Transcranial Direct Current Stimulation as a Treatment Tool for Mild Traumatic Brain Injury
Abstract
1. Introduction
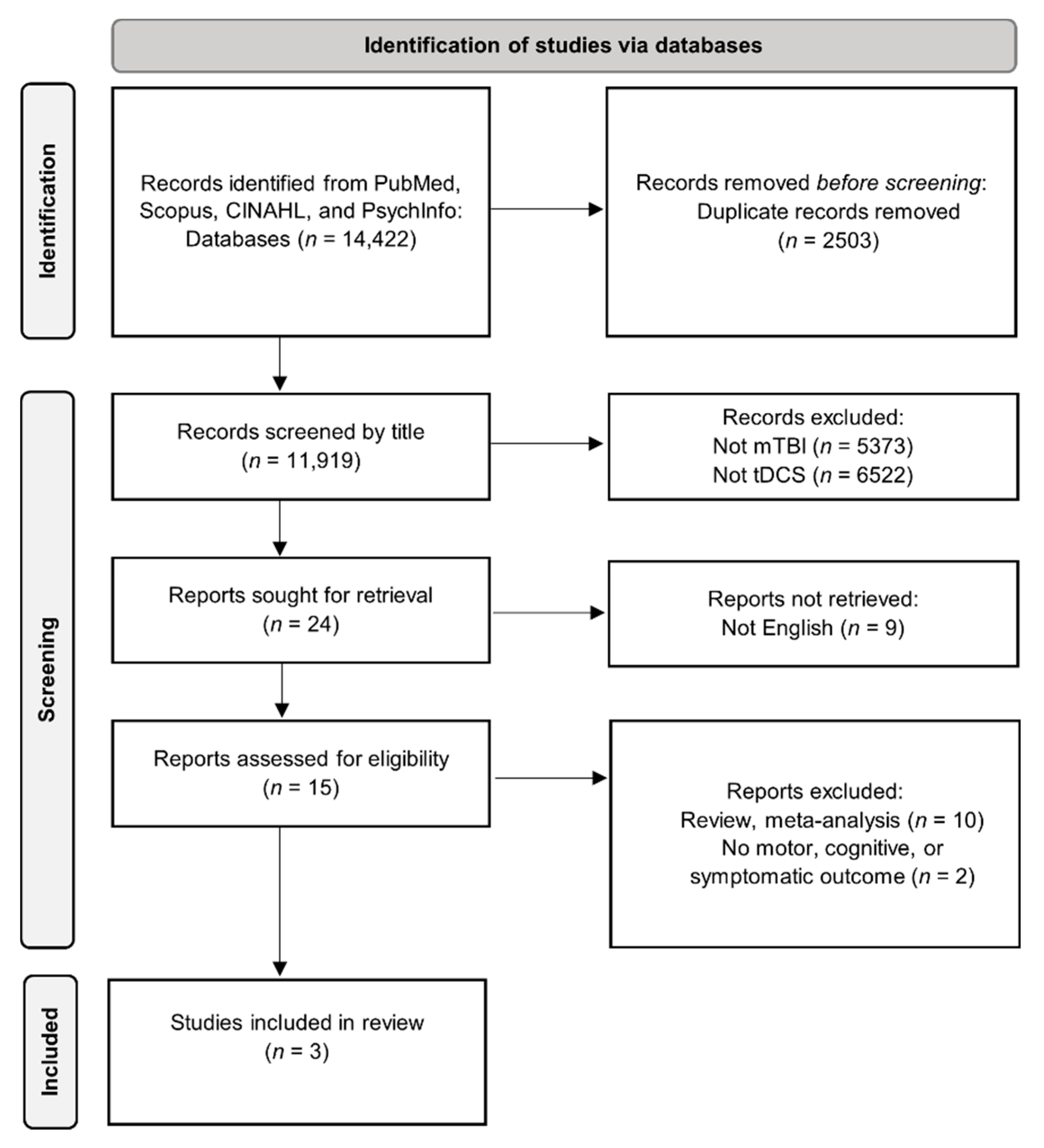
2. Search Methodology
3. Results
4. Discussion and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- American Academy of Neurology. Practice parameter: The management of concussion in sports (summary statement). Neurology 1997, 48, 581–585. [Google Scholar] [CrossRef]
- Howell, D.; Osternig, L.; Van Donkelaar, P.; Mayr, U.; Chou, L.S. Effects of concussion on attention and executive function in adolescents. Med. Sci. Sports Exerc. 2013, 45, 1030–1037. [Google Scholar] [CrossRef]
- Howell, D.R.; Osternig, L.R.; Chou, L.S. Dual-task effect on gait balance control in adolescents with concussion. Arch. Phys. Med. Rehabil. 2013, 94, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.R.; Yasen, A.L.; Maynard, L.F.; Chou, L.S.; Howell, D.R.; Christie, A.D. Acute and longitudinal changes in motor cortex function following mild traumatic brain injury. Brain Inj. 2014, 28, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.M.; Osternig, L.R.; van Donkelaar, P.; Chou, L.S. Recovery of cognitive and dynamic motor function following concussion. Br. J. Sports Med. 2007, 41, 868–873, discussion 873. [Google Scholar] [CrossRef] [PubMed]
- Cantu, R.C.; Aubry, M.; Dvorak, J.; Graf-Baumann, T.; Johnston, K.; Kelly, J.; Lovell, M.; McCrory, P.; Meeuwisse, W.; Schamasch, P.; et al. Overview of concussion consensus statements since 2000. Neurosurg. Focus 2006, 21, E3. [Google Scholar] [CrossRef]
- Guskiewicz, K.M.; McCrea, M.; Marshall, S.W.; Cantu, R.C.; Randolph, C.; Barr, W.; Onate, J.A.; Kelly, J.P. Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA concussion study. JAMA 2003, 290, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- McCrory, P.; Meeuwisse, W.H.; Aubry, M.; Cantu, R.C.; Dvořák, J.; Echemendia, R.J.; Engebretsen, L.; Johnston, K.; Kutcher, J.S.; Raftery, M.; et al. Consensus statement on concussion in sport: The 4th international conference on concussion in sport, Zurich, November 2012. J. Athl. Train. 2013, 48, 554–575. [Google Scholar] [CrossRef]
- Bleiberg, J.; Cernich, A.N.; Cameron, K.; Sun, W.; Peck, K.; Ecklund, P.J.; Reeves, D.; Uhorchak, J.; Sparling, M.B.; Warden, D.L. Duration of cognitive impairment after sports concussion. Neurosurgery 2004, 54, 1073–1078, discussion 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- McCrea, M.; Guskiewicz, K.M.; Marshall, S.W.; Barr, W.; Randolph, C.; Cantu, R.C.; Onate, J.A.; Yang, J.; Kelly, J.P. Acute effects and recovery time following concussion in collegiate football players: The NCAA concussion study. JAMA 2003, 290, 2556–2563. [Google Scholar] [CrossRef]
- De Beaumont, L.; Theoret, H.; Mongeon, D.; Messier, J.; Leclerc, S.; Tremblay, S.; Ellemberg, D.; Lassonde, M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain 2009, 132, 695–708. [Google Scholar] [CrossRef]
- Gray, C.; Cantagallo, A.; Della Sala, S.; Basaglia, N. Bradykinesia and bradyphrenia revisited: Patterns of subclinical deficit in motor speed and cognitive functioning in head-injured patients with good recovery. Brain Inj. 1998, 12, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; Osternig, L.R.; Chou, L.S. Return to activity after concussion affects dual-task gait balance control recovery. Med. Sci. Sports Exerc. 2015, 47, 673–680. [Google Scholar] [CrossRef]
