesia. Data from a Large Cohort of Patients Treated with Levodopa-Carbidopa Intestinal Gel for Advanced Parkinson’s Disease. Brain Sci. 2021, 11, 826. https://doi.org/10.3390/brainsci11070826
Szász JA, Constantin VA, Orbán-Kis K, Bancu LA, Ciorba M, Mihály I, Nagy EE, Szász RM, Kelemen K, Simu MA, et al. Management Challenges of Severe, Complex Dyskinesia. Data from a Large Cohort of Patients Treated with Levodopa-Carbidopa Intestinal Gel for Advanced Parkinson’s Disease. Brain Sciences. 2021; 11(7):826. https://doi.org/10.3390/brainsci11070826
Chicago/Turabian StyleSzász, József Attila, Viorelia Adelina Constantin, Károly Orbán-Kis, Ligia Ariana Bancu, Marius Ciorba, István Mihály, Előd Ernő Nagy, Róbert Máté Szász, Krisztina Kelemen, Mihaela Adriana Simu, and et al. 2021. "Management Challenges of Severe, Complex Dyskinesia. Data from a Large Cohort of Patients Treated with Levodopa-Carbidopa Intestinal Gel for Advanced Parkinson’s Disease" Brain Sciences 11, no. 7: 826. https://doi.org/10.3390/brainsci11070826
APA StyleSzász, J. A., Constantin, V. A., Orbán-Kis, K., Bancu, L. A., Ciorba, M., Mihály, I., Nagy, E. E., Szász, R. M., Kelemen, K., Simu, M. A., & Szatmári, S. (2021). Management Challenges of Severe, Complex Dyskinesia. Data from a Large Cohort of Patients Treated with Levodopa-Carbidopa Intestinal Gel for Advanced Parkinson’s Disease. Brain Sciences, 11(7), 826. https://doi.org/10.3390/brainsci11070826






