Transient Modulation of Working Memory Performance and Event-Related Potentials by Transcranial Static Magnetic Field Stimulation over the Dorsolateral Prefrontal Cortex
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. The n-Back Task
2.3. Study Procedure
2.4. Electroencephalography (EEG) Recording
2.5. Data Analysis
2.5.1. Behavioral Analysis
2.5.2. ERPs Analysis
2.5.3. Statistical Analysis
3. Results
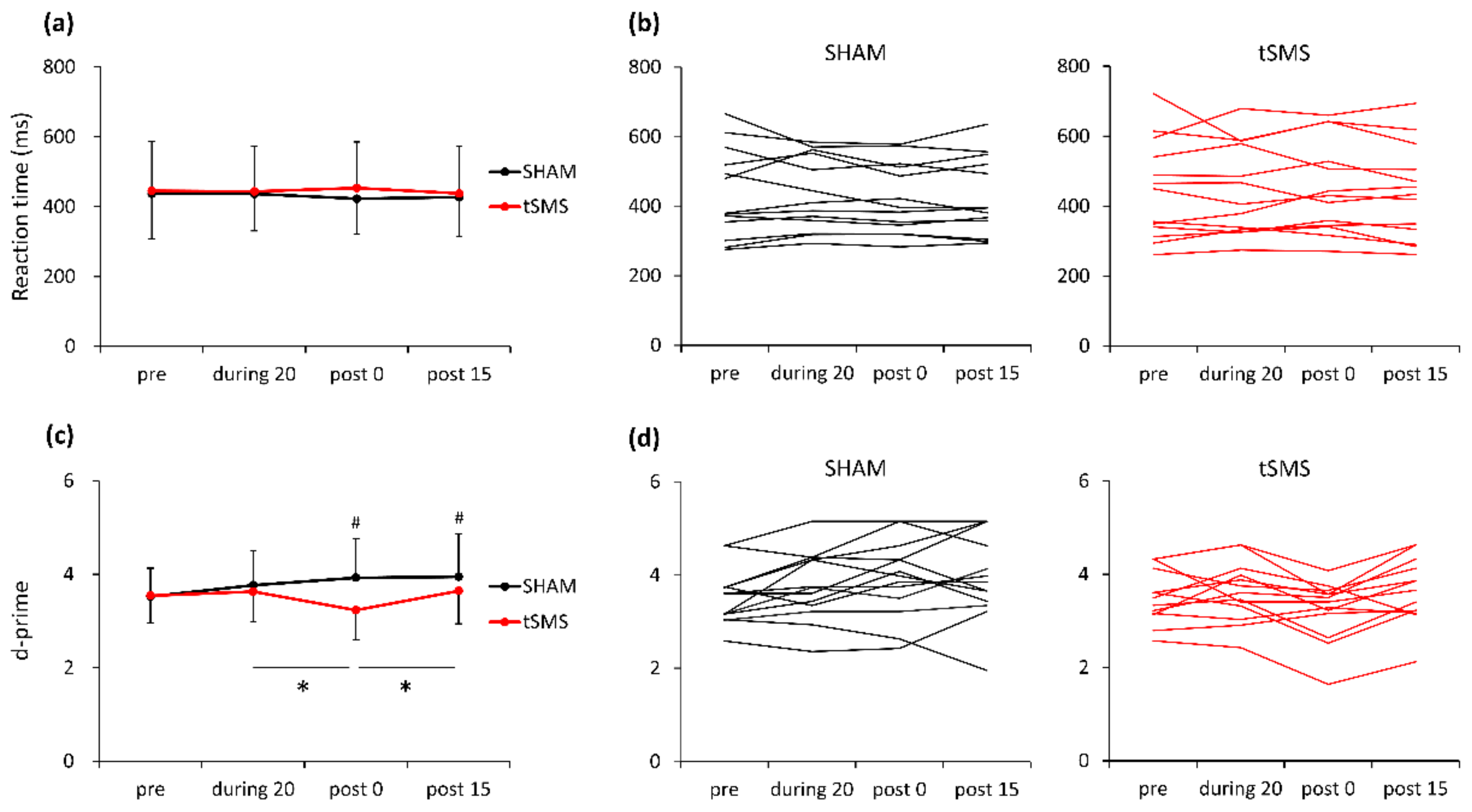
3.1. Behavioral Data
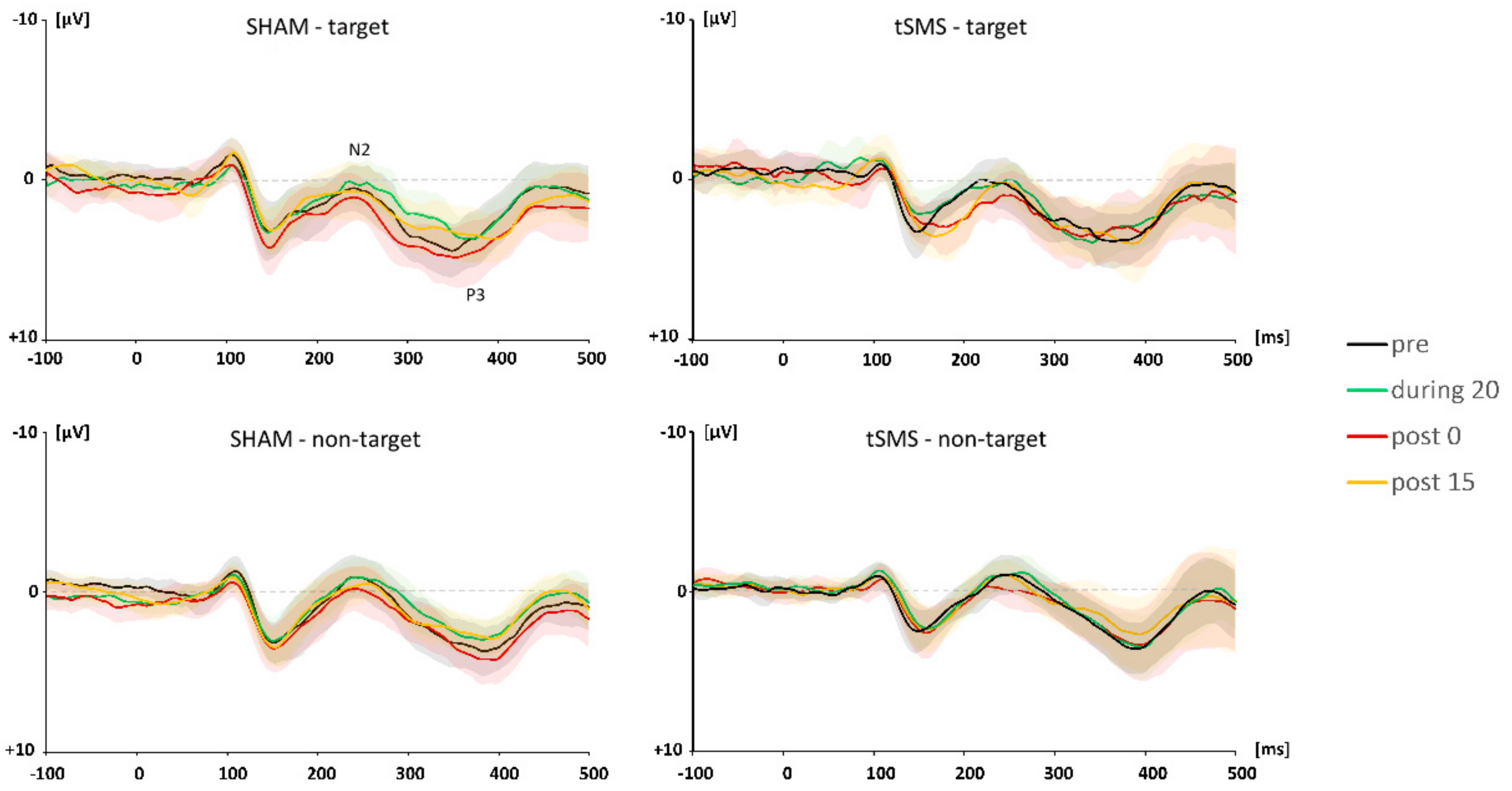
3.2. ERP Data
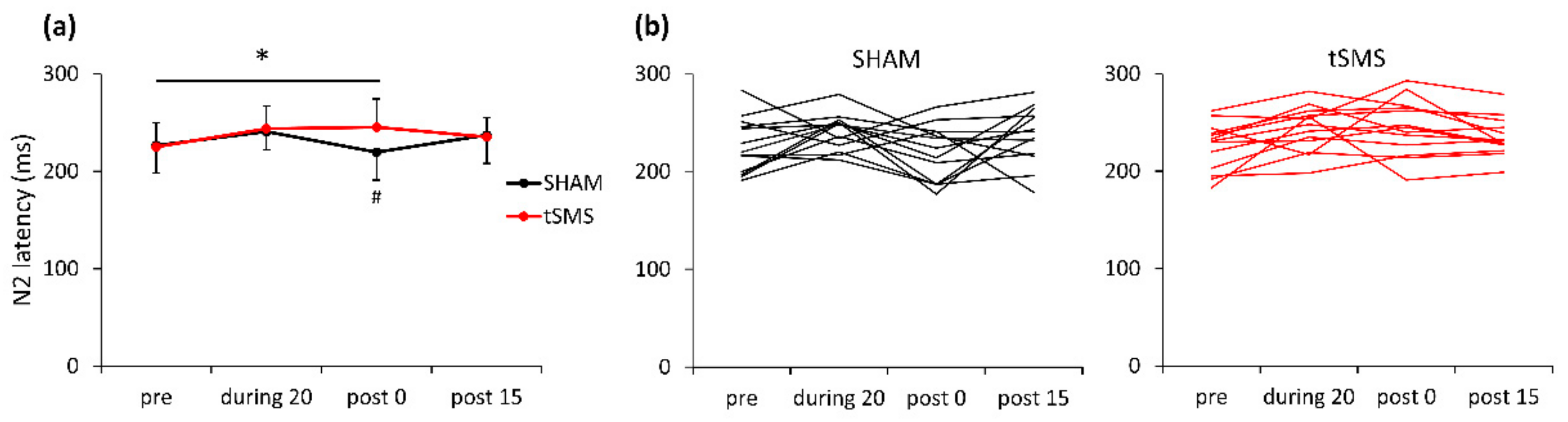
3.2.1. N2 Component
3.2.2. P3 Component
4. Discussion
4.1. The Effect of tSMS on Behavioral Performance
4.2. The Effect of tSMS on ERPs
4.2.1. The N2 Component
4.2.2. The P3 Component
4.3. Potential Clinical Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lefaucheur, J.P.; Ayache, S.S.; Sorel, M.; Farhat, W.H.; Zouari, H.G.; Ciampi De Andrade, D.; Ahdab, R.; Ménard-Lefaucheur, I.; Brugières, P.; Goujon, C. Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: Influence of theta burst stimulation priming. Eur. J. Pain 2012, 16, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.L.; Santarnecchi, E.; Buch, E.R.; Cohen, L.G. Non-invasive brain stimulation in neurorehabilitation: Local and distant effects for motor recovery. Front. Hum. Neurosci. 2014, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, A.; Mordillo-Mateos, L.; Arias, P.; Panyavin, I.; Foffani, G.; Aguilar, J. Transcranial static magnetic field stimulation of the human motor cortex. J. Physiol. 2011, 589, 4949–4958. [Google Scholar] [CrossRef] [PubMed]
- Kufner, M.; Brückner, S.; Kammer, T. No modulatory effects by transcranial static magnetic field stimulation of human motor and somatosensory cortex. Brain Stimul. 2017, 10, 703–710. [Google Scholar] [CrossRef][Green Version]
- Lorenz, S.; Alex, B.; Kammer, T. Ten minutes of transcranial static magnetic field stimulation does not reliably modulate motor cortex excitability. PLoS ONE 2020, 15, e0233614. [Google Scholar] [CrossRef]
- Watanabe, T.; Kubo, N.; Chen, X.; Yunoki, K.; Matsumoto, T.; Kuwabara, T.; Sunagawa, T.; Date, S.; Mima, T.; Kirimoto, H. Null Effect of Transcranial Static Magnetic Field Stimulation over the Dorsolateral Prefrontal Cortex on Behavioral Performance in a Go/NoGo Task. Brain Sci. 2021, 11, 483. [Google Scholar] [CrossRef]
