Tobacco and Nervous System Development and Function—New Findings 2015–2020
Abstract
1. Introduction
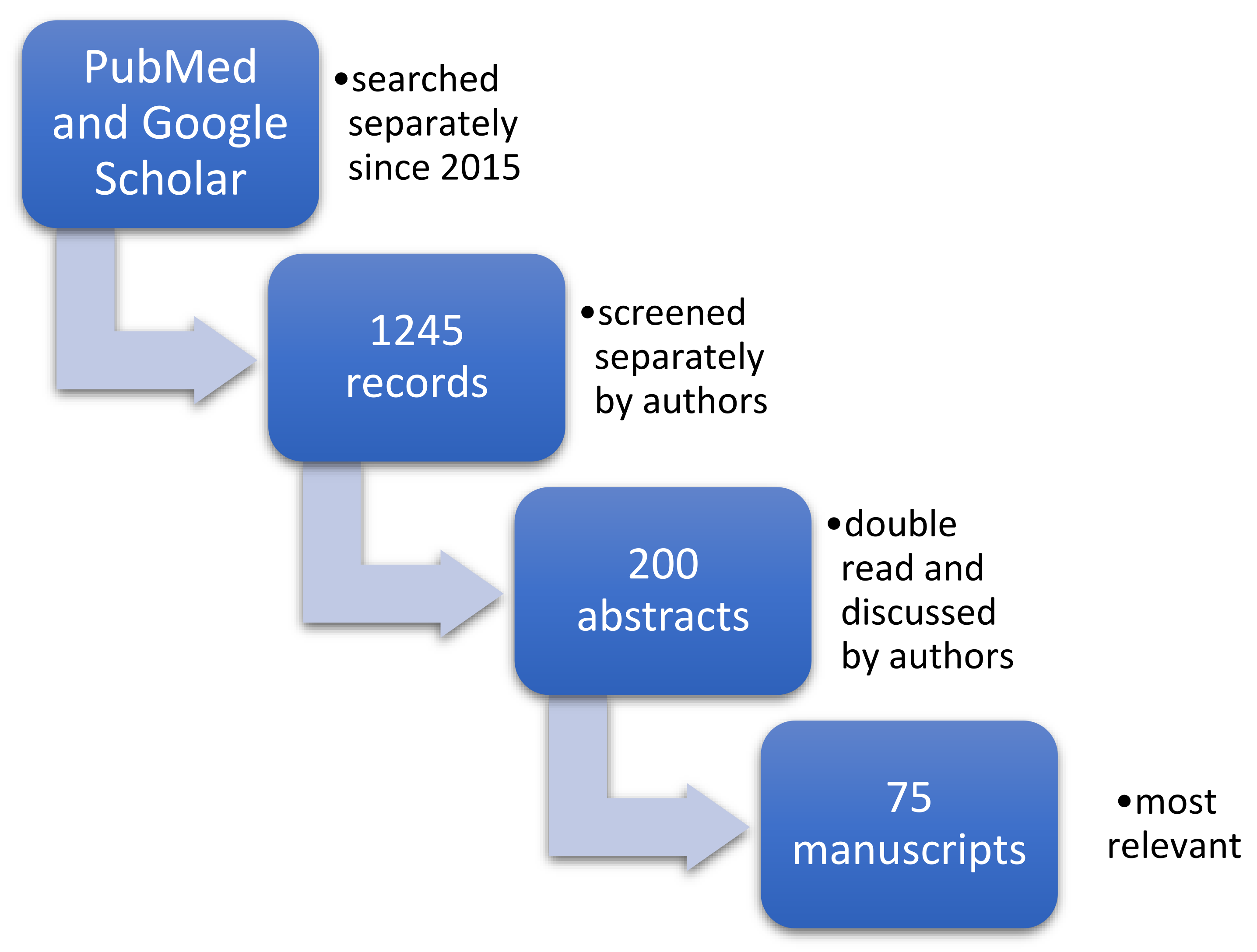
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction
2.3. Qualitive Analysis and Synthesis
3. Results
3.1. Nervous System Development, Embryology and Histology (Tobacco Impact on Formation and Differentiation of Neural Basic Structures)
Animal-Based Studies
3.2. Neurotransmitters and Tobacco—Receptors, Neurotransmitters, Signalling Pathways
Animal-Based Studies
3.3. Impact on Cognition, Higher-Order Brain Functions—Perception, Attention, Memory, (Nicotine Receptors in Hippocampus and Limbic Structure)
3.3.1. Impact on Cognition and Memory
Animal-Based Studies
3.3.2. Neurodegenerative Diseases
Animal-Based Studies
Human-Based Studies
3.3.3. Protective Influence of Nicotine on Dopaminergic System in Parkinson’s Disease
3.3.4. Insomnia and Affective Disorders
3.4. Impact of Tobacco on Other Neurovascular Diseases (Stroke, Aneurysm, Atherosclerosis), Migraine + Nicotinism—Significant Increase in Risk of Severe Strokes
3.5. Nicotine and Other Drugs and Stimulants—Additive Effect
Animal-Based Studies
3.6. Neuroradiological Examination
3.7. Grandmothers and Paternal Tobocco Smoke and Its Impact on Childen’s Health
4. Discussion
Strengths and Limitations
- The manuscript was prepared by a team not a single person.
- Record screening, evaluation of eligibility and data extraction were done by two people working independently at each stage and then synthesized.
- The review was based on independent assessments and the authors declared no conflict of interest.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025, 3rd ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Blair, W. An Obstruction of the Œsophagus Removed by a Tobacco Glyster, on the Third Day after the Accident. Lond. Med Phys. J. 1807, 17, 20. [Google Scholar]
