Relaying Aversive Ultrasonic Alarm Calls Depends on Previous Experience. Empathy, Social Buffering, or Panic?
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
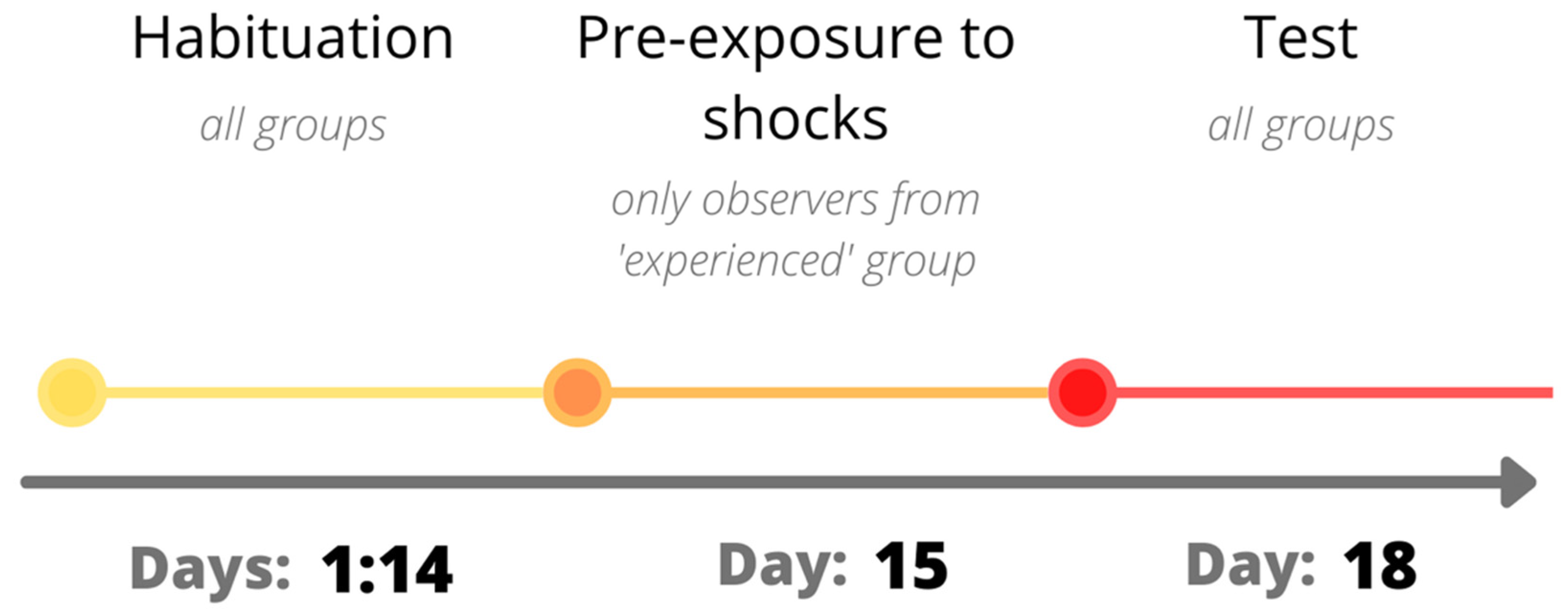
2.2. Experimental Procedure
2.2.1. Habituation
- Naive—4 demonstrators and 4 observers;
- Experienced—4 demonstrators and 4 observers;
- Control—4 animals, undivided into demonstrators and observers.
2.2.2. Pre-Exposure to Shocks
- The exposure was performed in a different behavioral room than the following test;
- The cage was illuminated with a bright, white light;
- The interior was sprayed with 1% acetic acid which left a strong smell;
- A plastic rooftop was installed to obtain a triangular shape for different spatial cues.
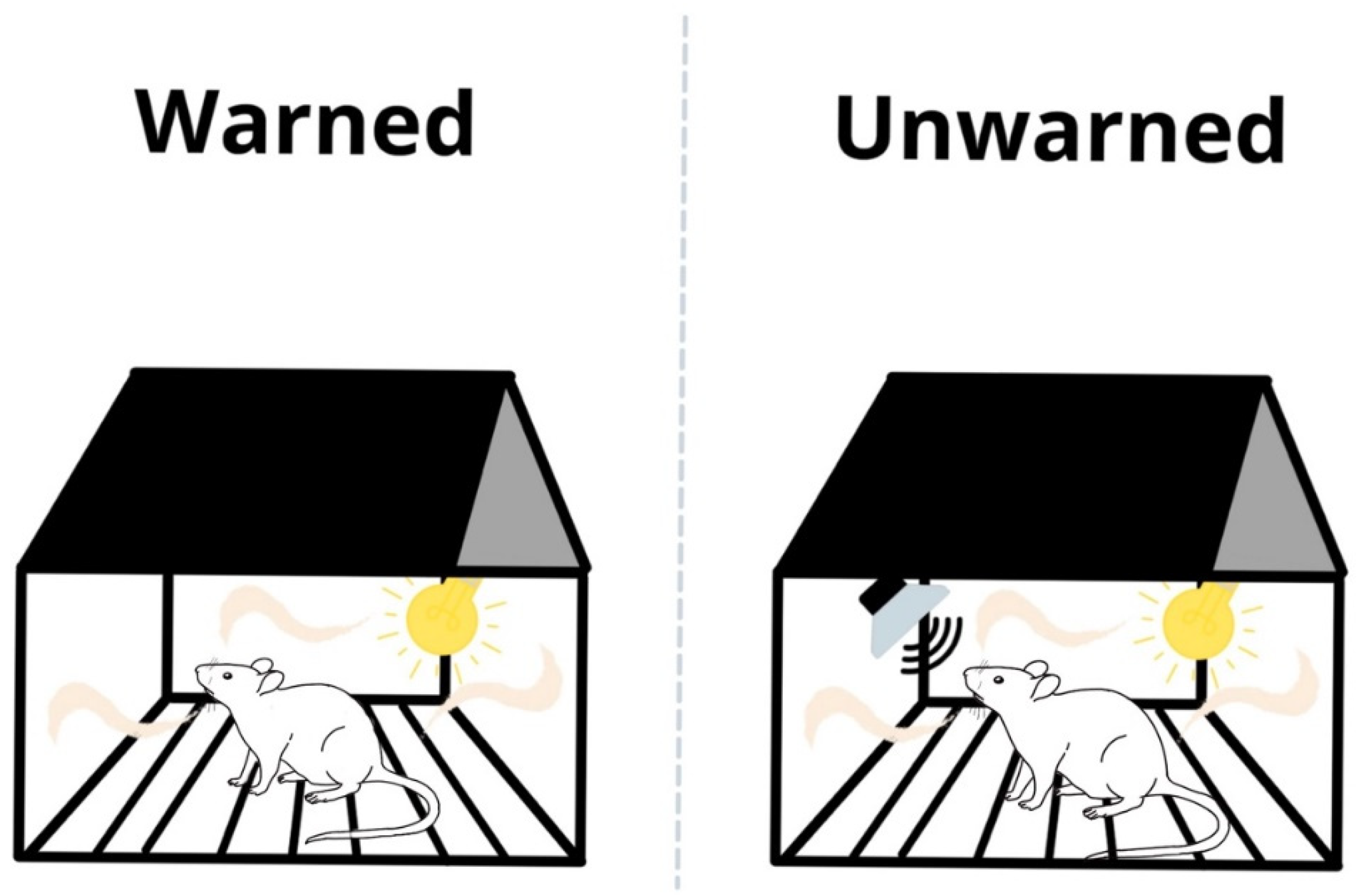
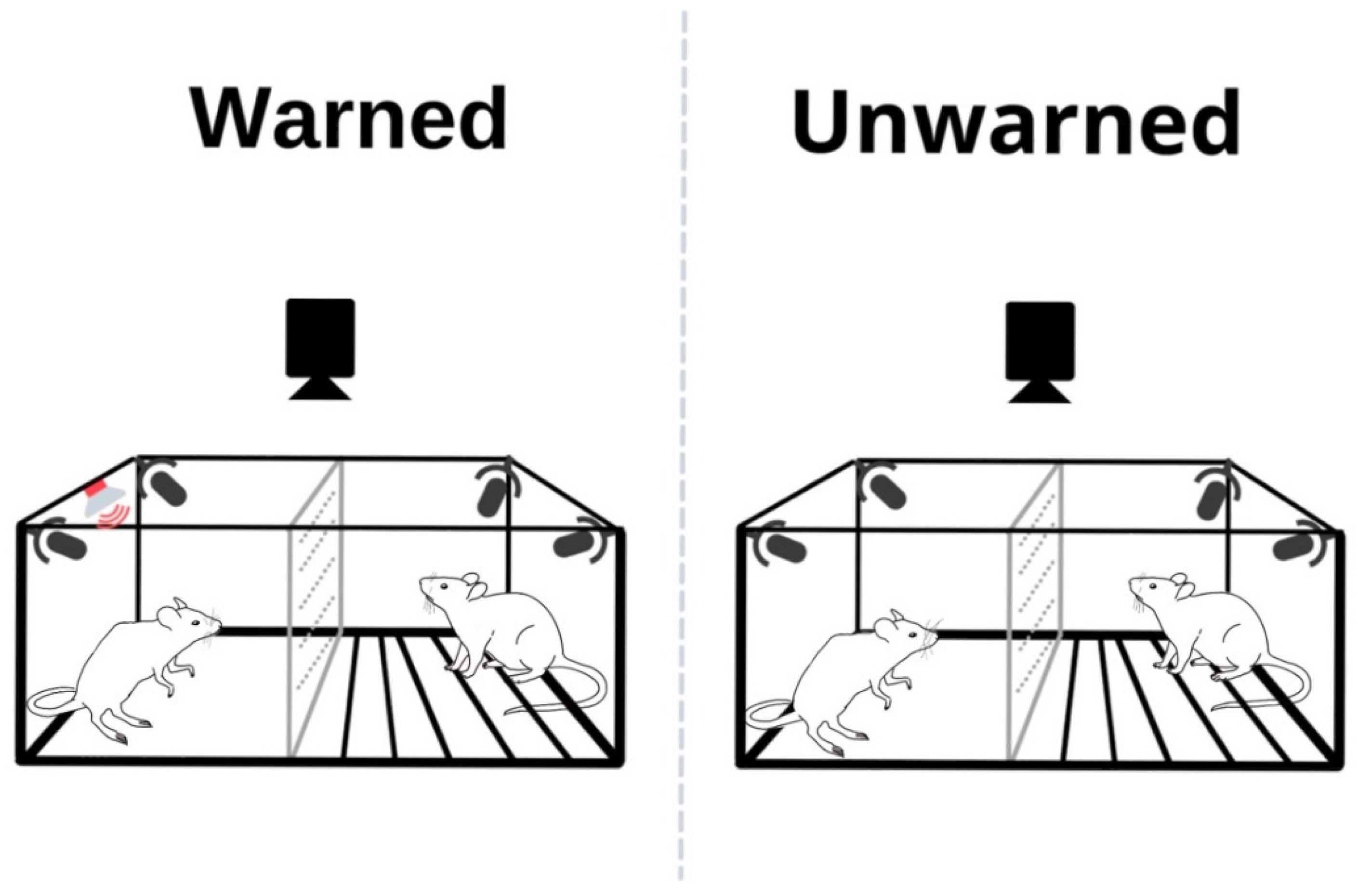
2.2.3. Test Day
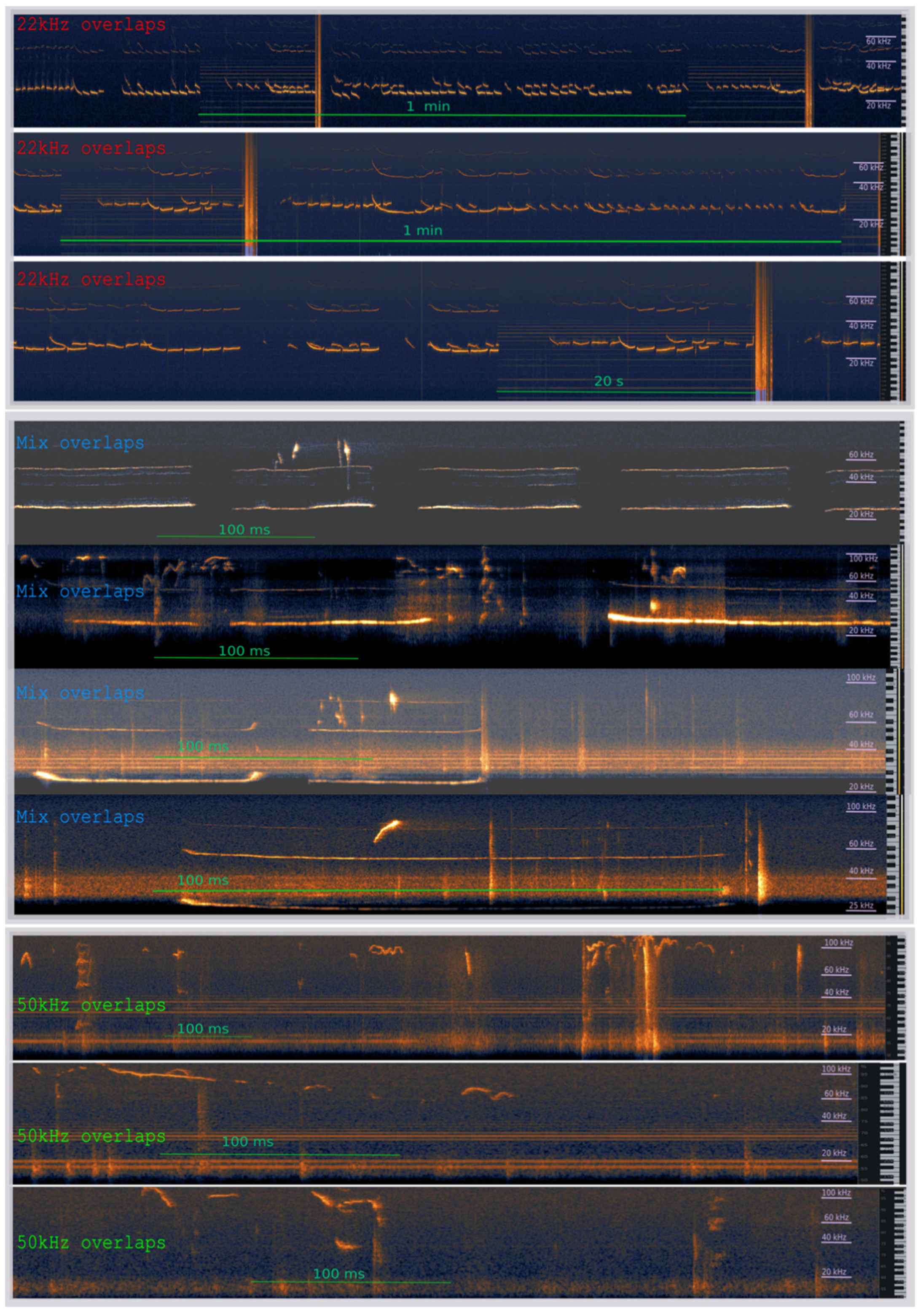
2.3. Apparatus and USV Recordings
2.4. Statistics
3. Results
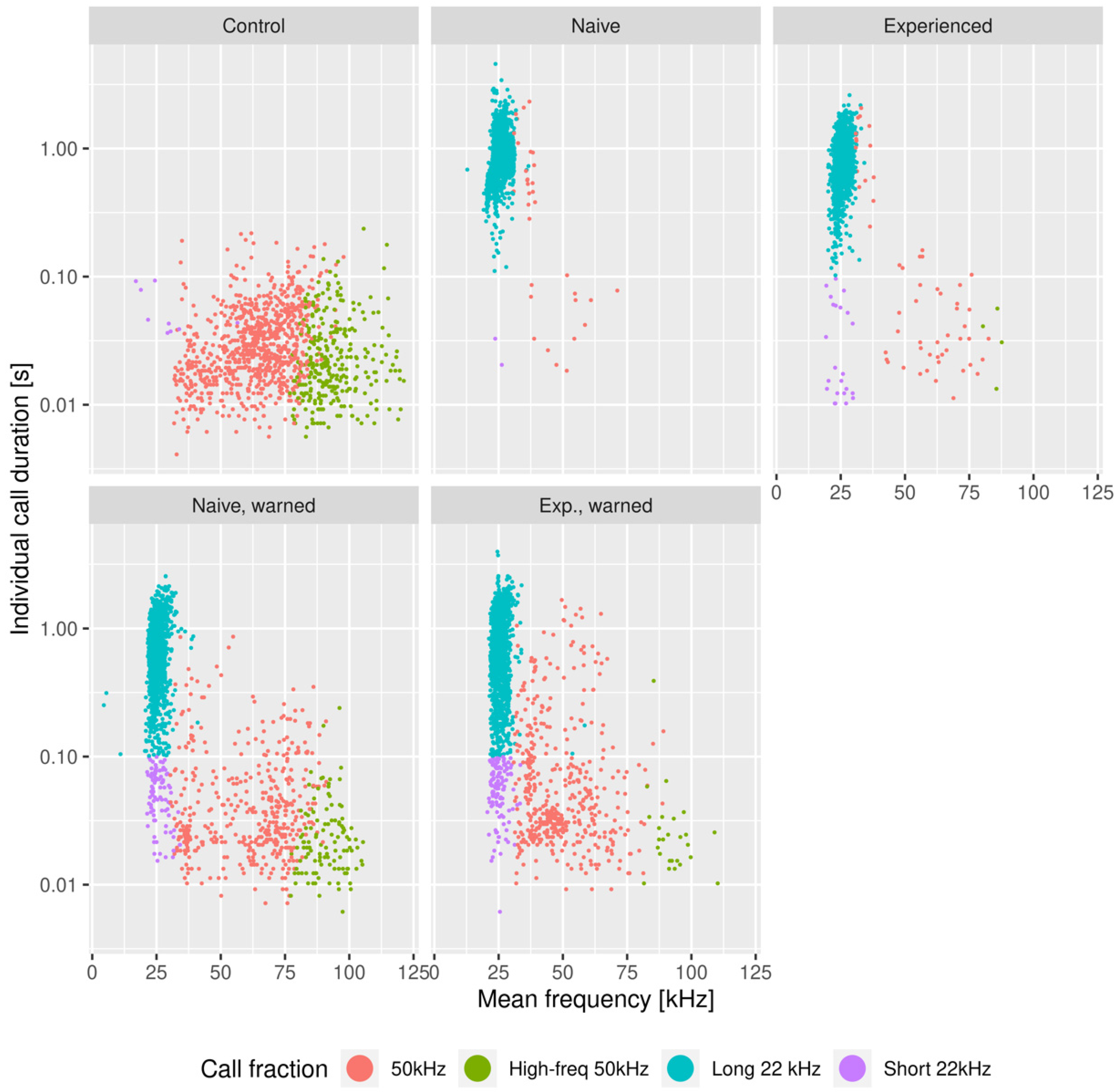
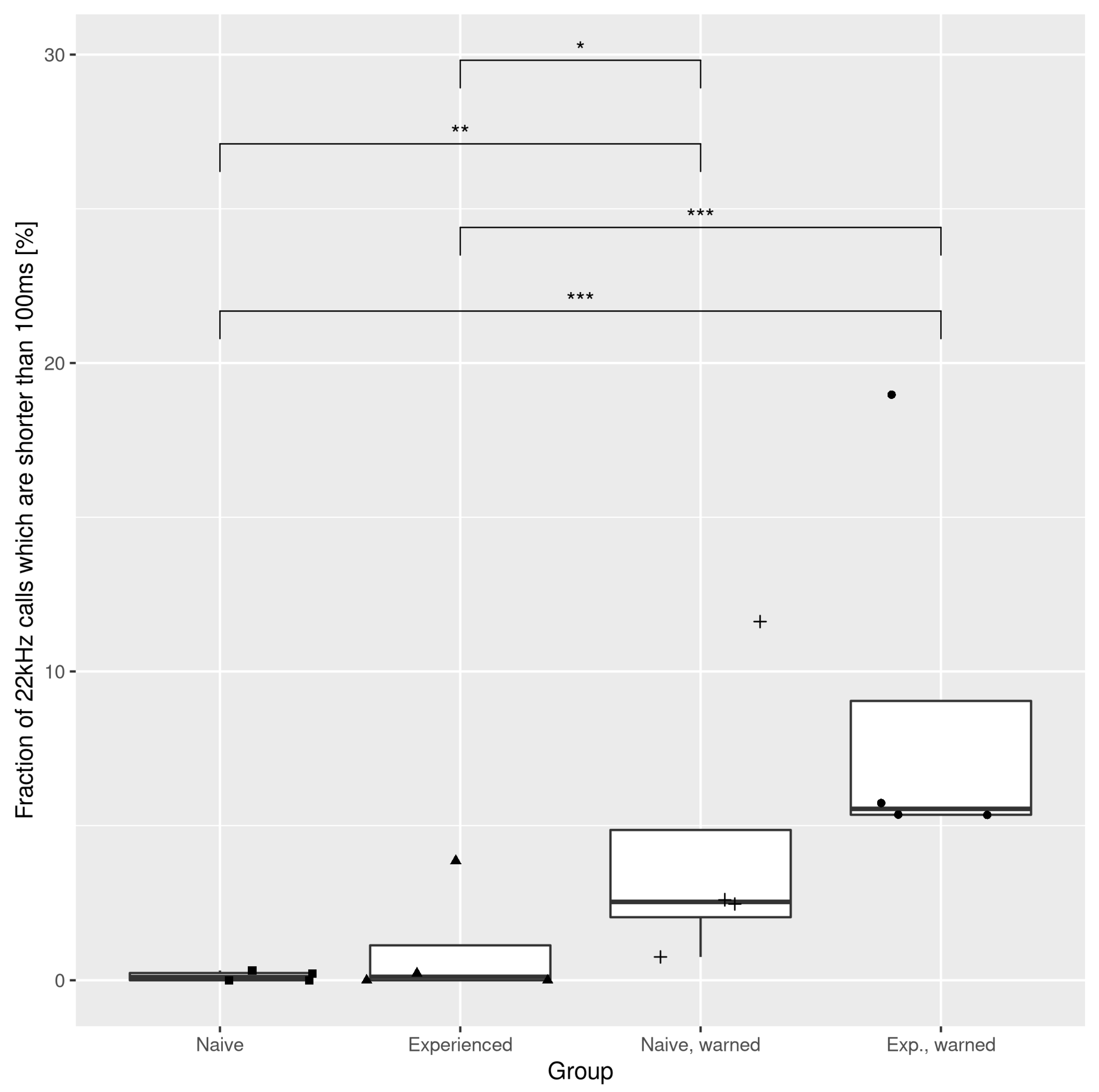
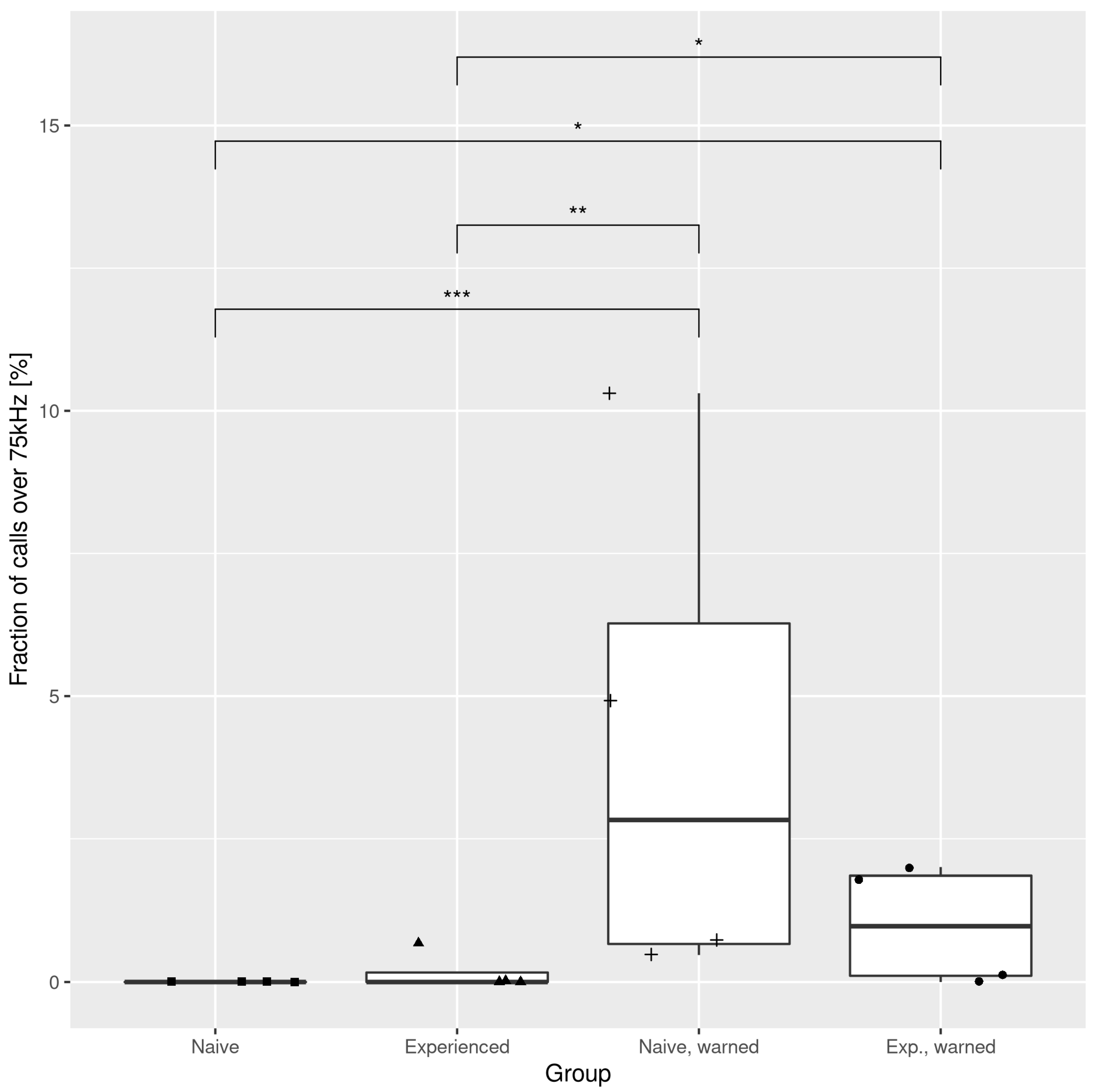
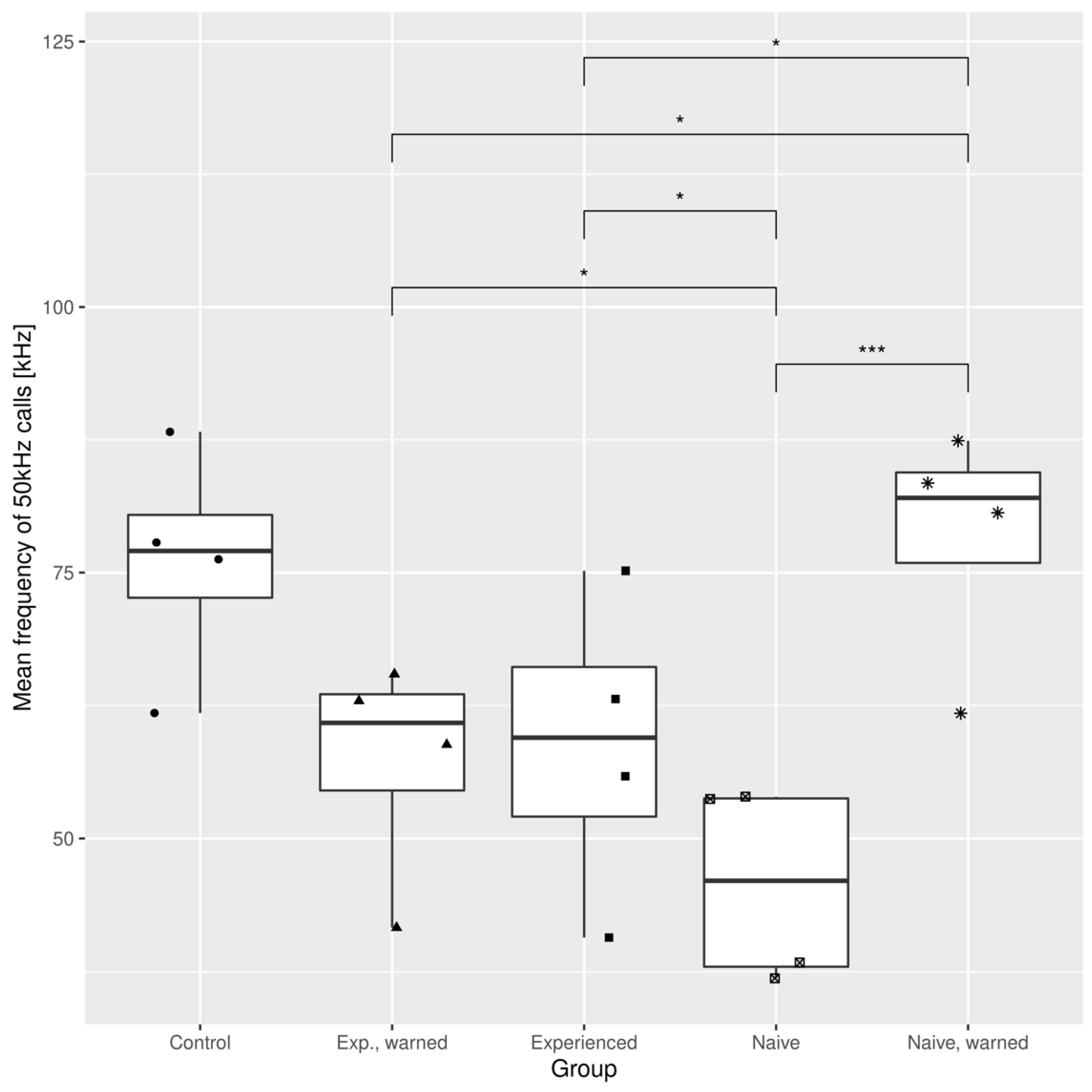
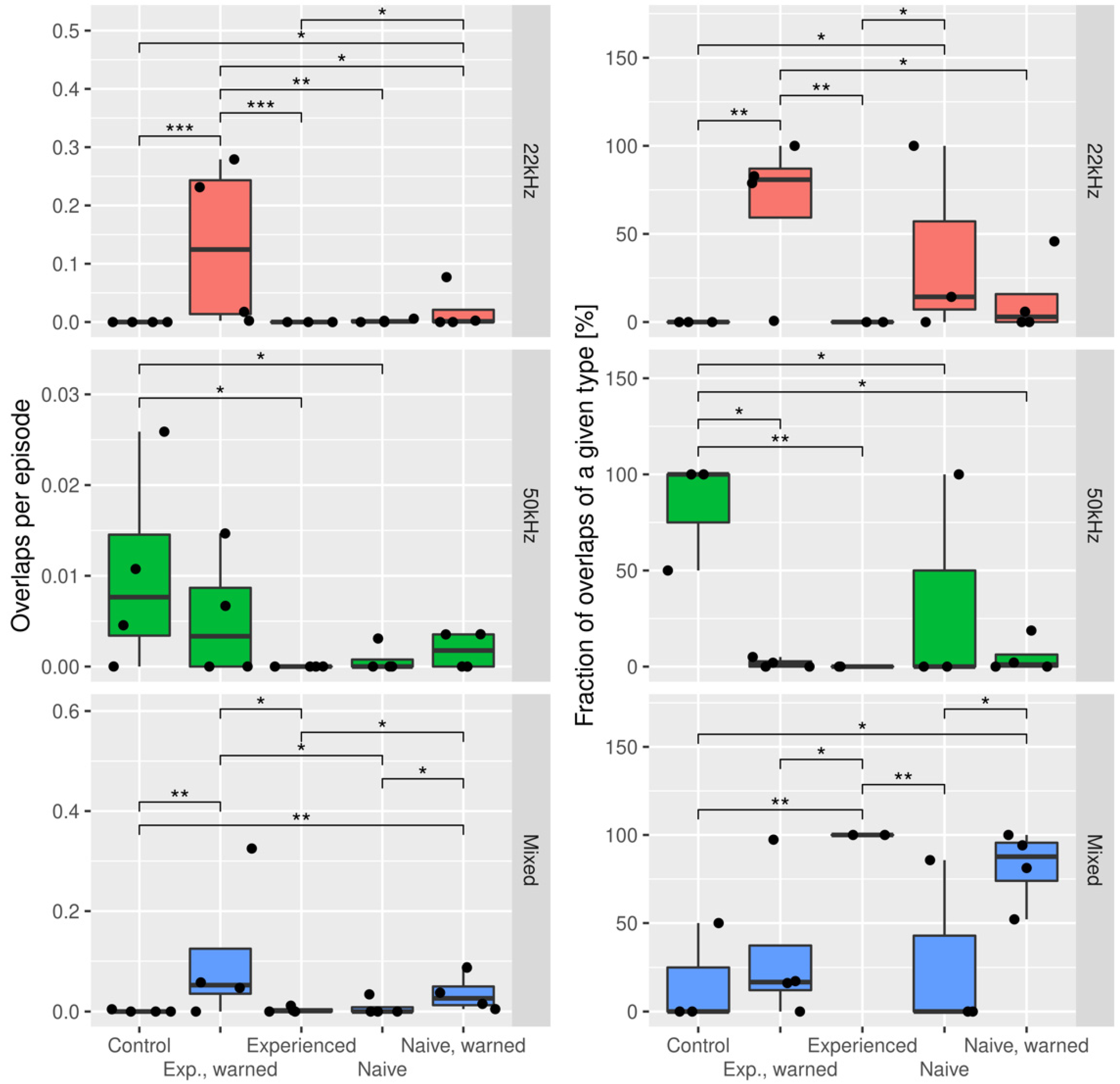
3.1. The Effects of the Warning Signal. Fractions of the Ultrasonic Vocalizations during Social Communication in the Social Transfer of Fear Paradigm
3.2. The Effects of the Warning Signal. Overlapping of the Ultrasonic Vocalizations during Social Communication in the Social Transfer of the Fear Paradigm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brudzynski, S.M. Ethotransmission: Communication of Emotional States through Ultrasonic Vocalization in Rats. Curr. Opin. Neurobiol. 2013, 23, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J.; Burgdorf, J. “Laughing” Rats and the Evolutionary Antecedents of Human Joy? Physiol. Behav. 2003, 79, 533–547. [Google Scholar] [CrossRef]
- Knutson, B.; Burgdorf, J.; Panksepp, J. Ultrasonic Vocalizations as Indices of Affective States in Rats. Psychol. Bull. 2002, 128, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Taracha, E.; Szyndler, J.; Krząścik, P.; Lehner, M.; Maciejak, P.; Skórzewska, A.; Płaźnik, A. The Effects of Morphine and Morphine Conditioned Context on 50kHz