Comparative Study of a Continuous Train of Theta-Burst Stimulation for a Duration of 20 s (cTBS 300) versus a Duration of 40 s (cTBS 600) in a Pre-Stimulation Relaxed Condition in Healthy Volunteers
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
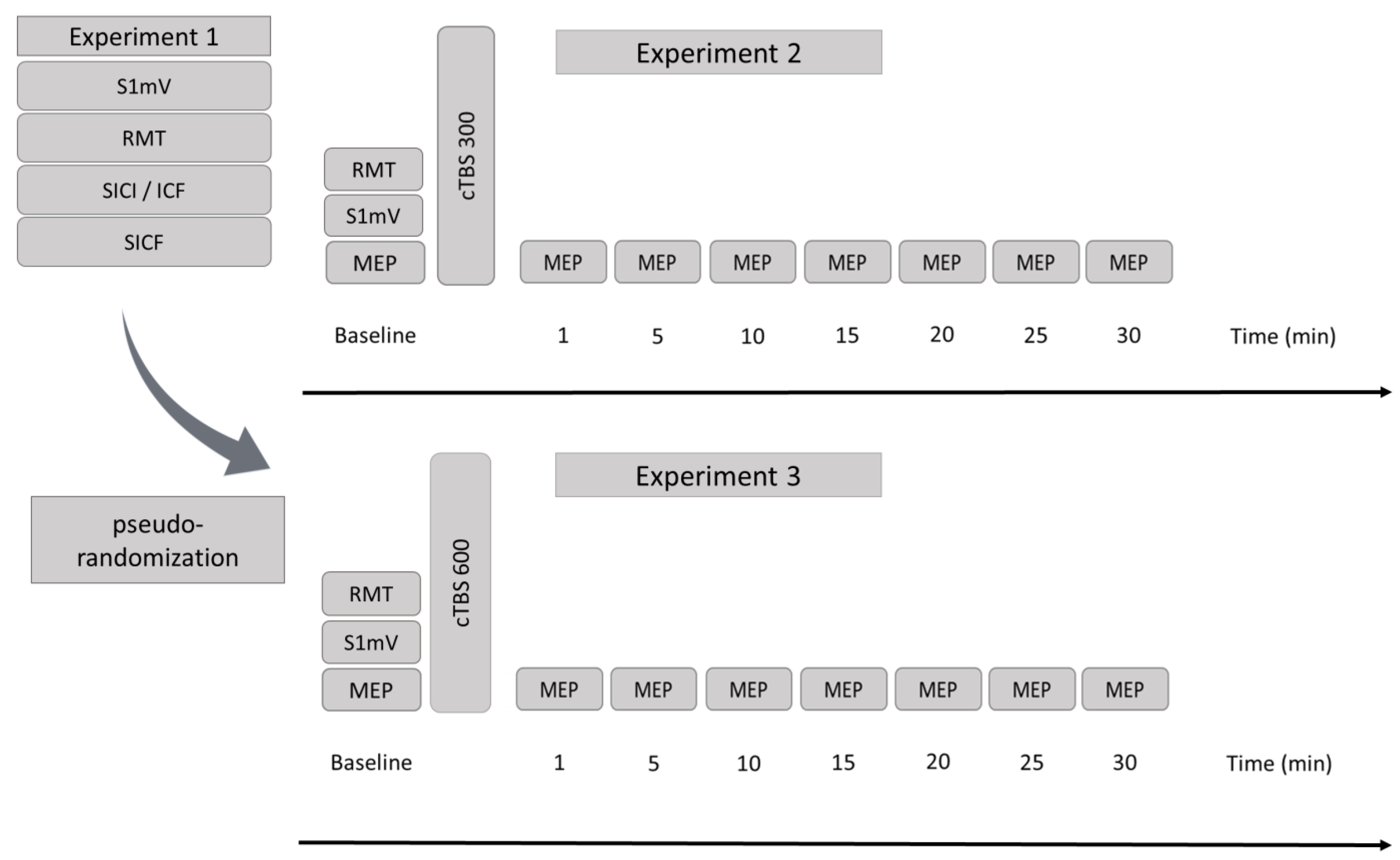
2.2. Design
2.3. Experimental Procedures
2.4. Baseline Excitability and Monitoring of Excitability Changes
2.5. Theta-Burst Stimulation
2.6. Statistical Methods
3. Results
3.1. Baseline Characteristics
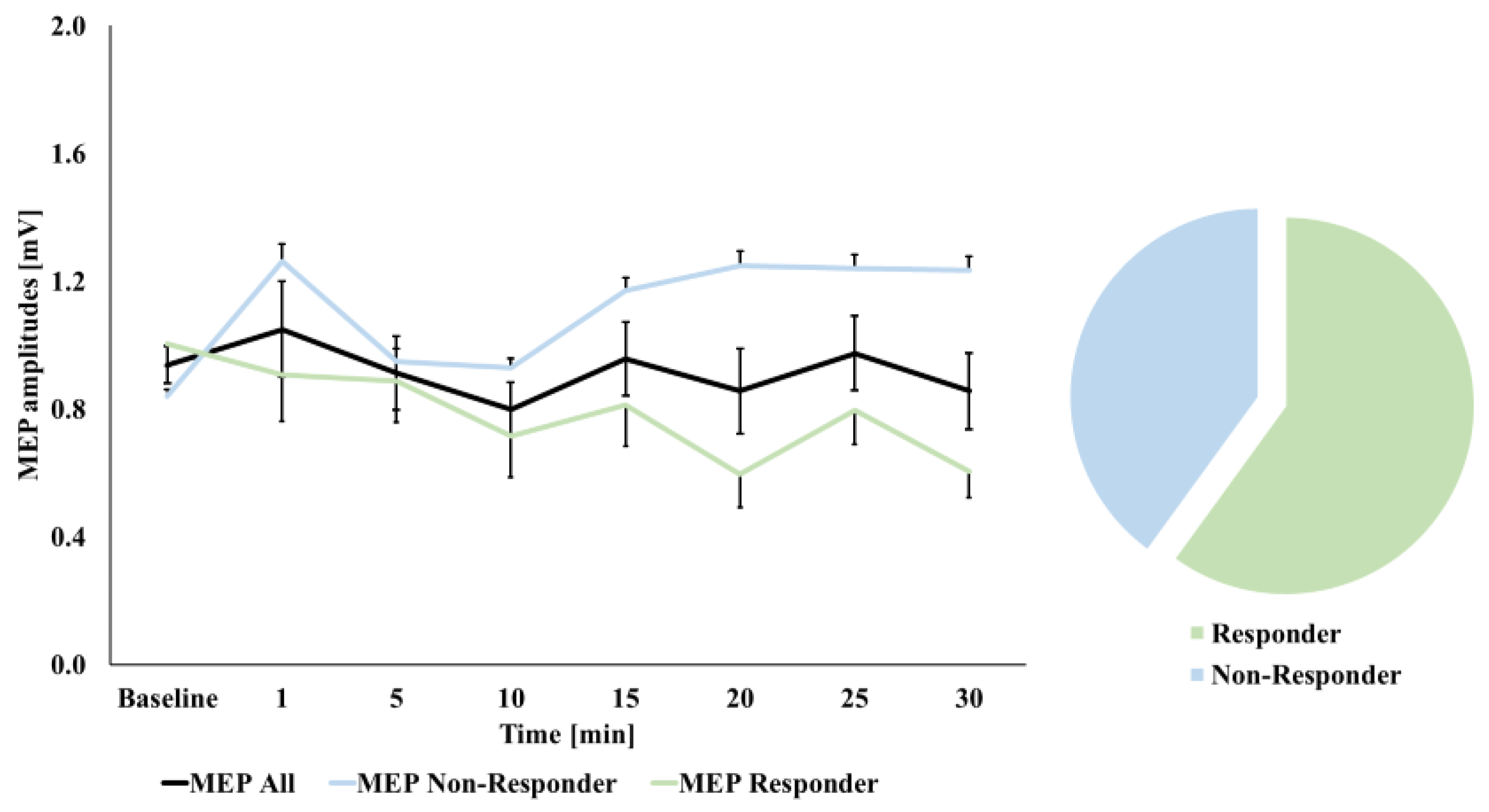
3.2. MEP Amplitude Changes over Time
3.3. Correlations with Baseline Excitability Measures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, S.W.; Hill, A.T.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. Use of Theta-Burst Stimulation in Changing Excitability of Motor Cortex: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2016, 63, 43–64. [Google Scholar] [CrossRef]
- Suppa, A.; Huang, Y.-Z.; Funke, K.; Ridding, M.C.; Cheeran, B.; Di Lazzaro, V.; Ziemann, U.; Rothwell, J.C. Ten Years of Theta Burst Stimulation in Humans: Established Knowledge, Unknowns and Prospects. Brain Stimul. 2016, 9, 323–335. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological After-Effects of Non-Invasive Brain Stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Paulus, W. Transcranial Electrical Stimulation (TES—TDCS; TRNS, TACS) Methods. Neuropsychol. Rehabil. 2011, 21, 602–617. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Rothwell, J.C. The Effect of Short-Duration Bursts of High-Frequency, Low-Intensity Transcranial Magnetic Stimulation on the Human Motor Cortex. Clin. Neurophysiol. 2004, 115, 1069–1075. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta Burst Stimulation of the Human Motor Cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Capocchi, G.; Zampolini, M.; Larson, J. Theta Burst Stimulation Is Optimal for Induction of LTP at Both Apical and Basal Dendritic Synapses on Hippocampal CA1 Neurons. Brain Res. 1992, 591, 332–336. [Google Scholar] [CrossRef]