- Campos, M.W.; Serebrisky, D.; Castaldelli-Maia, J.M. Smoking and Cognition. Curr. Drug Abus. Rev. 2016, 9, 76–79. [Google Scholar] [CrossRef]
- Alkam, T.; Nabeshima, T. Molecular mechanisms for nicotine intoxication. Neurochem. Int. 2019, 125, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Zhou, C.-Y.; Jiang, J.-H.; Yin, C. Neural circuits and nicotinic acetylcholine receptors mediate the cholinergic regulation of midbrain dopaminergic neurons and nicotine dependence. Acta Pharmacol. Sin. 2020, 41, 1–9. [Google Scholar] [CrossRef]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention USA: Atlanta, GA, USA, 2014. [Google Scholar]
- Talhout, R.; Schulz, T.; Florek, E.; Van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous Compounds in Tobacco Smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef]
- National Toxicology Program, U.S.; Department of Health and Human Services, Public Health Service. Report on Carcinogens, 14th Editon. 2016. Available online: https://ntp.niehs.nih.gov/go/roc14 (accessed on 3 June 2021).
- Jankowski, M.; Brożek, G.M.; Lawson, J.; Skoczyński, S.; Majek, P.; Zejda, J.E. New ideas, old problems? Heated tobacco products–A systematic review. Int. J. Occup. Med. Environ. Heal. 2019, 32, 595–634. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, S.C.; Zhu, J.; Qian, N. Analysis of Traces of Tobacco-Specific Nitrosamines (TSNAs) in USP Grade Nicotine, E-Liquids, and Particulate Phase Generated by the Electronic Smoking Devices. Contrib. Tob. Res. 2017, 27, 86–96. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control. 2013, 23, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, M.E.; Wang, T.W.; Jamal, A.; Loretan, C.G.; Neff, L.J. Tobacco Product Use Among Adults—United States, 2019. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Leading Cause of Death, Illness and Impoverishment. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 20 August 2020).