- Nojima, I.; Oliviero, A.; Mima, T. Transcranial static magnetic stimulation—From bench to bedside and beyond. Neurosci. Res. 2020, 156, 250–255. [Google Scholar] [CrossRef]
- Dileone, M.; Mordillo-Mateos, L.; Oliviero, A.; Foffani, G. Long-lasting effects of transcranial static magnetic field stimulation on motor cortex excitability. Brain Stimul. 2018, 11, 676–688. [Google Scholar] [CrossRef]
- Silbert, B.I.; Pevcic, D.D.; Patterson, H.I.; Windnagel, K.A.; Thickbroom, G.W. Inverse correlation between resting motor threshold and corticomotor excitability after static magnetic stimulation of human motor cortex. Brain Stimul. 2013, 6, 817–820. [Google Scholar] [CrossRef]
- Nojima, I.; Koganemaru, S.; Fukuyama, H.; Mima, T. Static magnetic field can transiently alter the human intracortical inhibitory system. Clin. Neurophysiol. 2015, 126, 2314–2319. [Google Scholar] [CrossRef]
- Nojima, I.; Koganemaru, S.; Mima, T.; Kida, T.; Brown, M.J.N.; Kirimoto, H. Combination of static magnetic fields and peripheral nerve stimulation can alter focal cortical excitability. Front. Hum. Neurosci. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Kirimoto, H.; Asao, A.; Tamaki, H.; Onishi, H. Non-invasive modulation of somatosensory evoked potentials by the application of static magnetic fields over the primary and supplementary motor cortices. Sci. Rep. 2016, 6, 4–11. [Google Scholar] [CrossRef]
- Kirimoto, H.; Tamaki, H.; Otsuru, N.; Yamashiro, K.; Onishi, H.; Nojima, I.; Oliviero, A. Transcranial static magnetic field stimulation over the primary motor cortex induces plastic changes in cortical nociceptive processing. Front. Hum. Neurosci. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Kirimoto, H.; Tamaki, H.; Matsumoto, T.; Sugawara, K.; Suzuki, M.; Oyama, M.; Onishi, H. Effect of transcranial static magnetic field stimulation over the sensorimotor cortex on somatosensory evoked potentials in humans. Brain Stimul. 2014, 7, 836–840. [Google Scholar] [CrossRef]