- Lima, D.P.D.; Simao, C.; Abib, S.D.V.; de Figueiredo, L.F.P. Quality of life and neuropsychological changes in mild head trauma—Late analysis and correlation with S100B protein and cranial CT scan performed at hospital admission. Inj. Int. J. Care Inj. 2008, 39, 604–611. [Google Scholar] [CrossRef]
- McCauley, S.R.; Boake, C.; Levin, H.S.; Contant, C.F.; Song, J.X. Postconcussional disorder following mild to moderate traumatic brain injury: Anxiety, depression, and social support as risk factors and comorbidities. J. Clin. Exp. Neuropsychol. 2001, 23, 792–808. [Google Scholar] [CrossRef]
- Ruffolo, C.F.; Friedland, J.F.; Dawson, D.R.; Colantonio, A.; Lindsay, P.H. Mild traumatic brain injury from motor vehicle accidents: Factors associated with return to work. Arch. Phys. Med. Rehabil. 1999, 80, 392–398. [Google Scholar] [CrossRef]
- Kashluba, S.; Paniak, C.; Blake, T.; Reynolds, S.; Toller-Lobe, G.; Nagy, J. A longitudinal, controlled study of patient complaints following treated mild traumatic brain injury. Arch. Clin. Neuropsychol. 2004, 19, 805–816. [Google Scholar] [CrossRef]
- Lundin, A.; Boussard, C.; Edman, G.; Borg, J. Symptoms and disability until 3 months after mild TBI. Brain Inj. 2006, 20, 799–806. [Google Scholar] [CrossRef]
- Bigler, E. The lesion(s) in traumatic brain injury: Implications for clinical neuropsychology. Arch. Clin. Neuropsychol. 2001, 16, 95–131. [Google Scholar] [CrossRef]
- King, N. Mild head injury: Neuropathology, sequelae, measurement and recovery. Br. J. Clin. Psychol. 1997, 36, 161–184. [Google Scholar] [CrossRef]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef]
- Stenberg, J.; Eikenes, L.; Moen, K.G.; Vik, A.; Håberg, A.K.; Skandsen, T. Acute diffusion tensor and kurtosis imaging and outcome following mild and traumatic brain injury. J. Neurotrauma 2021. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.C.; Tremblay, J.; Tremblay, S.; Lee, A.; Brun, C.; Lepore, N.; Theoret, H.; Ellemberg, D.; Lassonde, M. Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma 2011, 28, 2049–2059. [Google Scholar] [CrossRef]
- Tang, C.Y.; Eaves, E.; Dams-O’Connor, K.; Ho, L.; Leung, E.; Wong, E.; Carpenter, D.; Ng, J.; Gordon, W.; Pasinetti, G. Diffuse disconnectivity in TBI: A resting state fMRI and DTI study. Transl. Neurosci. 2012, 3, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Krach, L.; Ward, E.; Mueller, B.A.; Muetzel, R.; Schnoebelen, S.; Kiragu, A.; Lim, K.O. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: A diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 2007, 22, 555–568. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, R.K.; Husain, M.; Chaudhry, C.; Srivastava, A.; Saksena, S.; Rathore, R.K. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: Its correlation with neuropsychometric tests. Brain Inj. 2009, 23, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.G.; Pillai, S.V.; Rao, S.L.; Kovoor, J.M.; Chandramouli, B.A. Post-concussion syndrome: Correlation of neuropsychological deficits, structural lesions on magnetic resonance imaging and symptoms. Neurol. India 2009, 57, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Riggio, S.; Wong, M. Neurobehavioral sequelae of traumatic brain injury. Mt. Sinai J. Med. 2009, 76, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Giza, C.C.; Hovda, D.A. The neurometabolic cascade of concussion. J. Athl. Train 2001, 36, 228–235. [Google Scholar] [CrossRef]
