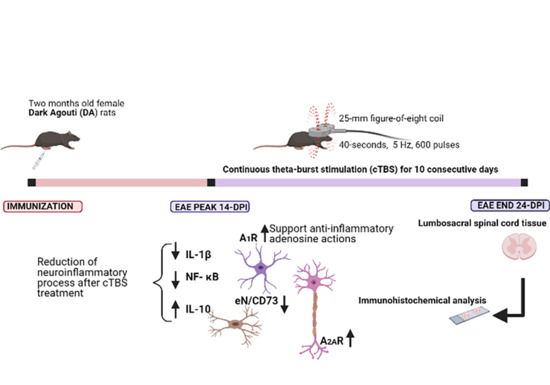
Downregulation of CD73/A2AR-Mediated Adenosine Signaling as a Potential Mechanism of Neuroprotective Effects of Theta-Burst Transcranial Magnetic Stimulation in Acute Experimental Autoimmune Encephalomyelitis
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Statement
2.2. Animals
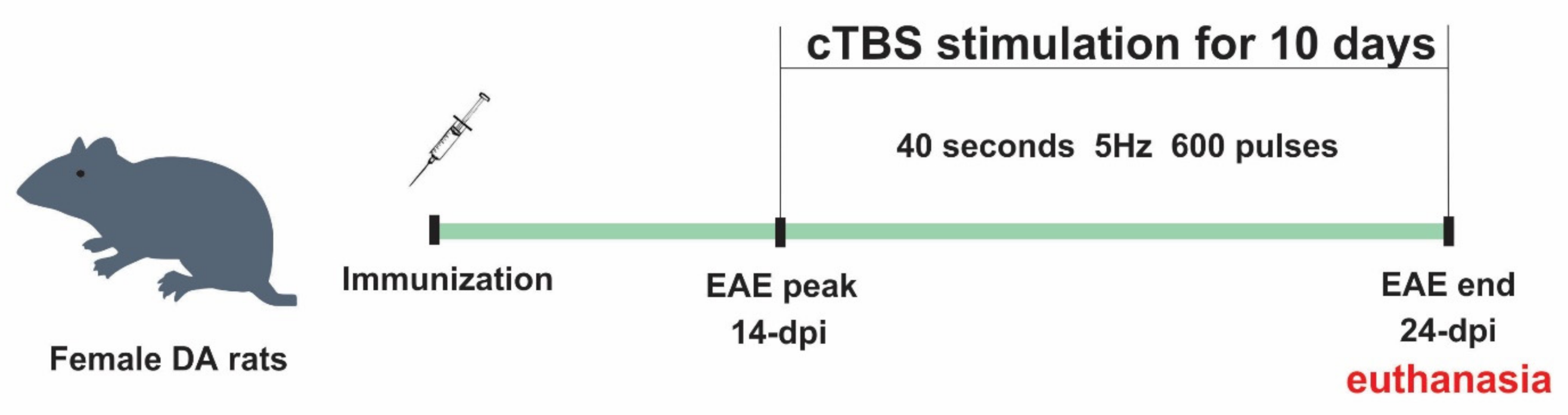
2.3. Induction of Experimental Autoimmune Encephalomyelitis
2.4. Theta-Burst Stimulation Protocol
2.5. Experimental Groups and Treatment
2.6. Enzyme Histochemistry
2.7. Immunofluorescence and Confocal Microscopy
2.8. Quantification of Immunofluorescence and Multi-Image Colocalization Analysis
2.9. Statistical Analysis
3. Results
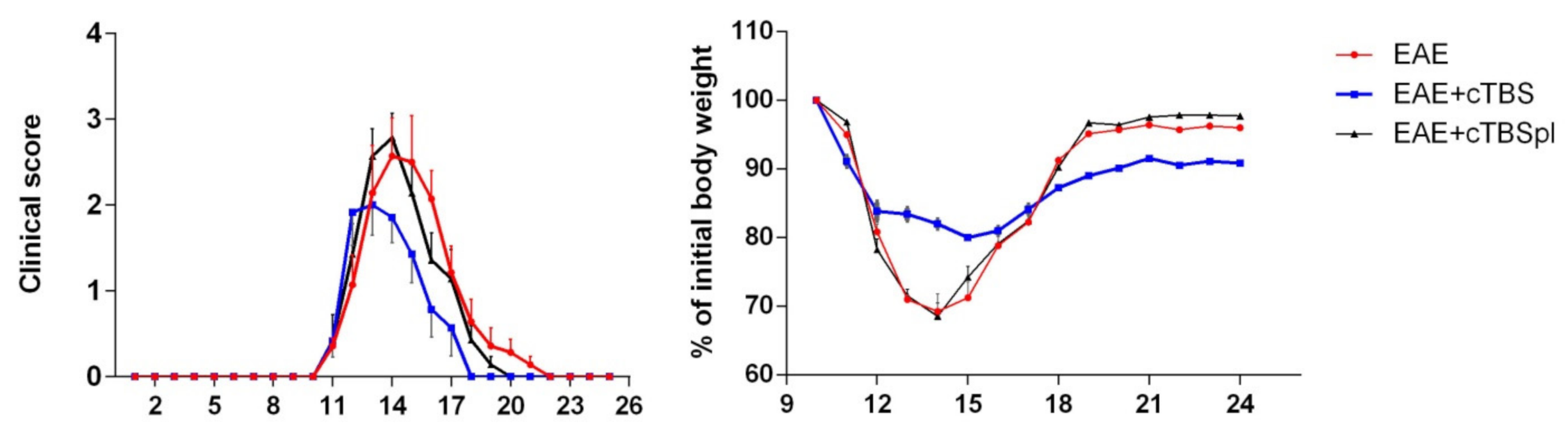
3.1. The Effect of Continuous Theta-Burst Stimulation on the Disease Course
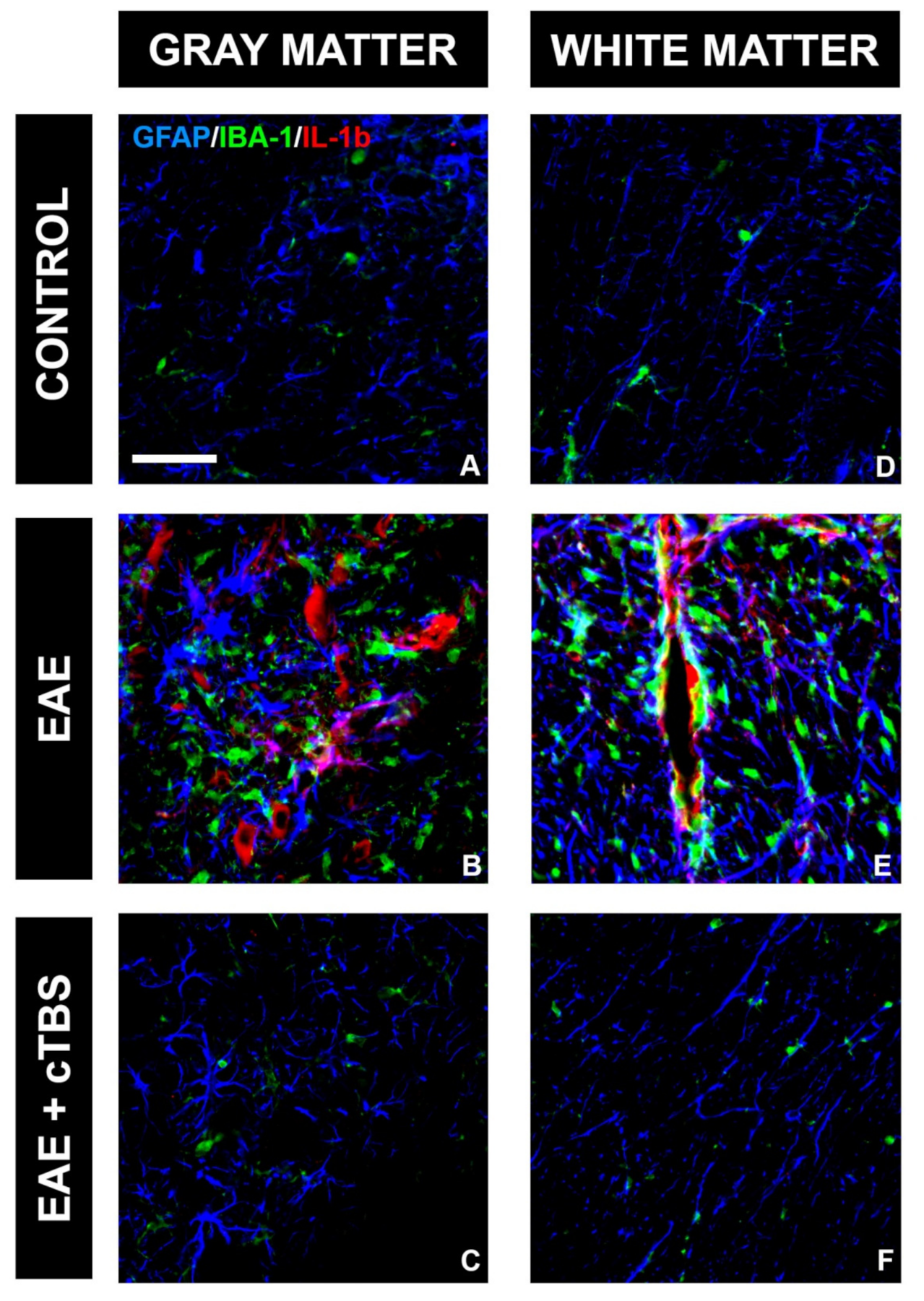
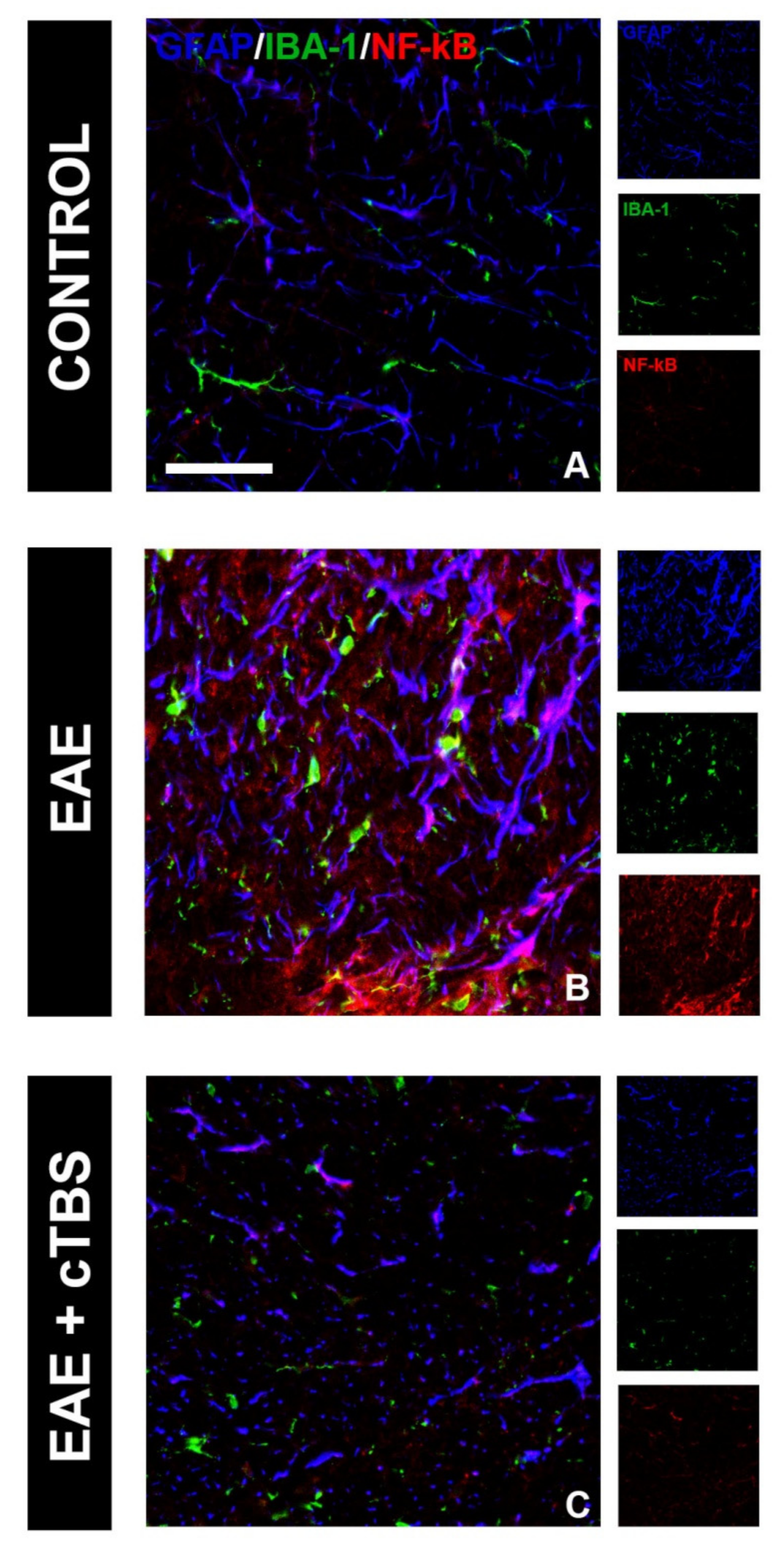