- Salminen, L.E.; Wilcox, R.R.; Zhu, A.H.; Riedel, B.C.; Ching, C.R.K.; Rashid, F.; Thomopoulos, S.I.; Saremi, A.; Harrison, M.B.; Ragothaman, A.; et al. Altered Cortical Brain Structure and Increased Risk for Disease Seen Decades After Perinatal Exposure to Maternal Smoking: A Study of 9000 Adults in the UK Biobank. Cereb. Cortex 2019, 29, 5217–5233. [Google Scholar] [CrossRef] [PubMed]
- Salihu, H.M.; Paothong, A.; Das, R.; King, L.M.; Pradhan, A.; Riggs, B.; Naik, E.; Siegel, E.M.; Whiteman, V.E. Evidence of altered brain regulatory gene expression in tobacco-exposed fetuses. J. Peérinat. Med. 2017, 45, 1045–1053. [Google Scholar] [CrossRef]
- Lewis, L.S.C.; Muldoon, P.P.; Pilaka, P.P.; Ottens, A.K. Frontal Cortex Proteome Perturbation after Juvenile Rat Secondhand Smoke Exposure. Proteomics 2018, 18, e1800268. [Google Scholar] [CrossRef] [PubMed]
- Dani, J.A. Neuronal Nicotinic Acetylcholine Receptor Structure and Function and Response to Nicotine. Int. Rev. Neurobiol. 2015, 124, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Ton, H.T.; Smart, A.E.; Aguilar, B.L.; Olson, T.T.; Kellar, K.J.; Ahern, G.P. Menthol Enhances the Desensitization of Human α3β4 Nicotinic Acetylcholine Receptors. Mol. Pharmacol. 2015, 88, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Subramaniyan, M.; Dani, J.A. Dopaminergic and cholinergic learning mechanisms in nicotine addiction. Ann. N. Y. Acad. Sci. 2015, 1349, 46–63. [Google Scholar] [CrossRef]
- Ashok, A.H.; Mizuno, Y.; Howes, O.D. Tobacco smoking and dopaminergic function in humans: A meta-analysis of molecular imaging studies. Psychopharmacology 2019, 236, 1119–1129. [Google Scholar] [CrossRef]
- Cao, J.; Nesil, T.; Wang, S.; Chang, S.L.; Tanseli, N. Expression profile of nicotinic acetylcholine receptor subunits in the brain of HIV-1 transgenic rats given chronic nicotine treatment. J. NeuroVirol. 2016, 22, 626–633. [Google Scholar] [CrossRef]
- Slotkin, T.A.; Skavicus, S.; Card, J.; Stadler, A.; Levin, E.D.; Seidler, F.J. Developmental Neurotoxicity of Tobacco Smoke Directed Toward Cholinergic and Serotonergic Systems: More Than Just Nicotine. Toxicol. Sci. 2015, 147, 178–189. [Google Scholar] [CrossRef][Green Version]
- Slotkin, T.A.; Stadler, A.; Skavicus, S.; Card, J.; Ruff, J.; Levin, E.D.; Seidler, F.J. Is There a Critical Period for the Developmental Neurotoxicity of Low-Level Tobacco Smoke Exposure? Toxicol. Sci. 2017, 155, 75–84. [Google Scholar] [CrossRef]
- Xia, H.; Du, X.; Yin, G.; Zhang, Y.; Li, X.; Cai, J.; Huang, X.; Ning, Y.; Soares, J.C.; Wu, F.; et al. Effects of smoking on cognition and BDNF levels in a male Chinese population: Relationship with BDNF Val66Met polymorphism. Sci. Rep. 2019, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Ha, M.; Hong, Y.-C.; Park, H.; Kim, Y.; Kim, E.-J.; Kim, Y.; Ha, E. Exposure to prenatal secondhand smoke and early neurodevelopment: Mothers and Children’s Environmental Health (MOCEH) study. Environ. Heal. 2019, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Shuffrey, L.C.; Myers, M.M.; Isler, J.R.; Lucchini, M.; Sania, A.; Pini, N.; Nugent, J.D.; Condon, C.; Ochoa, T.; Brink, L.; et al. Association Between Prenatal Exposure to Alcohol and Tobacco and Neonatal Brain Activity. JAMA Netw. Open 2020, 3, e204714. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Xu, Y.; Terrizzi, B.F.; Lanphear, B.; Chen, A.; Kalkbrenner, A.E.; Yolton, K. Associations Between Early Low-Level Tobacco Smoke Exposure and Executive Function at Age 8 Years. J. Pediatr. 2020, 221, 174–180. [Google Scholar] [CrossRef]