- Gasparovic, C.; Yeo, R.; Mannell, M.; Ling, J.; Elgie, R.; Phillips, J.; Doezema, D.; Mayer, A.R. Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: An 1H-magnetic resonance spectroscopy study. J. Neurotrauma 2009, 26, 1635–1643. [Google Scholar] [CrossRef]
- Harris, J.L.; Yeh, H.W.; Choi, I.Y.; Lee, P.; Berman, N.E.; Swerdlow, R.H.; Craciunas, S.C.; Brooks, W.M. Altered neurochemical profile after traumatic brain injury: (1)H-MRS biomarkers of pathological mechanisms. J. Cereb. Blood Flow Metab. 2012, 32, 2122–2134. [Google Scholar] [CrossRef]
- Tremblay, S.; Beaule, V.; Proulx, S.; Tremblay, S.; Marjanska, M.; Doyon, J.; Lassonde, M.; Theoret, H. Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clin. Neurophysiol. 2014, 125, 1371–1379. [Google Scholar] [CrossRef]
- Xu, S.; Zhuo, J.; Racz, J.; Shi, D.; Roys, S.; Fiskum, G.; Gullapalli, R. Early microstructural and metabolic changes following controlled cortical impact injury in rat: A magnetic resonance imaging and spectroscopy study. J. Neurotrauma 2011, 28, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- De Beaumont, L.; Mongeon, D.; Tremblay, S.; Messier, J.; Prince, F.; Leclerc, S.; Lassonde, M.; Theoret, H. Persistent motor system abnormalities in formerly concussed athletes. J. Athl. Train 2011, 46, 234–240. [Google Scholar] [CrossRef]
- De Beaumont, L.; Tremblay, S.; Poirier, J.; Lassonde, M.; Theoret, H. Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cereb. Cortex 2012, 22, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; de Beaumont, L.; Lassonde, M.; Theoret, H. Evidence for the specificity of intracortical inhibitory dysfunction in asymptomatic concussed athletes. J. Neurotrauma 2011, 28, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, A.V.; Soustiel, J.F.; Hafner, H.; Trubnik, M.; Levy, G.; Feinsod, M. Excitatory and inhibitory corticospinal responses to transcranial magnetic stimulation in patients with minor to moderate head injury. J. Neurol. Neurosurg. Psychiatry 2001, 70, 580–587. [Google Scholar] [CrossRef]
- De Beaumont, L.; Lassonde, M.; Leclerc, S.; Theoret, H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery 2007, 61, 329–336, discussion 336–327. [Google Scholar] [CrossRef]
- Pink, A.E.; Williams, C.; Alderman, N.; Stoffels, M. The use of repetitive transcranial magnetic stimulation (rTMS) following traumatic brain injury (TBI): A scoping review. Neuropsychol. Rehabil. 2021, 31, 479–505. [Google Scholar] [CrossRef] [PubMed]
- Cerruti, C.; Schlaug, G. Anodal transcranial direct current stimulation of the prefrontal cortex enhances complex verbal associative thought. J. Cogn. Neurosci. 2009, 21, 1980–1987. [Google Scholar] [CrossRef]
- Dockery, C.A.; Hueckel-Weng, R.; Birbaumer, N.; Plewnia, C. Enhancement of planning ability by transcranial direct current stimulation. J. Neurosci. 2009, 29, 7271–7277. [Google Scholar] [CrossRef] [PubMed]