Ultrasonic Vocalisation in Rats. Behav. Brain Res. 2012, 229, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Simola, N.; Fenu, S.; Costa, G.; Pinna, A.; Plumitallo, A.; Morelli, M. Pharmacological Characterization of 50-kHz Ultrasonic Vocalizations in Rats: Comparison of the Effects of Different Psychoactive Drugs and Relevance in Drug-Induced Reward. Neuropharmacology 2012, 63, 224–234. [Google Scholar] [CrossRef]
- Kuchniak, K.; Wyszogrodzka, E.; Chrapusta, S.J.; Czarna, M.; Michalak, M.; Płaźnik, A.; Krząścik, P.; Mierzejewski, P.; Taracha, E. Using Anticipatory and Drug-Evoked Appetitive Ultrasonic Vocalization for Monitoring the Rewarding Effect of Amphetamine in a Rat Model of Drug Self-Administration. Behav. Brain Res. 2019, 376, 112187. [Google Scholar] [CrossRef]
- Costa, G.; Serra, M.; Marongiu, J.; Morelli, M.; Simola, N. Influence of Dopamine Transmission in the Medial Prefrontal Cortex and Dorsal Striatum on the Emission of 50-kHz Ultrasonic Vocalizations in Rats Treated with Amphetamine: Effects on Drug-Stimulated and Conditioned Calls. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 79, 109797. [Google Scholar] [CrossRef]
- Simola, N.; Serra, M.; Marongiu, J.; Costa, G.; Morelli, M. Increased Emissions of 50-KHz Ultrasonic Vocalizations in Hemiparkinsonian Rats Repeatedly Treated with Dopaminomimetic Drugs: A Potential Preclinical Model for Studying the Affective Properties of Dopamine Replacement Therapy in Parkinson’s Disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110184. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, K.G.; Brudzynski, S.M. Non-Pharmacological Induction of Rat 50 kHz Ultrasonic Vocalization: Social and Non-Social Contexts Differentially Induce 50 kHz Call Subtypes. Physiol. Behav. 2018, 196, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Szyndler, J.; Taracha, E.; Turzyńska, D.; Sobolewska, A.; Lehner, M.; Krząścik, P.; Daszczuk, P. κ-Opioid Receptor as a Key Mediator in the Regulation of Appetitive 50-kHz Ultrasonic Vocalizations. Psychopharmacology 2015, 232, 1941–1955. [Google Scholar] [CrossRef]