- Ringin, E.; Cropley, V.; Zalesky, A.; Bruggemann, J.; Sundram, S.; Weickert, C.S.; Weickert, T.W.; Bousman, C.A.; Pantelis, C.; Van Rheenen, T.E. The impact of smoking status on cognition and brain morphology in schizophrenia spectrum disorders. Psychol. Med. 2021, 14, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Valentine, G. Cognitive Effects of Nicotine: Recent Progress. Curr. Neuropharmacol. 2018, 16, 403–414. [Google Scholar] [CrossRef]
- Zandonai, T.; Pizzolato, F.; Tam, E.; Bruseghini, P.; Chiamulera, C.; Cesari, P. The Effects of Nicotine on Cortical Excitability After Exercise. J. Clin. Psychopharmacol. 2020, 40, 495–498. [Google Scholar] [CrossRef]
- Jawinski, P.; Mauche, N.; Ulke, C.; Huang, J.; Spada, J.; Enzenbach, C.; Sander, C.; Hegerl, U.; Hensch, T. Tobacco use is associated with reduced amplitude and intensity dependence of the cortical auditory evoked N1-P2 component. Psychopharmacology 2016, 233, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, T.M.; Fleming, K.A.; Trela, C.J.; Bartholow, B.D. P3 event-related potential reactivity to smoking cues: Relations with craving, tobacco dependence, and alcohol sensitivity in young adult smokers. Psychol. Addict. Behav. 2017, 31, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Csabai, D.; Cseko, K.; Szaiff, L.; Varga, Z.; Miseta, A.; Helyes, Z.; Czéh, B. Low intensity, long term exposure to tobacco smoke inhibits hippocampal neurogenesis in adult mice. Behav. Brain Res. 2016, 302, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawalha, N.A.; Alzoubi, K.H.; Khabour, O.F.; Alyacoub, W.; Almahmood, Y. Effect of waterpipe tobacco smoke exposure during lactation on learning and memory of offspring rats: Role of oxidative stress. Life Sci. 2019, 227, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, K.H.; Khabour, O.F.; Alharahshah, E.A.; Alhashimi, F.H.; Shihadeh, A.; Eissenberg, T. The Effect of Waterpipe Tobacco Smoke Exposure on Learning and Memory Functions in the Rat Model. J. Mol. Neurosci. 2015, 57, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, A.-L.; Pang, Y.-P.; Cheng, X.-J.; Xu, T.; Li, X.-R.; Liu, J.; Zhang, Y.-Y.; Liu, Y. Astaxanthin Attenuates Environmental Tobacco Smoke-Induced Cognitive Deficits: A Critical Role of p38 MAPK. Mar. Drugs 2019, 17, 24. [Google Scholar] [CrossRef]
- Deochand, C.; Tong, M.; Agarwal, A.R.; Cadenas, E.; De La Monte, S.M. Tobacco Smoke Exposure Impairs Brain Insulin/IGF Signaling: Potential Co-Factor Role in Neurodegeneration. J. Alzheimer Dis. 2015, 50, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Deochand, C.; Krotow, A.; Leão, R.; Tong, M.; Agarwal, A.R.; Cadenas, E.; De La Monte, S.M. Tobacco Smoke-Induced Brain White Matter Myelin Dysfunction: Potential Co-Factor Role of Smoking in Neurodegeneration. J. Alzheimer Dis. 2016, 50, 133–148. [Google Scholar] [CrossRef]
- Torres, L.H.; Annoni, R.; Balestrin, N.T.; Coleto, P.L.; Duro, S.O.; Garcia, R.C.T.; Pacheco-Neto, M.; Mauad, T.; Camarini, R.; Britto, L.R.G.; et al. Environmental tobacco smoke in the early postnatal period induces impairment in brain myelination. Arch. Toxicol. 2014, 89, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Li, W.; Cai, X.; Yan, H.; Chen, M. Associations of cigarette smoking with memory decline and neurodegeneration among cognitively normal older individuals. Neurosci. Lett. 2020, 714, 134563. [Google Scholar] [CrossRef]
- Chen, M.; Hu, C.; Dong, H.; Yan, H.; Wu, P.; Initiative, A.D.N. A history of cigarette smoking is associated with faster functional decline and reduction of entorhinal cortex volume in mild cognitive impairment. Aging 2021, 13, 6205–6213. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Zhao, L.; Matloff, W.; Sun, F.; Toga, A.W. Association of relative brain age with tobacco smoking, alcohol consumption, and genetic variants. Sci. Rep. 2020, 10, 10. [Google Scholar] [CrossRef]