- Papazachariadis, O.; Dante, V.; Verschure, P.F.M.J.; Del Giudice, P.; Ferraina, S. ITBS-Induced LTP-like Plasticity Parallels Oscillatory Activity Changes in the Primary Sensory and Motor Areas of Macaque Monkeys. PLoS ONE 2014, 9, e112504. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Rothwell, J.C.; Chen, R.-S.; Lu, C.-S.; Chuang, W.-L. The Theoretical Model of Theta Burst Form of Repetitive Transcranial Magnetic Stimulation. Clin. Neurophysiol. 2011, 122, 1011–1018. [Google Scholar] [CrossRef]
- Gentner, R.; Wankerl, K.; Reinsberger, C.; Zeller, D.; Classen, J. Depression of Human Corticospinal Excitability Induced by Magnetic Theta-Burst Stimulation: Evidence of Rapid Polarity-Reversing Metaplasticity. Cereb. Cortex 2008, 18, 2046–2053. [Google Scholar] [CrossRef]
- Jannati, A.; Block, G.; Oberman, L.M.; Rotenberg, A.; Pascual-Leone, A. Interindividual Variability in Response to Continuous Theta-Burst Stimulation in Healthy Adults. Clin. Neurophysiol. 2017, 128, 2268–2278. [Google Scholar] [CrossRef]
- Vallence, A.-M.; Goldsworthy, M.R.; Hodyl, N.A.; Semmler, J.G.; Pitcher, J.B.; Ridding, M.C. Inter- and Intra-Subject Variability of Motor Cortex Plasticity Following Continuous Theta-Burst Stimulation. Neuroscience 2015, 304, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, E.; Conte, A.; Suppa, A.; Agostino, R.; Dinapoli, L.; Scontrini, A.; Berardelli, A. Phasic Voluntary Movements Reverse the Aftereffects of Subsequent Theta-Burst Stimulation in Humans. J. Neurophysiol. 2008, 100, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Wankerl, K.; Weise, D.; Gentner, R.; Rumpf, J.-J.; Classen, J. L-Type Voltage-Gated Ca2+ Channels: A Single Molecular Switch for Long-Term Potentiation/Long-Term Depression-like Plasticity and Activity-Dependent Metaplasticity in Humans. J. Neurosci. 2010, 30, 6197–6204. [Google Scholar] [CrossRef]
- Goldsworthy, M.R.; Müller-Dahlhaus, F.; Ridding, M.C.; Ziemann, U. Inter-Subject Variability of LTD-like Plasticity in Human Motor Cortex: A Matter of Preceding Motor Activation. Brain Stimul. 2014, 7, 864–870. [Google Scholar] [CrossRef]
- Van den Bos, M.A.J.; Menon, P.; Howells, J.; Geevasinga, N.; Kiernan, M.C.; Vucic, S. Physiological Processes Underlying Short Interval Intracortical Facilitation in the Human Motor Cortex. Front. Neurosci. 2018, 12, 240. [Google Scholar] [CrossRef]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical Inhibition in Human Motor Cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- Chen, R.; Tam, A.; Bütefisch, C.; Corwell, B.; Ziemann, U.; Rothwell, J.C.; Cohen, L.G. Intracortical Inhibition and Facilitation in Different Representations of the Human Motor Cortex. J. Neurophysiol. 1998, 80, 2870–2881. [Google Scholar] [CrossRef]