- Hamed, A.; Jaroszewski, T.; Maciejak, P.; Szyndler, J.; Lehner, M.; Kamecka, I.; Olczak, M.; Kuzinska, U.; Taracha, E.; Płaźnik, A. The Effects of Buspirone and Diazepam on Aversive Context- and Social Isolation-Induced Ultrasonic Vocalisation. Physiol. Behav. 2009, 98, 474–480. [Google Scholar] [CrossRef]
- Burgdorf, J.S.; Brudzynski, S.M.; Moskal, J.R. Using Rat Ultrasonic Vocalization to Study the Neurobiology of Emotion: From Basic Science to the Development of Novel Therapeutics for Affective Disorders. Curr. Opin. Neurobiol. 2020, 60, 192–200. [Google Scholar] [CrossRef]
- Lefebvre, E.; Granon, S.; Chauveau, F. Social Context Increases Ultrasonic Vocalizations during Restraint in Adult Mice. Anim. Cogn. 2020, 23, 351–359. [Google Scholar] [CrossRef]
- Brudzynski, S.M.; Pniak, A. Social Contacts and Production of 50-kHz Short Ultrasonic Calls in Adult Rats. J. Comp. Psychol. 2002, 116, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Wöhr, M. Ultrasonic Communication in Rats: Appetitive 50-kHz Ultrasonic Vocalizations as Social Contact Calls. Behav. Ecol. Sociobiol. 2017, 72. [Google Scholar] [CrossRef]
- Burgdorf, J.; Knutson, B.; Panksepp, J. Anticipation of Rewarding Electrical Brain Stimulation Evokes Ultrasonic Vocalization in Rats. Behav. Neurosci. 2000, 114, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Brenes, J.C.; Schwarting, R.K.W. Individual Differences in Anticipatory Activity to Food Rewards Predict Cue-Induced Appetitive 50-kHz Calls in Rats. Physiol. Behav. 2015, 149, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Kursa, M.B. Inter-Individual Differences in Serotonin and Glutamate Co-Transmission Reflect Differentiation in Context-Induced Conditioned 50-kHz USVs Response after Morphine Withdrawal. Brain Struct. Funct. 2018, 223, 3149–3167. [Google Scholar] [CrossRef]
- Meyer, P.J.; Ma, S.T.; Robinson, T.E. A Cocaine Cue Is More Preferred and Evokes More Frequency-Modulated 50-kHz Ultrasonic Vocalizations in Rats Prone to Attribute Incentive Salience to a Food Cue. Psychopharmacology 2012, 219, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.J.; Ma, S.T.; Robinson, T.E. Quantifying Individual Variation in the Propensity to Attribute Incentive Salience to Reward Cues. Psychopharmacology 2012, 7, 338987. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Ociepa, D. Ultrasonic Vocalization of Laboratory Rats in Response to Handling and Touch. Physiol. Behav. 1992, 52, 655–660. [Google Scholar] [CrossRef]