- Lavery, A.M.; Collins, B.N.; Waldman, A.T.; Hart, C.N.; Bar-Or, A.; Marrie, R.A.; Arnold, D.; O’Mahony, J.; Banwell, B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult. Scler. J. 2018, 25, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, R.; Hedström, A.-K.; Manouchehrinia, A.; Alfredsson, L.; Olsson, T.; Bottai, M.; Hillert, J. Effect of Smoking Cessation on Multiple Sclerosis Prognosis. JAMA Neurol. 2015, 72, 1117–1123. [Google Scholar] [CrossRef]
- Hopfner, F.; Höglinger, G.; Kuhlenbäumer, G.; Pottegård, A.; Wod, M.; Christensen, K.; Tanner, C.M.; Deuschl, G. β-adrenoreceptors and the risk of Parkinson’s disease. Lancet Neurol. 2020, 19, 247–254. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.-J. Tobacco smoking and the reduced risk of Parkinson disease. Neurology 2020, 94, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Dorn, H.F. Tobacco Consumption and Mortality from Cancer and Other Diseases. Public Heal. Rep. 1959, 74, 581–593. [Google Scholar] [CrossRef]
- Hasler, B.P.; Kirisci, L.; Clark, D.B. Restless Sleep and Variable Sleep Timing During Late Childhood Accelerate the Onset of Alcohol and Other Drug Involvement. J. Stud. Alcohol Drugs 2016, 77, 649–655. [Google Scholar] [CrossRef]
- Pasman, J.A.; Smit, D.J.; Kingma, L.; Vink, J.M.; Treur, J.L.; Verweij, K.J. Causal relationships between substance use and insomnia. Drug Alcohol Depend. 2020, 214, 108151. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.; Munafò, M.R.; E Taylor, A.; Treur, J.L. Evidence for Genetic Correlations and Bidirectional, Causal Effects Between Smoking and Sleep Behaviors. Nicotine Tob. Res. 2019, 21, 731–738. [Google Scholar] [CrossRef]
- Purani, H.; Friedrichsen, S.; Allen, A.M. Sleep quality in cigarette smokers: Associations with smoking-related outcomes and exercise. Addict. Behav. 2019, 90, 71–76. [Google Scholar] [CrossRef]
- Kondo, T.; Nakano, Y.; Adachi, S.; Murohara, T. Effects of Tobacco Smoking on Cardiovascular Disease. Circ. J. 2019, 83, 1980–1985. [Google Scholar] [CrossRef]
- Ambrose, A.J.; Barua, R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004, 43, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Chiolero, A.; Faeh, D.; Paccaud, F.; Cornuz, J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am. J. Clin. Nutr. 2008, 87, 801–809. [Google Scholar] [CrossRef]
- Digiacomo, S.I.; Jazayeri, M.-A.; Barua, R.S.; Ambrose, J.A. Environmental Tobacco Smoke and Cardiovascular Disease. Int. J. Environ. Res. Public Heal. 2018, 16, 96. [Google Scholar] [CrossRef]
- Taylor, F.R. Tobacco, Nicotine, and Headache. Headache J. Head Face Pain 2015, 55, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.E.; Baca, R.; Doran, N.; Jacobson, A.; Liu, T.T.; Jacobus, J. The effects of nicotine and cannabis co-use during adolescence and young adulthood on white matter cerebral blood flow estimates. Psychopharmacology 2020, 237, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Kaag, A.; Schulte, M.; Jansen, J.; Van Wingen, G.; Homberg, J.; Brink, W.V.D.; Wiers, R.; Schmaal, L.; Goudriaan, A.; Reneman, L. The relation between gray matter volume and the use of alcohol, tobacco, cocaine and cannabis in male polysubstance users. Drug Alcohol Depend. 2018, 187, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, R.R.; Jagannathan, M.K.; Hager, B.N.; Childress, A.R.; Rao, H.; Franklin, T.R. Cannabis, Cigarettes, and Their Co-Occurring Use: Disentangling Differences in Gray Matter Volume. Int. J. Neuropsychopharmacol. 2015, 18, pyv061. [Google Scholar] [CrossRef]