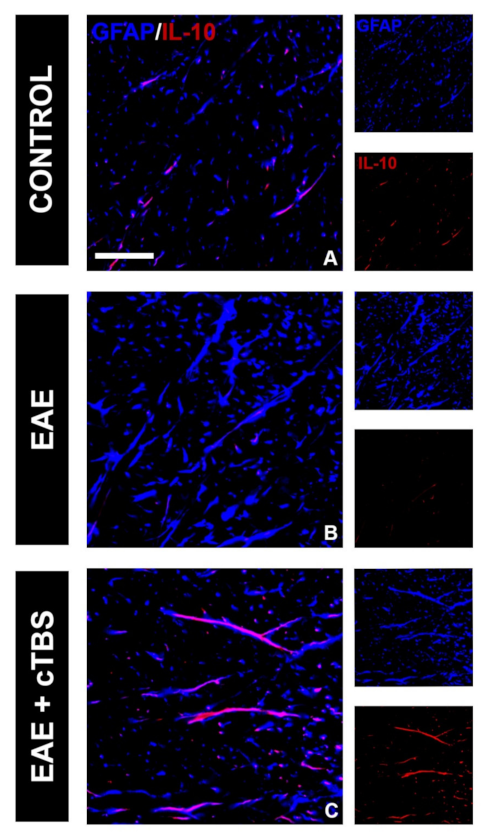
3.2. CTBS Promotes Anti-Inflammatory Milieu in EAE
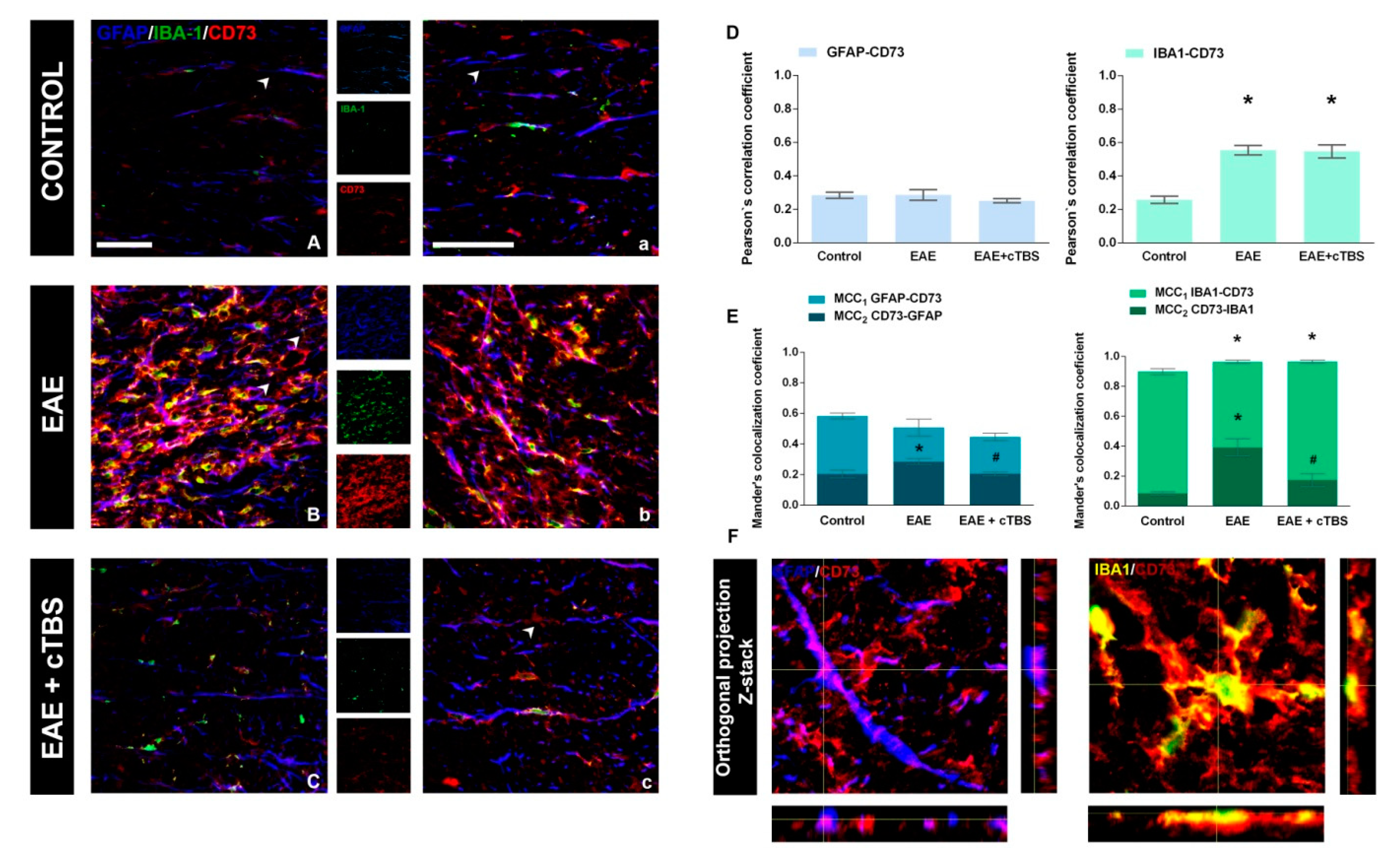
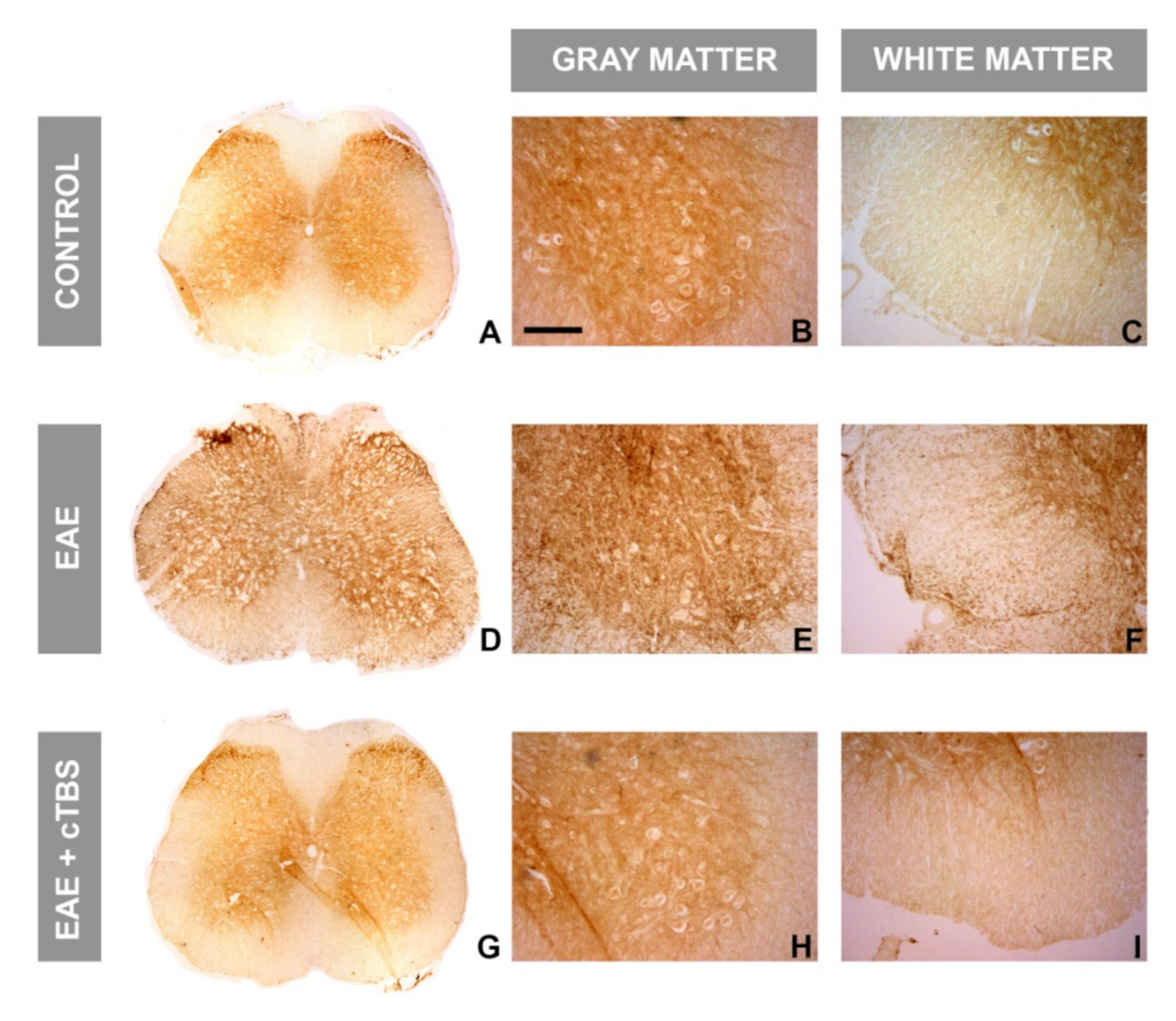
3.3. CTBS Attenuates EAE-Induced Expression of CD73
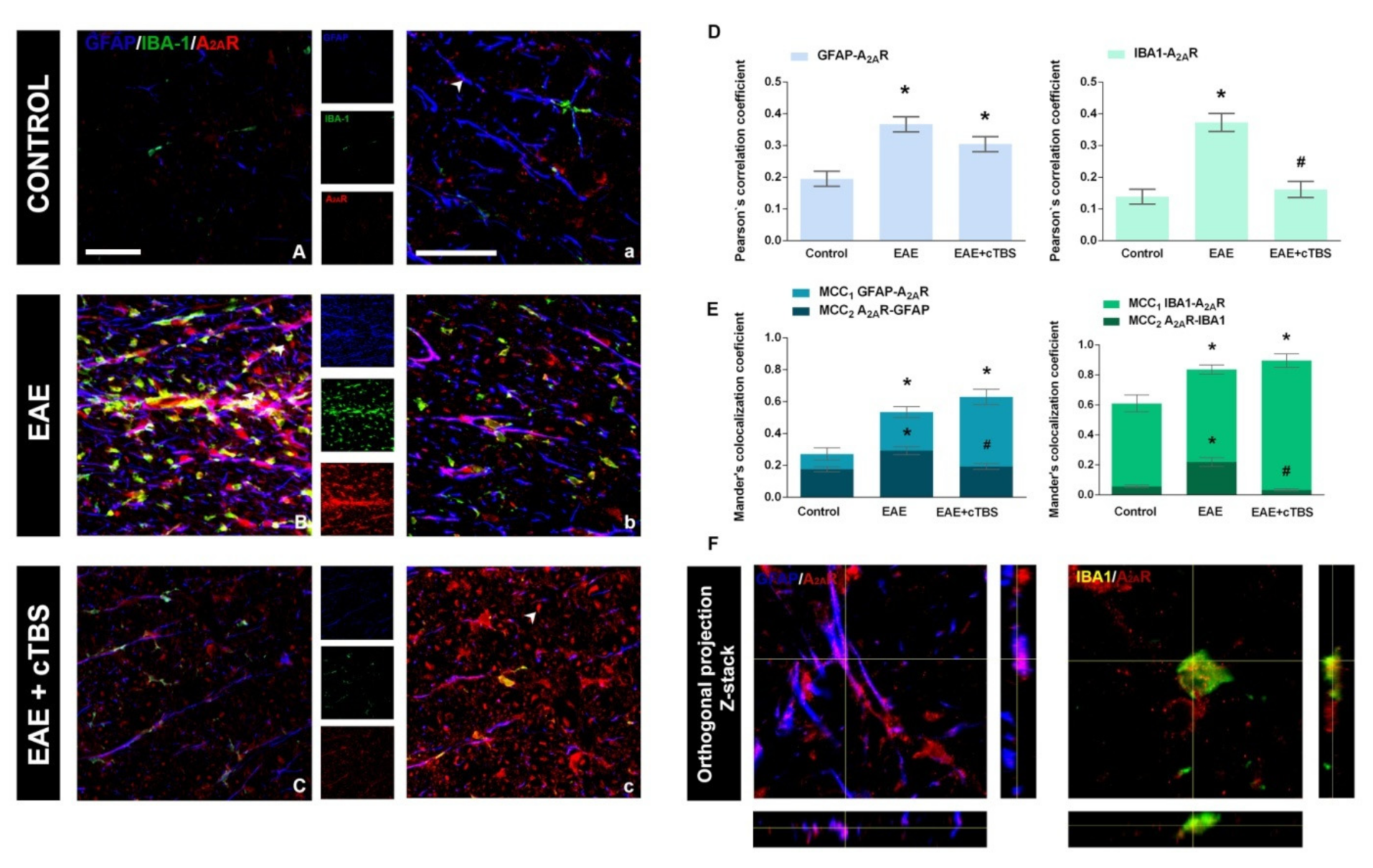
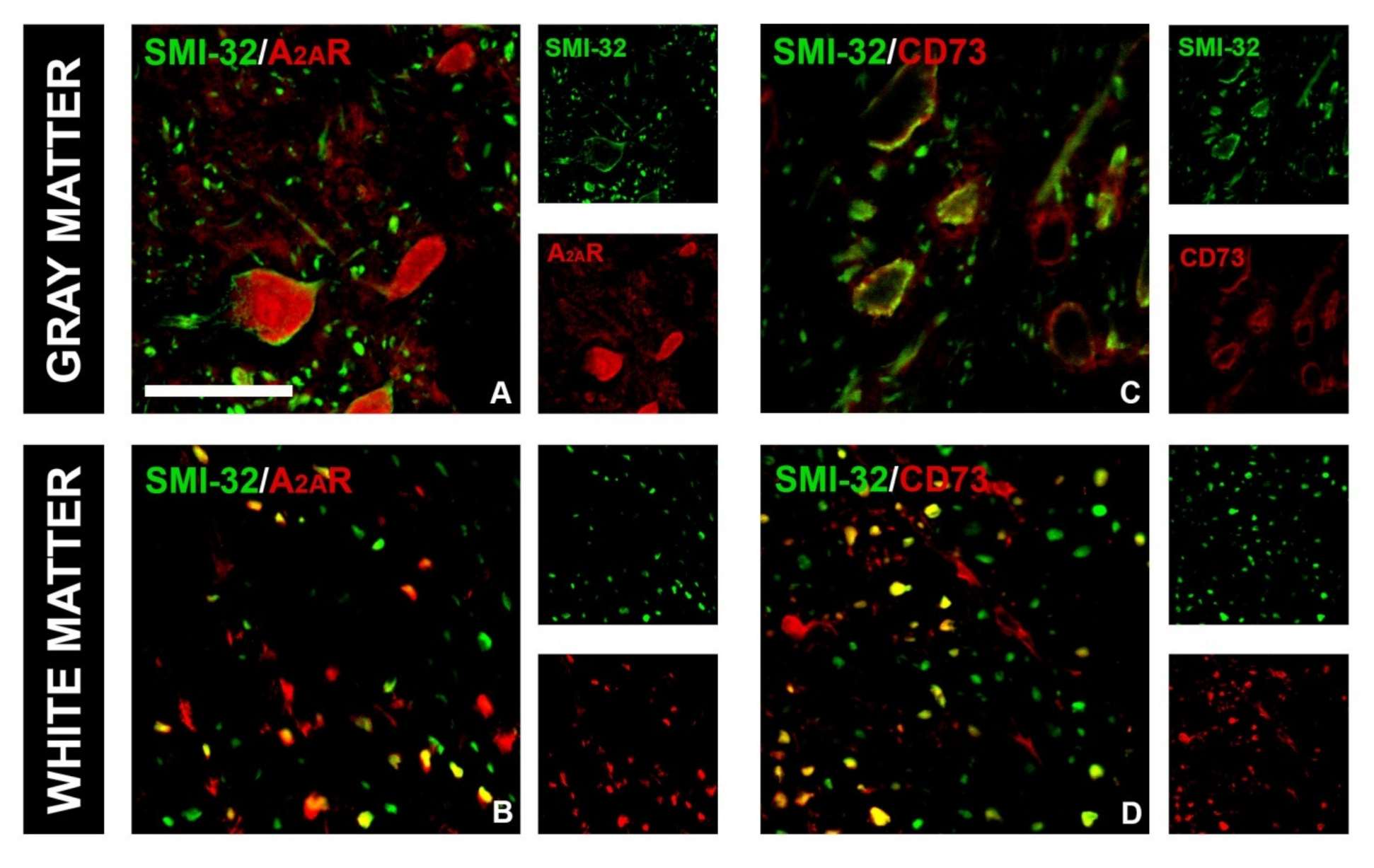
3.4. CTBS Attenuates EAE-Induced Upregulation of CD73 and Shift in A1R-to-A2AR Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The immunopathology of multiple sclerosis: An overview. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Preziosa, P.; Langdon, D.; Lassmann, H.; Paul, F.; Rovira, À.; Schoonheim, M.M.; Solari, A.; Stankoff, B.; Rocca, M.A. Identifying Progression in Multiple Sclerosis: New Perspectives. Ann. Neurol. 2020, 88, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2019, 137, 757–783. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.S.; Lees, J.G.; Moalem-Taylor, G. The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult. Scler. Int. 2014, 2014, 285245. [Google Scholar] [CrossRef] [PubMed]
- Compston, A.; Coles, A. Multiple sclerosis. Lancet 2002, 359, 1221–1231. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Lalor, S.J.; Sweeney, C.M.; Tubridy, N.; Mills, K.H.G. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sättler, M.B.; Bähr, M. Future neuroprotective strategies. Exp. Neurol. 2010, 225, 40–47. [Google Scholar] [CrossRef] [PubMed]
- di Virgilio, F.; Ceruti, S.; Bramanti, P.; Abbracchio, M.P. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009, 32, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.L.; Sarti, A.C.; di Virgilio, F. Ectonucleotidases in Acute and Chronic Inflammation. Front. Pharmacol. 2020, 11, 619458. [Google Scholar] [CrossRef]
- Yegutkin, G.G. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 2008, 1783, 673–694. [Google Scholar] [CrossRef]
- Adzic, M.; Nedeljkovic, N. Unveiling the Role of Ecto-5′-Nucleotidase/CD73 in Astrocyte Migration by Using Pharmacological Tools. Front. Pharmacol. 2018, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, G.; Amorini, A.M.; Eikelenboom, M.; Killestein, J.; Belli, A.; Di Pietro, V.; Tavazzi, B.; Barkhof, F.; Polman, C.; Uitdehaag, B.; et al. Cerebrospinal fluid ATP metabolites in multiple sclerosis. Mult. Scler. 2010, 16, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Lavrnja, I.; Bjelobaba, I.; Stojiljkovic, M.; Pekovic, S.; Mostarica-Stojkovic, M.; Stošić-Grujičić, S.; Nedeljkovic, N. Time-course changes in ectonucleotidase activities during experimental autoimmune encephalomyelitis. Neurochem. Int. 2009, 55, 193–198. [Google Scholar] [CrossRef]