- Takahashi, L.K.; Thomas, D.A.; Barfield, R.J. Analysis of Ultrasonic Vocalizations Emitted by Residents during Aggressive Encounters among Rats (Rattus norvegicus). J. Comp. Psychol. 1983, 97, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Sales, G.D. Ultrasound and Aggressive Behaviour in Rats and Other Small Mammals. Anim. Behav. 1972, 20, 88–100. [Google Scholar] [CrossRef]
- Panksepp, J. Toward a General Psychobiological Theory of Emotions. Behav. Brain Sci. 1982, 5, 407–422. [Google Scholar] [CrossRef]
- Portavella, M.; Depaulis, A.; Vergnes, M. 22–28 kHz Ultrasonic Vocalizations Associated with Defensive Reactions in Male Rats Do Not Result from Fear or Aversion. Psychopharmacology 1993, 111, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S.M.; Bihari, F.; Ociepa, D.; Fu, X.W. Analysis of 22 kHz Ultrasonic Vocalization in Laboratory Rats: Long and Short Calls. Physiol. Behav. 1993, 54, 215–221. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, E.S.; Covey, E.; Kim, J.J. Social Transmission of Fear in Rats: The Role of 22-kHz Ultrasonic Distress Vocalization. PLoS ONE 2010, 5, e15077. [Google Scholar] [CrossRef]
- Sewell, G.D. Ultrasound in Adult Rodents. Nature 1967, 215, 512. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Blanchard, D.C.; Agullana, R.; Weiss, S.M. Twenty-Two kHz Alarm Cries to Presentation of a Predator, by Laboratory Rats Living in Visible Burrow Systems. Physiol. Behav. 1991, 50, 967–972. [Google Scholar] [CrossRef]
- Fendt, M.; Brosch, M.; Wernecke, K.E.A.; Willadsen, M.; Wöhr, M. Predator Odour but Not TMT Induces 22-kHz Ultrasonic Vocalizations in Rats That Lead to Defensive Behaviours in Conspecifics upon Replay. Sci. Rep. 2018, 8, 11041. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Agullana, R.; McGee, L.; Weiss, S.; Blanchard, D.C. Sex Differences in the Incidence and Sonographic Characteristics of Antipredator Ultrasonic Cries in the Laboratory Rat (Rattus norvegicus). J. Comp. Psychol. 1992, 106, 270–277. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Blanchard, D.C. Defensive Reactions in the Albino Rat. Learn. Motiv. 1971, 2, 351–362. [Google Scholar] [CrossRef]