- Schuetze, P.; Zhao, J.; Eiden, R.D.; Shisler, S.; Huestis, M.A. Prenatal exposure to tobacco and marijuana and child autonomic regulation and reactivity: An analysis of indirect pathways via maternal psychopathology and parenting. Dev. Psychobiol. 2019, 61, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- El Marroun, H.; Tiemeier, H.; Franken, I.H.; Jaddoe, V.W.; van der Lugt, A.; Verhulst, F.C.; Lahey, B.B.; White, T. Prenatal Cannabis and Tobacco Exposure in Relation to Brain Morphology: A Prospective Neuroimaging Study in Young Children. Biol. Psychiatry 2016, 79, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Mejia, M.H.; Wade, N.E.; Baca, R.; Diaz, V.G.; Jacobus, J. The Influence of Cannabis and Nicotine Co-use on Neuromaturation: A Systematic Review of Adolescent and Young Adult Studies. Biol. Psychiatry 2021, 89, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Quinteiros, D.A.; Hansen, A.W.; Bellaver, B.; Bobermin, L.D.; Pulcinelli, R.R.; Bandiera, S.; Caletti, G.; Bitencourt, P.E.R.; Quincozes-Santos, A.; Gomez, R. Combined Exposure to Alcohol and Tobacco Smoke Changes Oxidative, Inflammatory, and Neurotrophic Parameters in Different Areas of the Brains of Rats. ACS Chem. Neurosci. 2019, 10, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Bandiera, S.; Caletti, G.; Giustina, C.L.; Hansen, A.W.; Deniz, B.F.; Confortim, H.D.; Pulcinelli, R.R.; Nin, M.S.; Silva, L.O.; Gomez, R. Changes in behavioral and neuronal parameters by alcohol, cigarette, or their combined use in rats. Behav. Pharmacol. 2019, 30, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Vňuková, M.; Ptáček, R.; Raboch, J.; Stefano, G.B. Decreased Central Nervous System Grey Matter Volume (GMV) in Smokers Affects Cognitive Abilities: A Systematic Review. Med Sci. Monit. 2017, 23, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, P.; Shen, Z.; Qian, W.; Li, K.; Luo, X.; Zeng, Q.; Guo, T.; Yu, H.; Yang, Y.; et al. Gray matter volumes of insular subregions are not correlated with smoking cessation outcomes but negatively correlated with nicotine dependence severity in chronic smokers. Neurosci. Lett. 2019, 696, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Shi, H.; Shen, Y.; Dai, Z.; Zhu, Y.; Ma, H.; Sheng, L. Voxelwise meta-analysis of gray matter anomalies in chronic cigarette smokers. Behav. Brain Res. 2016, 311, 39–45. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Y.; Cheng, J.; Wang, W.; Wen, M. Evaluating the Changes of White Matter Microstructures in Tobacco Addicts Based on Diffusion Tensor Imaging. Med Sci. Monit. 2020, 26, e919105. [Google Scholar] [CrossRef]
- Liberman, K.; Van Schuerbeek, P.; Herremans, S.; Meysman, M.; De Mey, J.; Buls, N. The effect of nicotine patches on craving in the brain. Medicine 2018, 97, e12415. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Suderman, M.; Guggenheim, J.A.; Ellis, G.; Gregory, S.; Iles-Caven, Y.; Northstone, K.; Golding, J.; Pembrey, M. Grandmothers’ smoking in pregnancy is associated with a reduced prevalence of early-onset myopia. Sci. Rep. 2019, 9, 15413. [Google Scholar] [CrossRef]
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdóttir, B.; Bertelsen, R.; Bråbäck, L.; Carsin, A.-E.; Dharmage, S.C.; Dratva, J.; et al. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018, 47, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Magnus, M.C.; Håberg, S.E.; Øystein, K.; Nafstad, P.; London, S.J.; Nystad, W. Grandmother’s smoking when pregnant with the mother and asthma in the grandchild: The Norwegian Mother and Child Cohort Study. Thorax 2015, 70, 237–243. [Google Scholar] [CrossRef]
- Wu, C.-C.; Hsu, T.-Y.; Chang, J.-C.; Ou, C.-Y.; Kuo, H.-C.; Liu, C.-A.; Wang, C.-L.; Chuang, H.; Chen, C.-P.; Yang, K.D. Paternal Tobacco Smoke Correlated to Offspring Asthma and Prenatal Epigenetic Programming. Front. Genet. 2019, 10, 471. [Google Scholar] [CrossRef]
- Cochrane Training. Available online: https://training.cochrane.org/handbook/current/chapter-01 (accessed on 3 June 2021).