- Ni, Z.; Gunraj, C.; Chen, R. Short Interval Intracortical Inhibition and Facilitation during the Silent Period in Human. J. Physiol. 2007, 583, 971–982. [Google Scholar] [CrossRef]
- Tokimura, H.; Ridding, M.C.; Tokimura, Y.; Amassian, V.E.; Rothwell, J.C. Short Latency Facilitation between Pairs of Threshold Magnetic Stimuli Applied to Human Motor Cortex. Electroencephalogr. Clin. Neurophysiol. 1996, 101, 263–272. [Google Scholar] [CrossRef]
- Plewnia, C.; Pasqualetti, P.; Große, S.; Schlipf, S.; Wasserka, B.; Zwissler, B.; Fallgatter, A. Treatment of Major Depression with Bilateral Theta Burst Stimulation: A Randomized Controlled Pilot Trial. J. Affect. Disord. 2014, 156, 219–223. [Google Scholar] [CrossRef]
- Chistyakov, A.V.; Kreinin, B.; Marmor, S.; Kaplan, B.; Khatib, A.; Darawsheh, N.; Koren, D.; Zaaroor, M.; Klein, E. Preliminary Assessment of the Therapeutic Efficacy of Continuous Theta-Burst Magnetic Stimulation (CTBS) in Major Depression: A Double-Blind Sham-Controlled Study. J. Affect. Disord. 2015, 170, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-M.; Juan, C.-H.; Chen, M.-H.; Chang, C.-F.; Lu, H.J.; Su, T.-P.; Lee, Y.-C.; Li, C.-T. Different Forms of Prefrontal Theta Burst Stimulation for Executive Function of Medication- Resistant Depression: Evidence from a Randomized Sham-Controlled Study. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 66, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Hamada, M.; Nitsche, M.A.; Ruge, D.; Galea, J.M.; Wobrock, T.; Rothwell, J.C. Direct-Current-Dependent Shift of Theta-Burst-Induced Plasticity in the Human Motor Cortex. Exp. Brain Res. 2012, 217, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Restuccia, D.; Oliviero, A.; Profice, P.; Ferrara, L.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Effects of Voluntary Contraction on Descending Volleys Evoked by Transcranial Stimulation in Conscious Humans. J. Physiol. 1998, 508 Pt 2, 625–633. [Google Scholar] [CrossRef]