- Sales, G.; Pye, D. Ultrasonic Communication by Animals; Springer: Berlin/Heidelberg, Germany, 1974. [Google Scholar] [CrossRef]
- Fu, X.W.; Brudzynski, S.M. High-Frequency Ultrasonic Vocalization Induced by Intracerebral Glutamate in Rats. Pharmacol. Biochem. Behav. 1994, 49, 835–841. [Google Scholar] [CrossRef]
- Van der Poel, A.M.; Miczek, K.A. Long Ultrasonic Calls in Male Rats Following Mating, Defeat and Aversive Stimulation: Frequency Modulation and Bout Structure. Behaviour 1991, 119, 127–142. [Google Scholar] [CrossRef]
- De Vry, J.; Benz, U.; Schreiber, R.; Traber, J. Shock-Induced Ultrasonic Vocalization in Young Adult Rats: A Model for Testing Putative Anti-Anxiety Drugs. Eur. J. Pharmacol. 1993, 249, 331–339. [Google Scholar] [CrossRef]
- Kaltwasser, M.T. Acoustic Startle Induced Ultrasonic Vocalization in the Rat: A Novel Animal Model of Anxiety? Behav. Brain Res. 1991, 43, 133–137. [Google Scholar] [CrossRef]
- Knapp, D.J.; Pohorecky, L.A. An Air-Puff Stimulus Method for Elicitation of Ultrasonic Vocalizations in Rats. J. Neurosci. Methods 1995, 62, 1–5. [Google Scholar] [CrossRef]
- Brudzynski, S.M.; Chiu, E.M.C. Behavioural Responses of Laboratory Rats to Playback of 22 kHz Ultrasonic Calls. Physiol. Behav. 1995, 57, 1039–1044. [Google Scholar] [CrossRef]
- Burgdorf, J.; Kroes, R.A.; Moskal, J.R.; Pfaus, J.G.; Brudzynski, S.M.; Panksepp, J. Ultrasonic Vocalizations of Rats (Rattus norvegicus) During Mating, Play, and Aggression: Behavioral Concomitants, Relationship to Reward, and Self-Administration of Playback. J. Comp. Psychol. 2008, 122, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Sadananda, M.; Wöhr, M.; Schwarting, R.K.W. Playback of 22-kHz and 50-kHz Ultrasonic Vocalizations Induces Differential c-Fos Expression in Rat Brain. Neurosci. Lett. 2008, 435, 17–23. [Google Scholar] [CrossRef]
- Panksepp, J.; Burgdorf, J.; Beinfeld, M.C.; Kroes, R.A.; Moskal, J.R. Regional Brain Cholecystokinin Changes as a Function of Friendly and Aggressive Social Interactions in Rats. Brain Res. 2004, 1025, 75–84. [Google Scholar] [CrossRef]