- Lavrnja, I.; Laketa, D.; Savić, D.; Bozic, I.; Bjelobaba, I.; Pekovic, S.; Nedeljkovic, N. Expression of a second ecto-5’-nucleotidase variant besides the usual protein in symptomatic phase of experimental autoimmune encephalomyelitis. J. Mol. Neurosci. MN 2015, 55, 898–911. [Google Scholar] [CrossRef]
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Ceruti, S. Roles of P2 receptors in glial cells: Focus on astrocytes. Purinergic Signal. 2006, 2, 595–604. [Google Scholar] [CrossRef]
- di Virgilio, F.; Vuerich, M. Purinergic signaling in the immune system. Auton. Neurosci. Basic Clin. 2015, 191, 117–123. [Google Scholar] [CrossRef]
- Nedeljkovic, N. Complex regulation of ecto-5’-nucleotidase/CD73 and A(2A)R-mediated adenosine signaling at neurovascular unit: A link between acute and chronic neuroinflammation. Pharmacol. Res. 2019, 144, 99–115. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Chen, J.-F.; Cunha, R.A.; Svenningsson, P.; Vaugeois, J.-M. Adenosine and brain function. Int. Rev. Neurobiol. 2005, 63, 191–270. [Google Scholar]
- Smith, G.E.; Pankratz, V.S.; Negash, S.; Machulda, M.M.; Petersen, R.C.; Boeve, B.F.; Knopman, D.S.; Lucas, J.A.; Ferman, T.J.; Graff-Radford, N.; et al. A plateau in pre-Alzheimer memory decline: Evidence for compensatory mechanisms? Neurology 2007, 69, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, M.R.; Vance, C.O.; Morschl, E.; Wilson, C.N. Adenosine receptors and inflammation. Handb. Exp. Pharmacol. 2009, 193, 215–269. [Google Scholar]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-invasive magnetic stimulation of human motor cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Hallett, M. Transcranial magnetic stimulation: A primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014-2018). Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Rothwell, J.C.; Chen, R.-S.; Lu, C.-S.; Chuang, W.