- Dresler, M.; Sandberg, A.; Ohla, K.; Bublitz, C.; Trenado, C.; Mroczko-Wasowicz, A.; Kuhn, S.; Repantis, D. Non-pharmacological cognitive enhancement. Neuropharmacology 2013, 64, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.K.; Kim, D.Y.; Paik, N.J. Transcranial direct current stimulation of the left prefrontal cortex improves attention in patients with traumatic brain injury: A pilot study. J. Rehabil. Med. 2012, 44, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Koski, L.; Kolivakis, T.; Yu, C.; Chen, J.K.; Delaney, S.; Ptito, A. Noninvasive brain stimulation for persistent postconcussion symptoms in mild traumatic brain injury. J. Neurotrauma 2015, 32, 38–44. [Google Scholar] [CrossRef]
- Leśniak, M.; Polanowska, K.; Seniów, J.; Członkowska, A. Effects of repeated anodal tDCS coupled with cognitive training for patients with severe traumatic brain injury: A pilot randomized controlled trial. J. Head Trauma Rehabil 2014, 29, E20–E29. [Google Scholar] [CrossRef]
- Ulam, F.; Shelton, C.; Richards, L.; Davis, L.; Hunter, B.; Fregni, F.; Higgins, K. Cumulative effects of transcranial direct current stimulation on EEG oscillations and attention/working memory during subacute neurorehabilitation of traumatic brain injury. Clin. Neurophysiol. 2015, 126, 486–496. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Flöel, A. tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage 2014, 85 Pt 3, 934–947. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef]
- Gough, N.; Brkan, L.; Subramaniam, P.; Chiuccariello, L.; De Petrillo, A.; Mulsant, B.H.; Bowie, C.R.; Rajji, T.K. Feasibility of remotely supervised transcranial direct current stimulation and cognitive remediation: A systematic review. PLoS ONE 2020, 15, e0223029. [Google Scholar] [CrossRef]
- List, J.; Lesemann, A.; Kubke, J.C.; Kulzow, N.; Schreiber, S.J.; Floel, A. Impact of tDCS on cerebral autoregulation in aging and in patients with cerebrovascular diseases. Neurology 2015, 84, 626–628. [Google Scholar] [CrossRef]
- Bikson, M.; Grossman, P.; Thomas, C.; Zannou, A.L.; Jiang, J.; Adnan, T.; Mourdoukoutas, A.P.; Kronberg, G.; Truong, D.; Boggio, P.; et al. Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 2016, 9, 641–661. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, V.; Near, J.; Johansen-Berg, H.; Stagg, C.J. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. Elife 2015, 4, e08789. [Google Scholar] [CrossRef]
- Kim, S.; Stephenson, M.C.; Morris, P.G.; Jackson, S.R. tDCS-induced alterations in GABA concentration within primary motor cortex predict motor learning and motor memory: A 7T magnetic resonance spectroscopy study. Neuroimage 2014, 99, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. What are we measuring with GABA magnetic resonance spectroscopy? Commun. Integr. Biol. 2011, 4, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Best, J.G.; Stephenson, M.C.; O’Shea, J.; Wylezinska, M.; Kincses, Z.T.; Morris, P.G.; Matthews, P.M.; Johansen-Berg, H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J. Neurosci. 2009, 29, 5202–5206. [Google Scholar] [CrossRef]
- Tremblay, S.; Beaule, V.; Lepage, J.F.; Theoret, H. Anodal transcranial direct current stimulation modulates GABAB-related intracortical inhibition in the M1 of healthy individuals. Neuroreport 2013, 24, 46–50. [Google Scholar] [CrossRef]
- Guerriero, R.M.; Giza, C.C.; Rotenberg, A. Glutamate and GABA imbalance following traumatic brain injury. Curr. Neurol. Neurosci. Rep. 2015, 15, 27. [Google Scholar] [CrossRef]
- Dhaliwal, S.K.; Meek, B.P.; Modirrousta, M.M. Non-invasive brain stimulation for the treatment of symptoms following traumatic brain injury. Front. Psychiatry. 2015, 6, 119. [Google Scholar] [CrossRef]