- Litvin, Y.; Blanchard, D.C.; Blanchard, R.J. Rat 22 kHz Ultrasonic Vocalizations as Alarm Cries. Behav. Brain Res. 2007, 182, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Mori, Y. The Emission of Stress-Induced 22-kHz Calls in Female Rats Is Independent of Testosterone Levels. Horm. Behav. 2015, 69, 116–118. [Google Scholar] [CrossRef]
- Brudzynski, S.M.; Holland, G. Acoustic Characteristics of Air Puff-Induced 22-kHz Alarm Calls in Direct Recordings. Neurosci. Biobehav. Rev. 2005, 29, 1169–1180. [Google Scholar] [CrossRef]
- Brudzynski, S.M.; Barnabi, F. Contribution of the Ascending Cholinergic Pathways in the Production of Ultrasonic Vocalization in the Rat. Behav. Brain Res. 1996, 80, 145–152. [Google Scholar] [CrossRef]
- Schwarting, R.K.W.; Wöhr, M. On the Relationships between Ultrasonic Calling and Anxiety-Related Behavior in Rats. Braz. J. Med. Biol. Res. 2012, 45, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.J.G.; Maier, E.; Brecht, M. Involvement of Rat Posterior Prelimbic and Cingulate Area 2 in Vocalization Control. Eur. J. Neurosci. 2019, 50, 3164–3180. [Google Scholar] [CrossRef]
- Dupin, M.; Garcia, S.; Boulanger-Bertolus, J.; Buonviso, N.; Mouly, A.M. New Insights from 22-kHz Ultrasonic Vocalizations to Characterize Fear Responses: Relationship with Respiration and Brain Oscillatory Dynamics. eNeuro 2019, 6. [Google Scholar] [CrossRef]
- Kondrakiewicz, K.; Rokosz-Andraka, K.; Nikolaev, T.; Górkiewicz, T.; Danielewski, K.; Gruszczyńska, A.; Meyza, K.; Knapska, E. Social Transfer of Fear in Rodents. Curr. Protoc. Neurosci. 2019, 90, e85. [Google Scholar] [CrossRef] [PubMed]
- Andraka, K.; Kondrakiewicz, K.; Rojek-Sito, K.; Ziegart-Sadowska, K.; Meyza, K.; Nikolaev, T.; Hamed, A.; Kursa, M.; Wójcik, M.; Danielewski, K.; et al. Distinct Circuits in Rat Central Amygdala for Defensive Behaviors Evoked by Socially Signaled Imminent versus Remote Danger. Curr. Biol. 2021. [Google Scholar] [CrossRef]
- Langford, D.J.; Crager, S.E.; Shehzad, Z.; Smith, S.B.; Sotocinal, S.G.; Levenstadt, J.S.; Chanda, M.L.; Levitin, D.J.; Mogil, J.S. Social Modulation of Pain as Evidence for Empathy in Mice. Science 2006, 312, 1967–1970. [Google Scholar] [CrossRef]