- Gonzalez-Rosa, J.J.; Soto-Leon, V.; Real, P.; Carrasco-Lopez, C.; Foffani, G.; Strange, B.A.; Oliviero, A. Static magnetic field stimulation over the visual cortex increases alpha oscillations and slows visual search in humans. J. Neurosci. 2015, 35, 9182–9193. [Google Scholar] [CrossRef]
- Carrasco-López, C.; Soto-León, V.; Céspedes, V.; Profice, P.; Strange, B.A.; Foffani, G.; Oliviero, A. Static magnetic field stimulation over parietal cortex enhances somatosensory detection in humans. J. Neurosci. 2017, 37, 3840–3847. [Google Scholar] [CrossRef]
- Shibata, S.; Watanabe, T.; Yukawa, Y.; Minakuchi, M.; Shimomura, R.; Mima, T. Effect of transcranial static magnetic stimulation on intracortical excitability in the contralateral primary motor cortex. Neurosci. Lett. 2020, 723, 134871. [Google Scholar] [CrossRef]
- Pineda-Pardo, J.A.; Obeso, I.; Guida, P.; Dileone, M.; Strange, B.A.; Obeso, J.A.; Oliviero, A.; Foffani, G. Static magnetic field stimulation of the supplementary motor area modulates resting-state activity and motor behavior. Commun. Biol. 2019, 2. [Google Scholar] [CrossRef]
- Takamatsu, Y.; Koganemaru, S.; Watanabe, T.; Shibata, S.; Yukawa, Y.; Minakuchi, M.; Shimomura, R.; Mima, T. Transcranial static magnetic stimulation over the motor cortex can facilitate the contralateral cortical excitability in human. Sci. Rep. 2021, 11, 5370. [Google Scholar] [CrossRef]
- Shibata, S.; Watanabe, T.; Yukawa, Y.; Minakuchi, M.; Shimomura, R.; Ichimura, S.; Kirimoto, H.; Mima, T. Effects of transcranial static magnetic stimulation over the primary motor cortex on local and network spontaneous electroencephalogram oscillations. Sci. Rep. 2021, 11, 8261. [Google Scholar] [CrossRef]
- Nojima, I.; Watanabe, T.; Gyoda, T.; Sugata, H.; Ikeda, T.; Mima, T. Transcranial static magnetic stimulation over the primary motor cortex alters sequential implicit motor learning. Neurosci. Lett. 2019, 696, 33–37. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sasaki, A.; Nakazawa, K. Accuracy in Pinch Force Control Can Be Altered by Static Magnetic Field Stimulation Over the Primary Motor Cortex. Neuromodulation 2019, 22, 871–876. [Google Scholar] [CrossRef]
- Kirimoto, H.; Watanabe, T.; Kubo, N.; Date, S.; Sunagawa, T.; Mima, T.; Ogata, K.; Nakazono, H.; Tobimatsu, S.; Oliviero, A. Influence of static magnetic field stimulation on the accuracy of tachystoscopically presented line bisection. Brain Sci. 2020, 10, 1006. [Google Scholar] [CrossRef]