- Ziemann, U.; Tergau, F.; Wassermann, E.M.; Wischer, S.; Hildebrandt, J.; Paulus, W. Demonstration of Facilitatory I Wave Interaction in the Human Motor Cortex by Paired Transcranial Magnetic Stimulation. J. Physiol. 1998, 511 Pt 1, 181–190. [Google Scholar] [CrossRef]
- Ziemann, U.; Lönnecker, S.; Steinhoff, B.J.; Paulus, W. The Effect of Lorazepam on the Motor Cortical Excitability in Man. Exp. Brain Res. 1996, 109, 127–135. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Rothwell, J.C.; Oliviero, A.; Profice, P.; Insola, A.; Mazzone, P.; Tonali, P. Intracortical Origin of the Short Latency Facilitation Produced by Pairs of Threshold Magnetic Stimuli Applied to Human Motor Cortex. Exp. Brain Res. 1999, 129, 494–499. [Google Scholar] [CrossRef]
- Ziemann, U. I-Waves in Motor Cortex Revisited. Exp. Brain Res. 2020, 238, 1601–1610. [Google Scholar] [CrossRef]
- Goldsworthy, M.R.; Pitcher, J.B.; Ridding, M.C. The Application of Spaced Theta Burst Protocols Induces Long-Lasting Neuroplastic Changes in the Human Motor Cortex. Eur. J. Neurosci. 2012, 35, 125–134. [Google Scholar] [CrossRef]
- Gamboa, O.L.; Antal, A.; Moliadze, V.; Paulus, W. Simply Longer Is Not Better: Reversal of Theta Burst after-Effect with Prolonged Stimulation. Exp. Brain Res. 2010, 204, 181–187. [Google Scholar] [CrossRef] [PubMed]
- López-Alonso, V.; Cheeran, B.; Río-Rodríguez, D.; Fernández-Del-Olmo, M. Inter-Individual Variability in Response to Non-Invasive Brain Stimulation Paradigms. Brain Stimul. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Tiksnadi, A.; Murakami, T.; Wiratman, W.; Matsumoto, H.; Ugawa, Y. Direct Comparison of Efficacy of the Motor Cortical Plasticity Induction and the Interindividual Variability between TBS and QPS. Brain Stimul. 2020, 13, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; López-Alonso, V.; Cheeran, B.; Suppa, A. Variability in Non-Invasive Brain Stimulation Studies: Reasons and Results. Neurosci. Lett. 2020, 719, 133330. [Google Scholar] [CrossRef]
- Bonett, D.G.; Wright, T.A. Sample Size Requirements for Estimating Pearson, Kendall and Spearman Correlations. Psychometrika 2000, 65, 23–28. [Google Scholar] [CrossRef]
- Doeltgen, S.H.; Ridding, M.C. Low-Intensity, Short-Interval Theta Burst Stimulation Modulates Excitatory but Not Inhibitory Motor Networks. Clin. Neurophysiol. 2011, 122, 1411–1416. [Google Scholar] [CrossRef]