- Panksepp, J.B.; Lahvis, G.P. Rodent Empathy and Affective Neuroscience. Neurosci. Biobehav. Rev. 2011, 35, 1864–1875. [Google Scholar] [CrossRef]
- De Waal, F.B.M. Putting the Altruism Back into Altruism: The Evolution of Empathy. Annu. Rev. Psychol. 2008, 59, 279–300. [Google Scholar] [CrossRef]
- Atsak, P.; Orre, M.; Bakker, P.; Cerliani, L.; Roozendaal, B.; Gazzola, V.; Moita, M.; Keysers, C. Experience Modulates Vicarious Freezing in Rats: A Model for Empathy. PLoS ONE 2011, 6, e21855. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, S. Pharmacology of Ultrasonic Vocalizations in Adult Rats: Significance, Call Classification and Neural Substrate. Curr. Neuropharmacol. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.Z. Stress of Predictable and Unpredictable Shock. Psychol. Bull. 1986, 100, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Beckett, S.R.G.; Duxon, M.S.; Aspley, S.; Marsden, C.A. Central C-Fos Expression Following 20kHz Ultrasound Induced Defence Behaviour in the Rat. Brain Res. Bull. 1997, 42, 421–426. [Google Scholar] [CrossRef]
- Allen, T.A.; Furtak, S.C.; Brown, T.H. Single-Unit Responses to 22 kHz Ultrasonic Vocalizations in Rat Perirhinal Cortex. Behav. Brain Res. 2007, 182, 327–336. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Blanchard, D.C. Crouching as an Index of Fear. J. Comp. Physiol. Psychol. 1969, 67, 370–375. [Google Scholar] [CrossRef]
- Gorman, J.M.; Askanazi, J.; Liebowitz, M.R.; Fyer, A.J.; Stein, J.; Kinney, J.M.; Klein, D.F. Response to Hyperventilation in a Group of Patients with Panic Disorder. Am. J. Psychiatry 1984, 141, 857–861. [Google Scholar] [CrossRef]
- Olsson, M.; Annerbrink, K.; Westberg, L.; Melke, J.; Baghaei, F.; Rosmond, R.; Holm, G.; Andersch, S.; Allgulander, C.; Eriksson, E. Angiotensin-Related Genes in Patients with Panic Disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2004, 127B, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, M.; Uengoer, M.; Schwarting, R.; Homberg, J.; Wöhr, M. Reduced Emission of Alarm 22-kHz Ultrasonic Vocalizations during Fear Conditioning in Rats Lacking the Serotonin Transporter. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 108, 110072. [Google Scholar] [CrossRef]