- Tsuru, D.; Watanabe, T.; Chen, X.; Kubo, N.; Sunagawa, T.; Mima, T.; Kirimoto, H. The effects of transcranial static magnetic fields stimulation over the supplementary motor area on anticipatory postural adjustments. Neurosci. Lett. 2020, 723, 134863. [Google Scholar] [CrossRef]
- Rose, E.J.; Ebmeier, K.P. Pattern of impaired working memory during major depression. J. Affect. Disord. 2006, 90, 149–161. [Google Scholar] [CrossRef]
- Santarnecchi, E.; Brem, A.K.; Levenbaum, E.; Thompson, T.; Kadosh, R.C.; Pascual-Leone, A. Enhancing cognition using transcranial electrical stimulation. Curr. Opin. Behav. Sci. 2015, 4, 171–178. [Google Scholar] [CrossRef]
- Curtis, C.E.; D’Esposito, M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 2003, 7, 415–423. [Google Scholar] [CrossRef]
- Smith, E.E.; Jonides, J. Storage and Executive Processes in the Frontal Lobes. Science 1999, 283, 1657. [Google Scholar] [CrossRef]
- Tsuchida, A.; Fellows, L.K. Lesion evidence that two distinct regions within prefrontal cortex are critical for n-back performance in humans. J. Cogn. Neurosci. 2009, 21, 2263–2275. [Google Scholar] [CrossRef]
- Mull, B.R.; Seyal, M. Transcranial magnetic stimulation of left prefrontal cortex impairs working memory. Clin. Neurophysiol. 2001, 112, 1672–1675. [Google Scholar] [CrossRef]
- Osaka, N.; Otsuka, Y.; Hirose, N.; Ikeda, T. Transcranial magnetic stimulation (TMS) applied to left dorsolateral prefrontal cortex disrupts verbal working memory performance in humans. Neurosci. Lett. 2007, 418, 232–235. [Google Scholar] [CrossRef]
- Hill, A.T.; Fitzgerald, P.B.; Hoy, K.E. Effects of Anodal Transcranial Direct Current Stimulation on Working Memory: A Systematic Review and Meta-Analysis of Findings from Healthy and Neuropsychiatric Populations. Brain Stimul. 2016, 9, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Vanderhasselt, M.A. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain Cogn. 2014, 86, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dedoncker, J.; Brunoni, A.; Stimulation, C.B.-B. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: Influence of stimulation parameters. Brain Stimul. 