- Hara, T.; Shanmugalingam, A.; McIntyre, A.; Burhan, A.M. The effect of non-invasive brain stimulation (NIBS) on executive functioning, attention and memory in rehabilitation patients with traumatic brain injury: A systematic review. Diagnostics 2021, 11, 627. [Google Scholar] [CrossRef]
- Kim, W.S.; Lee, K.; Kim, S.; Cho, S.; Paik, N.J. Transcranial direct current stimulation for the treatment of motor impairment following traumatic brain injury. J. Neuroeng. Rehabil. 2019, 16, 14. [Google Scholar] [CrossRef]
- Nardone, R.; Sebastianelli, L.; Versace, V.; Brigo, F.; Golaszewski, S.; Manganotti, P.; Saltuari, L.; Trinka, E. Repetitive transcranial magnetic stimulation in traumatic brain injury: Evidence from animal and human studies. Brain Res. Bull 2020, 159, 44–52. [Google Scholar] [CrossRef]
- Zaninotto, A.L.; El-Hagrassy, M.M.; Green, J.R.; Babo, M.; Paglioni, V.M.; Benute, G.G.; Paiva, W.S. Transcranial direct current stimulation (tDCS) effects on traumatic brain injury (TBI) recovery: A systematic review. Dement. Neuropsychol. 2019, 13, 172–179. [Google Scholar] [CrossRef]
- Pinchuk, D.; Pinchuk, O.; Sirbiladze, K.; Shugar, O. Clinical effectiveness of primary and secondary headache treatment by transcranial direct current stimulation. Front. Neurol. 2013, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, M.; Abu-Arafeh, I. Chronic posttraumatic headache in children and adolescents. Dev. Med. Child. Neurol. 2001, 43, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Kryzhanovsky, G. Central Nervous System Pathology: A New Approach; Consultants Bureau: New York, NY, USA, 2012. [Google Scholar]
- Tsirkin, V.; Trukhina, S. The physiological basis of mental activity and behavior. In Moscow Medical Book; Novgorod, N., Ed.; Publishing House of the NGMA: Nizhny Novgorod, Russia, 2001. [Google Scholar]
- Andrasik, F. Biofeedback in headache: An overview of approaches and evidence. Clevel. Clin. J. Med. 2010, 77 (Suppl. 3), S72–S76. [Google Scholar] [CrossRef]
- Bryans, R.; Descarreaux, M.; Duranleau, M.; Marcoux, H.; Potter, B.; Ruegg, R.; Shaw, L.; Watkin, R.; White, E. Evidence-based guidelines for the chiropractic treatment of adults with headache. J. Manip. Physiol. Ther. 2011, 34, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Haag, G. Headache and medication overuse: Are clinical case series appropriate to reveal differential risks of different medications? Expert Opin. Drug Saf. 2010, 9, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Quinn, D.K.; Upston, J.; Jones, T.; Brandt, E.; Story-Remer, J.; Fratzke, V.; Wilson, J.K.; Rieger, R.; Hunter, M.A.; Gill, D.; et al. Cerebral perfusion effects of cognitive training and transcranial direct current stimulation in mild-moderate TBI. Front. Neurol. 2020, 11, 545174. [Google Scholar] [CrossRef] [PubMed]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain 3-Dimensional Proportional System: An Approach to Cerebral Imaging; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 1988. [Google Scholar]
- Coffman, B.A.; Trumbo, M.C.; Clark, V.P. Enhancement of object detection with transcranial direct current stimulation is associated with increased attention. BMC Neurosci. 2012, 13. [Google Scholar] [CrossRef]