- Kikusui, T.; Winslow, J.; Mori, Y. Social Buffering: Relief from Stress and Anxiety. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2007, 361, 2215–2228. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Yuki, S.; Seki, Y.; Kagawa, H.; Okanoya, K. Cognitive Bias in Rats Evoked by Ultrasonic Vocalizations Suggests Emotional Contagion. Behav. Process. 2016, 132, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, Y.; Hennessy, M.B. Comparative Studies of Social Buffering: A Consideration of Approaches, Terminology, and Pitfalls. Neurosci. Biobehav. Rev. 2018, 86, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, Y.; Honda, A.; Takeuchi, Y.; Mori, Y. A Familiar Conspecific Is More Effective than an Unfamiliar Conspecific for Social Buffering of Conditioned Fear Responses in Male Rats. Behav. Brain Res. 2014, 267, 189–193. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwicka, W.; Wiatrowska, M.; Kondrakiewicz, K.; Knapska, E.; Kursa, M.B.; Hamed, A. Relaying Aversive Ultrasonic Alarm Calls Depends on Previous Experience. Empathy, Social Buffering, or Panic? Brain Sci. 2021, 11, 759. https://doi.org/10.3390/brainsci11060759
Karwicka W, Wiatrowska M, Kondrakiewicz K, Knapska E, Kursa MB, Hamed A. Relaying Aversive Ultrasonic Alarm Calls Depends on Previous Experience. Empathy, Social Buffering, or Panic? Brain Sciences. 2021; 11(6):759. https://doi.org/10.3390/brainsci11060759
Chicago/Turabian StyleKarwicka, Wiktoria, Marta Wiatrowska, Kacper Kondrakiewicz, Ewelina Knapska, Miron Bartosz Kursa, and Adam Hamed. 2021. "Relaying Aversive Ultrasonic Alarm Calls Depends on Previous Experience. Empathy, Social Buffering, or Panic?" Brain Sciences 11, no. 6: 759. https://doi.org/10.3390/brainsci11060759
APA StyleKarwicka, W., Wiatrowska, M., Kondrakiewicz, K., Knapska, E., Kursa, M. B., & Hamed, A. (2021). Relaying Aversive Ultrasonic Alarm Calls Depends on Previous Experience. Empathy, Social Buffering, or Panic? Brain Sciences, 11(6), 759. https://doi.org/10.3390/brainsci11060759