2016, 9, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, L.E.; Ilieva, I.P.; Hamilton, R.H.; Farah, M.J. Does transcranial direct current stimulation improve healthy working memory?: A meta-analytic review. J. Cogn. Neurosci. 2016, 28, 1063–1089. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, S.M.; Buschkuehl, M.; Perrig, W.J.; Meier, B. The concurrent validity of the N-back task as a working memory measure. Memory 2010, 18, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef]
- Daffner, K.R.; Chong, H.; Sun, X.; Tarbi, E.C.; Riis, J.L.; McGinnis, S.M.; Holcomb, P.J. Mechanisms underlying age-and performance-related differences in working memory. J. Cogn. Neurosci. 2011, 23, 1298–1314. [Google Scholar] [CrossRef]
- Donchin, E.; Ritter, W.; Mccallum, W.C. Cognitive Psychophysiology: The Endogenous Components of the ERP. Event Relat. Brain Potentials Man 1978, 349, 411. [Google Scholar]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Falkenstein, M. Neurocognition of aging in working environments. Z. Arb. 2011, 44, 307–320. [Google Scholar] [CrossRef]
- Wiedemann, G.; Pauli, P.; Dengler, W.; Lutzenberger, W.; Birbaumer, N.; Buchkremer, G. Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Arch. Gen. Psychiatry 1999, 56, 78–84. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsutou, K.; Saito, K.; Ishida, K.; Tanabe, S.; Nojima, I. Performance monitoring and response conflict resolution associated with choice stepping reaction tasks. Exp. Brain Res. 2016, 234, 3355–3365. [Google Scholar] [CrossRef]
- Haatveit, B.C.; Sundet, K.; Hugdahl, K.; Ueland, T.; Melle, I.; Andreassen, O.A. The validity of d prime as a working memory index: Results from the Bergen n-back task. J. Clin. Exp. Neuropsychol. 2010, 32, 871–880. [Google Scholar] [CrossRef]
- Macmillan, N.A.; Creelman, C.D. Detection Theory: A User’s Guide; Cambridge University Press: New York, NY, USA, 1991; ISBN 0-521-36359-4. [Google Scholar]
- Muller, A.; Sirianni, L.A.; Addante, R.J. Neural correlates of the Dunning–Kruger effect. Eur. J. Neurosci. 2021, 53, 460–484. [Google Scholar] [CrossRef]
- Addante, R.J.; Ranganath, C.; Yonelinas, A.P. Examining ERP correlates of recognition memory: Evidence of accurate source recognition without recollection. Neuroimage 2012, 62, 439–450. [Google Scholar] [CrossRef]