| Subsection | Human Based | Animal Based |
|---|---|---|
| 3.1 NS development | Perinatal: Maternal smoking impairs brain development and sensory cortices | Postnatal: 800 proteins of prefrontal cortex responses to tobacco |
| 3.2 Neurotransmitters | Lowered transporter for dopamine and unaffected D2 receptor in smokers | HIV-1 proteins may commit to susceptibility for nicotine and smoking; tobacco has more adverse effect than pure nicotine |
| 3.3 cognition and brain functions | Perinatal: Smoking impairs infant postnatal development; Postnatal: Chronic smoking impairs memory; impact to cognition depends on prior smoking history; ETS contributes to Alzheimer disease and multiple sclerosis and sleep disorders; Tobacco alleviate Parkinson’s disease symptoms | Postnatal: Smoking during lactations impairs offspring memory; ETS impairs myelination of NS; Tobacco causes reduction in antioxidant enzymes |
| 3.4 Neurovascular diseases | Risk depends on number of cigarettes smoked but its not linear; tobacco increases oxidative stress | - |
| 3.5 Tobacco and stimulants | Mixing impairs emotional and physical functioning, reduce grey matter volume (GMV); | Mixing contributes to oxidative stress in hippocampus and decreases brain-derived neurotrophic factor |
| 3.6 Neuroradiology | Postnatal: Smoking reduces GMV; nicotine contributes to destruction of white matter and neurons; nicotine deprivation is associates with lower activity in attention brain areas; Perinatal: effect on sensory brain structures | - |
| 3.7 Paternal and grand-maternal smoking | Perinatal: Grandmothers and paternal smoking associated with offspring’s asthma | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajdusianek, W.; Żórawik, A.; Waliszewska-Prosół, M.; Poręba, R.; Gać, P. Tobacco and Nervous System Development and Function—New Findings 2015–2020. Brain Sci. 2021, 11, 797. https://doi.org/10.3390/brainsci11060797
Hajdusianek W, Żórawik A, Waliszewska-Prosół M, Poręba R, Gać P. Tobacco and Nervous System Development and Function—New Findings 2015–2020. Brain Sciences. 2021; 11(6):797. https://doi.org/10.3390/brainsci11060797
Chicago/Turabian StyleHajdusianek, Wojciech, Aleksandra Żórawik, Marta Waliszewska-Prosół, Rafał Poręba, and Paweł Gać. 2021. "Tobacco and Nervous System Development and Function—New Findings 2015–2020" Brain Sciences 11, no. 6: 797. https://doi.org/10.3390/brainsci11060797
APA StyleHajdusianek, W., Żórawik, A., Waliszewska-Prosół, M., Poręba, R., & Gać, P. (2021). Tobacco and Nervous System Development and Function—New Findings 2015–2020. Brain Sciences, 11(6), 797. https://doi.org/10.3390/brainsci11060797