- Stefan, K.; Gentner, R.; Zeller, D.; Dang, S.; Classen, J. Theta-Burst Stimulation: Remote Physiological and Local Behavioral after-Effects. Neuroimage 2008, 40, 265–274. [Google Scholar] [CrossRef]
- Fang, J.-H.; Huang, Y.-Z.; Hwang, I.-S.; Chen, J.-J.J. Selective Modulation of Motor Cortical Plasticity during Voluntary Contraction of the Antagonist Muscle. Eur. J. Neurosci. 2014, 39, 2083–2088. [Google Scholar] [CrossRef]
- Huang, Y.-Z. What Do We Learn from the Influence of Motor Activities on the After-Effect of Non-Invasive Brain Stimulation? Clin. Neurophysiol. 2016, 127, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Dileone, M.; Pilato, F.; Capone, F.; Musumeci, G.; Ranieri, F.; Ricci, V.; Bria, P.; Di Iorio, R.; de Waure, C.; et al. Modulation of Motor Cortex Neuronal Networks by RTMS: Comparison of Local and Remote Effects of Six Different Protocols of Stimulation. J. Neurophysiol. 2011, 105, 2150–2156. [Google Scholar] [CrossRef]
- Doeltgen, S.H.; Ridding, M.C. Modulation of Cortical Motor Networks Following Primed θ Burst Transcranial Magnetic Stimulation. Exp. Brain Res. 2011, 215, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Goldsworthy, M.R.; Pitcher, J.B.; Ridding, M.C. Neuroplastic Modulation of Inhibitory Motor Cortical Networks by Spaced Theta Burst Stimulation Protocols. Brain Stimul. 2013, 6, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Murase, N.; Hasan, A.; Balaratnam, M.; Rothwell, J.C. The Role of Interneuron Networks in Driving Human Motor Cortical Plasticity. Cereb. Cortex 2013, 23, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Hordacre, B.; Goldsworthy, M.R.; Vallence, A.-M.; Darvishi, S.; Moezzi, B.; Hamada, M.; Rothwell, J.C.; Ridding, M.C. Variability in Neural Excitability and Plasticity Induction in the Human Cortex: A Brain Stimulation Study. Brain Stimul. 2017, 10, 588–595. [Google Scholar] [CrossRef]


| Experiment 1 | Experiment 2 cTBS 300 | Experiment 3 cTBS 600 | Dependent Samples t-Tests (Experiment 2 vs. Experiment 3) | ||
|---|---|---|---|---|---|
| Test Statistic | p-Values | ||||
| Baseline-MEP [mV] | - | 0.934 ± 0.244 | 0.938 ± 0.262 | t(19) = 0.067 | 0.947 |