- Friedman, D.; Cycowicz, Y.M.; Gaeta, H. The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci. Biobehav. Rev. 2001, 25, 355–373. [Google Scholar] [CrossRef]
- Dubreuil-Vall, L.; Chau, P.; Ruffini, G.; Widge, A.S.; Camprodon, J.A. tDCS to the left DLPFC modulates cognitive and physiological correlates of executive function in a state-dependent manner. Brain Stimul. 2019, 12, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, W.W.C.; Costa, R.M.P.B.; de Salazar e Fernandes, T.; Porto, A.L.F. Evidences of the static magnetic field influence on cellular systems. Prog. Biophys. Mol. Biol. 2016, 121, 16–28. [Google Scholar] [CrossRef]
- Rosen, A.D. Membrane response to static magnetic fields: Effect of exposure duration. BBA Biomembr. 1993, 1148, 317–320. [Google Scholar] [CrossRef]
- Dobson, J.; Stewart, Z.; Martinac, B. Preliminary evidence for weak magnetic field effects on mechanosensitive ion channel subconducting states in Escherichia coli. Electromagn. Biol. Med. 2002, 21, 89–95. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.S.; Nitsche, M.; Bermpohl, F.; Antal, A.; Feredoes, E.; Marcolin, M.A.; Rigonatti, S.P.; Silva, M.T.A.; Paulus, W.; et al. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp. Brain Res. 2005, 166, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Brzezicka, A.; Kamiński, J.; Reed, C.M.; Chung, J.M.; Mamelak, A.N.; Rutishauser, U. Working memory load related theta power decreases in dorsolateral prefrontal cortex predict individual differences in performance. J. Cogn. Neurosci. 2019, 31, 1290–1307. [Google Scholar] [CrossRef] [PubMed]
- Osaka, M.; Osaka, N.; Kondo, H.; Morishita, M.; Fukuyama, H.; Aso, T.; Shibasaki, H. The neural basis of individual differences in working memory capacity: An fMRI study. Neuroimage 2003, 18, 789–797. [Google Scholar] [CrossRef]
- Watanabe, T.; Mima, T.; Shibata, S.; Kirimoto, H. Midfrontal theta as moderator between beta oscillations and precision control. Neuroimage 2021, 235, 118022. [Google Scholar] [CrossRef]
- Rodriguez Merzagora, A.C.; Izzetoglu, M.; Onaral, B.; Schultheis, M.T. Verbal working memory impairments following traumatic brain injury: An fNIRS investigation. Brain Imaging Behav. 2014, 8, 446–459. [Google Scholar] [CrossRef]