- Doshi, H.; Wiseman, N.; Liu, J.; Wang, W.; Welch, R.D.; O’Neil, B.J.; Zuk, C.; Wang, X.; Mika, V.; Szaflarski, J.P.; et al. Cerebral hemodynamic changes of mild traumatic brain injury at the acute stage. PLoS ONE 2015, 10, e0118061. [Google Scholar] [CrossRef]
- Kim, J.; Whyte, J.; Patel, S.; Avants, B.; Europa, E.; Wang, J.; Slattery, J.; Gee, J.C.; Coslett, H.B.; Detre, J.A. Resting cerebral blood flow alterations in chronic traumatic brain injury: An arterial spin labeling perfusion fmri study. J. Neurotrauma 2010, 27, 1399–1411. [Google Scholar] [CrossRef]
- Lin, C.M.; Tseng, Y.C.; Hsu, H.L.; Chen, C.J.; Chen, D.Y.; Yan, F.X.; Chiu, W.T. Arterial spin labeling perfusion study in the patients with subacute mild traumatic brain injury. PLoS ONE 2016, 11, e0149109. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Fischer, T.; Saiote, C.; Miller, R.; Chaieb, L.; Wang, D.J.; Plessow, F.; Paulus, W.; Kirschbaum, C. Transcranial electrical stimulation modifies the neuronal response to psychosocial stress exposure. Hum. Brain Mapp. 2014, 35, 3750–3759. [Google Scholar] [CrossRef]
- Stagg, C.J.; Lin, R.L.; Mezue, M.; Segerdahl, A.; Kong, Y.; Xie, J.; Tracey, I. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J. Neurosci. 2013, 33, 11425–11431. [Google Scholar] [CrossRef] [PubMed]
- Lipszyc, J.; Levin, H.; Hanten, G.; Hunter, J.; Dennis, M.; Schachar, R. Frontal white matter damage impairs response inhibition in children following traumatic brain injury. Arch. Clin. Neuropsychol. 2014, 29, 289–299. [Google Scholar] [CrossRef]
- Mayer, A.R.; Stephenson, D.D.; Wertz, C.J.; Dodd, A.B.; Shaff, N.A.; Ling, J.M.; Park, G.; Oglesbee, S.J.; Wasserott, B.C.; Meier, T.B.; et al. Proactive inhibition deficits with normal perfusion after pediatric mild traumatic brain injury. Hum. Brain Mapp. 2019, 40, 5370–5381. [Google Scholar] [CrossRef]
- Mendez, M.F.; Owens, E.M.; Reza Berenji, G.; Peppers, D.C.; Liang, L.J.; Licht, E.A. Mild traumatic brain injury from primary blast vs. blunt forces: Post-concussion consequences and functional neuroimaging. NeuroRehabilitation 2013, 32, 397–407. [Google Scholar] [CrossRef]
- Cunillera, T.; Brignani, D.; Cucurell, D.; Fuentemilla, L.; Miniussi, C. The right inferior frontal cortex in response inhibition: A tDCS-ERP co-registration study. Neuroimage 2016, 140, 66–75. [Google Scholar] [CrossRef]
- Giglia, G.; Brighina, F.; Rizzo, S.; Puma, A.; Indovino, S.; Maccora, S.; Baschi, R.; Cosentino, G.; Fierro, B. Anodal transcranial direct current stimulation of the right dorsolateral prefrontal cortex enhances memory-guided responses in a visuospatial working memory task. Funct. Neurol. 2014, 29, 189–193. [Google Scholar] [PubMed]
- Kito, S.; Hasegawa, T.; Koga, Y. Neuroanatomical correlates of therapeutic efficacy of low-frequency right prefrontal transcranial magnetic stimulation in treatment-resistant depression. Psychiatry Clin. Neurosci. 2011, 65, 175–182. [Google Scholar] [CrossRef]
- Clark, L.; Manes, F.; Antoun, N.; Sahakian, B.J.; Robbins, T.W. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia 2003, 41, 1474–1483. [Google Scholar] [CrossRef]
- Min, S.K.; Lee, B.O. Laterality in somatization. Psychosom. Med. 1997, 59, 236–240. [Google Scholar] [CrossRef]