| RMT [%] | 47.25 ± 8.30 | 50.30 ± 9.69 | 49.05 ± 9.90 | t(19) = 1.403 | 0.177 |
| S1 mV [%] | 55.85 ± 10.42 | 58.00 ± 10.58 | 57.68 ± 9.29 | t(18) = 0.344 | 0.735 |
| ISI | N | Mean ± SD | |
|---|---|---|---|
| S1 mV | 20 | 55.85 ± 10.42 | |
| RMT | 20 | 47.25 ± 8.30 | |
| SICI | 2 ms | 20 | 0.75 ± 0.45 |
| 3 ms | 20 | 0.89 ± 0.70 | |
| ICF | 9 ms | 20 | 2.16 ± 1.28 |
| 12 ms | 20 | 2.22 ± 1.17 | |
| SICF | 1.4 ms | 20 | 3.44 ± 1.47 |
| 1.6 ms | 20 | 3.43 ± 2.10 | |
| 1.8 ms | 20 | 2.09 ± 1.33 | |
| 2.0 ms | 20 | 1.54 ± 0.69 | |
| 2.2 ms | 20 | 1.36 ± 0.55 | |
| 2.4 ms | 20 | 1.97 ± 0.66 | |
| 2.6 ms | 20 | 2.57 ± 0.76 | |
| 2.8 ms | 20 | 2.13 ± 0.69 | |
| 3.0 ms | 20 | 2.17 ± 0.99 | |
| 3.2 ms | 20 | 1.87 ± 1.01 | |
| cTBS 300 | mean post rel. MEP | 20 | 1.12 ± 0.42 |
| cTBS 600 | mean post rel. MEP | 20 | 0.99 ± 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haeckert, J.; Rothwell, J.; Hannah, R.; Hasan, A.; Strube, W. Comparative Study of a Continuous Train of Theta-Burst Stimulation for a Duration of 20 s (cTBS 300) versus a Duration of 40 s (cTBS 600) in a Pre-Stimulation Relaxed Condition in Healthy Volunteers. Brain Sci. 2021, 11, 737. https://doi.org/10.3390/brainsci11060737
Haeckert J, Rothwell J, Hannah R, Hasan A, Strube W. Comparative Study of a Continuous Train of Theta-Burst Stimulation for a Duration of 20 s (cTBS 300) versus a Duration of 40 s (cTBS 600) in a Pre-Stimulation Relaxed Condition in Healthy Volunteers. Brain Sciences. 2021; 11(6):737. https://doi.org/10.3390/brainsci11060737
Chicago/Turabian StyleHaeckert, Jan, John Rothwell, Ricci Hannah, Alkomiet Hasan, and Wolfgang Strube. 2021. "Comparative Study of a Continuous Train of Theta-Burst Stimulation for a Duration of 20 s (cTBS 300) versus a Duration of 40 s (cTBS 600) in a Pre-Stimulation Relaxed Condition in Healthy Volunteers" Brain Sciences 11, no. 6: 737. https://doi.org/10.3390/brainsci11060737
APA StyleHaeckert, J., Rothwell, J., Hannah, R., Hasan, A., & Strube, W. (2021). Comparative Study of a Continuous Train of Theta-Burst Stimulation for a Duration of 20 s (cTBS 300) versus a Duration of 40 s (cTBS 600) in a Pre-Stimulation Relaxed Condition in Healthy Volunteers. Brain Sciences, 11(6), 737. https://doi.org/10.3390/brainsci11060737