- Gajewski, P.D.; Falkenstein, M. Age-Related Effects on ERP and Oscillatory EEG-Dynamics in a 2-Back Task. J. Psychophysiol. 2014, 28, 162–177. [Google Scholar] [CrossRef]
- Polich, J. Meta-analysis of P300 normative aging studies. Psychophysiology 1996, 33, 334–353. [Google Scholar] [CrossRef]
- Zaehle, T.; Sandmann, P.; Thorne, J.D.; Jäncke, L.; Herrmann, C.S. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: Combined behavioural and electrophysiological evidence. BMC Neurosci. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet 2012, 379, 1045–1055. [Google Scholar] [CrossRef]
- Mann, J.J. The Medical Management of Depression. N. Engl. J. Med. 2005, 353, 1819–1834. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Fava, M. Pharmacotherapy for Depression and Treatment-Resistant Depression; World Scientific: Singapore, 2010; ISBN 981428758X. [Google Scholar]
- Knott, V.; Mahoney, C.; Kennedy, S.; Evans, K. EEG power, frequency, asymmetry and coherence in male depression. Psychiatry Res. Neuroimaging 2001, 106, 123–140. [Google Scholar] [CrossRef]
- Diego, M.A.; Field, T.; Hernandez-Reif, M. CES-D depression scores are correlated with frontal EEG alpha asymmetry. Depress. Anxiety 2001, 13, 32–37. [Google Scholar] [CrossRef]
- Bench, C.J.; Frackowiak, R.S.; Dolan, R.J. Changes in regional cerebral blood flow on recovery from depression. Psychol. Med. 1995, 25, 247–261. [Google Scholar] [CrossRef]
- Berlim, M.T.; Van Den Eynde, F.; Tovar-Perdomo, S.; Daskalakis, Z.J. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: A systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol. Med. 2014, 44, 225–239. [Google Scholar] [CrossRef]
- Berlim, M.T.; Van Den Eynde, F.; Daskalakis, Z.J. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: A meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology 2013, 38, 543–551. [Google Scholar] [CrossRef]

| SHAM | tSMS | ||
|---|---|---|---|
| RT (ms) | pre | 436.87 ± 128.74 | 445.16 ± 141.71 |
| during 20 | 436.73 ± 106.05 | 443.59 ± 129.99 | |
| post 0 | 422.82 ± 101.02 | 453.31 ± 132.31 | |
| post 15 | 426 ± 111.88 | 437.93 ± 135.18 | |
| Hit rate | pre | 0.86 ± 0.10 | 0.83 ± 0.14 |