- Richard, N.M.; O’Connor, C.; Dey, A.; Robertson, I.H.; Levine, B. External modulation of the sustained attention network in traumatic brain injury. Neuropsychology 2018, 32, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Wilson, C.M.; Brabazon, F.; von Leden, R.; Jurgens, J.S.; Oakes, T.R.; Selwyn, R.G. FDG-PET imaging in mild traumatic brain injury: A critical review. Front. Neuroenergetics 2014, 5, 13. [Google Scholar] [CrossRef]
- Kushner, D. Mild traumatic brain injury: Toward understanding manifestations and treatment. Arch. Intern. Med. 1998, 158, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Iodice, R.; Manganelli, F.; Dubbioso, R. The therapeutic use of non-invasive brain stimulation in multiple sclerosis—A review. Restor. Neurol. Neurosci. 2017, 35, 497–509. [Google Scholar] [CrossRef]
- Sánchez-Kuhn, A.; Pérez-Fernández, C.; Cánovas, R.; Flores, P.; Sánchez-Santed, F. Transcranial direct current stimulation as a motor neurorehabilitation tool: An empirical review. Biomed. Eng. Online 2017, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Model. Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Suh, J.H.; Han, S.J. Transcranial direct current stimulation for balance and gait in repetitive mild traumatic brain injury in rats. BMC Neurosci. 2021, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Ikoma, K.; Samii, A.; Mercuri, B.; Wassermann, E.M.; Hallett, M. Abnormal cortical motor excitability in dystonia. Neurology 1996, 46, 1371–1376. [Google Scholar] [CrossRef]
- Sacco, K.; Galetto, V.; Dimitri, D.; Geda, E.; Perotti, F.; Zettin, M.; Geminiani, G.C. Concomitant use of transcranial direct current stimulation and computer-assisted training for the rehabilitation of attention in traumatic brain injured patients: Behavioral and neuroimaging results. Front. Behav. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, F.; Constantinidis, C. Bottom-up and top-down attention: Different processes and overlapping neural systems. Neuroscientist 2014, 20, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Deliagina, T.G.; Zelenin, P.V.; Beloozerova, I.N.; Orlovsky, G.N. Nervous mechanisms controlling body posture. Physiol. Behav. 2007, 92, 148–154. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Cortical control of postural responses. J. Neural. Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef]
- Yosephi, M.H.; Ehsani, F.; Zoghi, M.; Jaberzadeh, S. Multi-session anodal tDCS enhances the effects of postural training on balance and postural stability in older adults with high fall risk: Primary motor cortex versus cerebellar stimulation. Brain Stimul. 2018, 11, 1239–1250. [Google Scholar] [CrossRef]
- Sussman, D.; da Costa, L.; Chakravarty, M.M.; Pang, E.W.; Taylor, M.J.; Dunkley, B.T. Concussion induces focal and widespread neuromorphological changes. Neurosci. Lett. 2017, 650, 52–59. [Google Scholar] [CrossRef]
- Opie, G.M.; Liao, W.Y.; Semmler, J.G. Interactions between cerebellum and the intracortical excitatory circuits of motor cortex: A mini-review. Cerebellum 2021. [Google Scholar] [CrossRef]
- Fonteneau, C.; Redoute, J.; Haesebaert, F.; Le Bars, D.; Costes, N.; Suaud-Chagny, M.F.; Brunelin, J. Frontal transcranial direct current stimulation induces dopamine release in the ventral striatum in human. Cereb. Cortex 2018, 28, 2636–2646. [Google Scholar] [CrossRef]