| during 20 | 0.87 ± 0.13 | 0.86 ± 0.12 | |
| post 0 | 0.87 ± 0.15 | 0.80 ± 0.15 | |
| post 15 | 0.87 ± 0.15 | 0.85 ± 0.15 | |
| False alarm rate | pre | 0.01 ± 0.01 | 0.01 ± 0.01 |
| during 20 | 0.01 ± 0.01 | 0.01 ± 0.00 | |
| post 0 | 0.01 ± 0.00 | 0.01 ± 0.01 | |
| post 15 | 0.01 ± 0.01 | 0.01 ± 0.01 | |
| d-prime | pre | 3.53 ± 0.60 | 3.54 ± 0.59 |
| during 20 | 3.77 ± 0.74 | 3.63 ± 0.64 * | |
| post 0 | 3.93 ± 0.84 | 3.23 ± 0.64 # | |
| post 15 | 3.95 ± 0.92 | 3.64 ± 0.70 * | |
| criterion | pre | 0.57 ± 0.28 | 0.67 ± 0.29 |
| during 20 | 0.50 ± 0.37 | 0.59 ± 0.25 | |
| post 0 | 0.54 ± 0.36 | 0.70 ± 0.27 | |
| post 15 | 0.51 ± 0.39 | 0.62 ± 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Watanabe, T.; Kubo, N.; Yunoki, K.; Matsumoto, T.; Kuwabara, T.; Sunagawa, T.; Date, S.; Mima, T.; Kirimoto, H. Transient Modulation of Working Memory Performance and Event-Related Potentials by Transcranial Static Magnetic Field Stimulation over the Dorsolateral Prefrontal Cortex. Brain Sci. 2021, 11, 739. https://doi.org/10.3390/brainsci11060739
Chen X, Watanabe T, Kubo N, Yunoki K, Matsumoto T, Kuwabara T, Sunagawa T, Date S, Mima T, Kirimoto H. Transient Modulation of Working Memory Performance and Event-Related Potentials by Transcranial Static Magnetic Field Stimulation over the Dorsolateral Prefrontal Cortex. Brain Sciences. 2021; 11(6):739. https://doi.org/10.3390/brainsci11060739
Chicago/Turabian StyleChen, Xiaoxiao, Tatsunori Watanabe, Nami Kubo, Keisuke Yunoki, Takuya Matsumoto, Takayuki Kuwabara, Toru Sunagawa, Shota Date, Tatsuya Mima, and Hikari Kirimoto. 2021. "Transient Modulation of Working Memory Performance and Event-Related Potentials by Transcranial Static Magnetic Field Stimulation over the Dorsolateral Prefrontal Cortex" Brain Sciences 11, no. 6: 739. https://doi.org/10.3390/brainsci11060739
APA StyleChen, X., Watanabe, T., Kubo, N., Yunoki, K., Matsumoto, T., Kuwabara, T., Sunagawa, T., Date, S., Mima, T., & Kirimoto, H. (2021). Transient Modulation of Working Memory Performance and Event-Related Potentials by Transcranial Static Magnetic Field Stimulation over the Dorsolateral Prefrontal Cortex. Brain Sciences, 11(6), 739. https://doi.org/10.3390/brainsci11060739