- Fukai, M.; Bunai, T.; Hirosawa, T.; Kikuchi, M.; Ito, S.; Minabe, Y.; Ouchi, Y. Endogenous dopamine release under transcranial direct-current stimulation governs enhanced attention: A study with positron emission tomography. Transl. Psychiatry 2019, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Grami, F.; de Marco, G.; Bodranghien, F.; Manto, M.; Habas, C. Cerebellar transcranial direct current stimulation reconfigurates static and dynamic functional connectivity of the resting-state networks. Cerebellum Ataxias 2021, 8, 7. [Google Scholar] [CrossRef]
- Rudroff, T.; Workman, C.D.; Fietsam, A.C.; Ponto, L.L.B. Imaging transcranial direct current stimulation (tDCS) with positron emission tomography (PET). Brain Sci. 2020, 10, 236. [Google Scholar] [CrossRef]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef]
- Schmidt, C.; Wagner, S.; Burger, M.; Rienen, U.; Wolters, C.H. Impact of uncertain head tissue conductivity in the optimization of transcranial direct current stimulation for an auditory target. J. Neural. Eng. 2015, 12, 046028. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I.; Tanaka, S.; Koyama, S.; De Santis, V.; Hirata, A. Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimul. 2015, 8, 906–913. [Google Scholar] [CrossRef]
- Saturnino, G.B.; Antunes, A.; Thielscher, A. On the importance of electrode parameters for shaping electric field patterns generated by tDCS. Neuroimage 2015, 120, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Laakso, I.; Tanaka, S.; Mikkonen, M.; Koyama, S.; Sadato, N.; Hirata, A. Electric fields of motor and frontal tDCS in a standard brain space: A computer simulation study. Neuroimage 2016, 137, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Workman, C.D.; Fietsam, A.C.; Kamholz, J. Response variability in transcranial direct current stimulation: Why sex matters. Front. Psychiatry 2020, 11, 585. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Rudroff, T. Transcranial direct current stimulation at 4 mA induces greater leg muscle fatigability in women compared to men. Brain Sci. 2020, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.F.; Paulus, W.; Nitsche, M.A. Sex differences in cortical neuroplasticity in humans. Neuroreport 2006, 17, 1703–1707. [Google Scholar] [CrossRef]
- Inghilleri, M.; Conte, A.; Currà, A.; Frasca, V.; Lorenzano, C.; Berardelli, A. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin. Neurophysiol. 2004, 115, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.; Cohen Kadosh, R. Not all brains are created equal: The relevance of individual differences in responsiveness to transcranial electrical stimulation. Front. Syst. Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudroff, T.; Workman, C.D. Transcranial Direct Current Stimulation as a Treatment Tool for Mild Traumatic Brain Injury. Brain Sci. 2021, 11, 806. https://doi.org/10.3390/brainsci11060806
Rudroff T, Workman CD. Transcranial Direct Current Stimulation as a Treatment Tool for Mild Traumatic Brain Injury. Brain Sciences. 2021; 11(6):806. https://doi.org/10.3390/brainsci11060806
Chicago/Turabian StyleRudroff, Thorsten, and Craig D. Workman. 2021. "Transcranial Direct Current Stimulation as a Treatment Tool for Mild Traumatic Brain Injury" Brain Sciences 11, no. 6: 806. https://doi.org/10.3390/brainsci11060806
APA StyleRudroff, T., & Workman, C. D. (2021). Transcranial Direct Current Stimulation as a Treatment Tool for Mild Traumatic Brain Injury. Brain Sciences, 11(6), 806. https://doi.org/10.3390/brainsci11060806