-L. The theoretical model of theta burst form of repetitive transcranial magnetic stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2011, 122, 1011–1018. [Google Scholar] [CrossRef]
- Koch, G.; Brusa, L.; Caltagirone, C.; Peppe, A.; Oliveri, M.; Stanzione, P.; Centonze, D. rTMS of supplementary motor area modulates therapy-induced dyskinesias in Parkinson disease. Neurology 2005, 65, 623–625. [Google Scholar] [CrossRef]
- George, M.S.; Lisanby, S.H.; Avery, D.; McDonald, W.M.; Durkalski, V.; Pavlicova, M.; Anderson, B.; Nahas, Z.; Bulow, P.; Zarkowski, P.; et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: A sham-controlled randomized trial. Arch. Gen. Psychiatry 2010, 67, 507–516. [Google Scholar] [CrossRef]
- Khedr, E.M.; Fetoh, N.A.-E. Short- and long-term effect of rTMS on motor function recovery after ischemic stroke. Restor. Neurol. Neurosci. 2010, 28, 545–559. [Google Scholar] [CrossRef]
- Ridding, M.C.; Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 2010, 588, 2291–2304. [Google Scholar] [CrossRef]
- Hasan, A.; Guse, B.; Cordes, J.; Wölwer, W.; Winterer, G.; Gaebel, W.; Langguth, B.; Landgrebe, M.; Eichhammer, P.; Frank, E.; et al. Cognitive Effects of High-Frequency rTMS in Schizophrenia Patients With Predominant Negative Symptoms: Results From a Multicenter Randomized Sham-Controlled Trial. Schizophr. Bull. 2016, 42, 608–618. [Google Scholar] [CrossRef]
- Rabey, J.M.; Dobronevsky, E. Repetitive transcranial magnetic stimulation (rTMS) combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: Clinical experience. J. Neural Transm. 2016, 123, 1449–1455. [Google Scholar] [CrossRef]
- Nasios, G.; Messinis, L.; Dardiotis, E.; Papathanasopoulos, P. Repetitive Transcranial Magnetic Stimulation, Cognition, and Multiple Sclerosis: An Overview. Behav. Neurol. 2018, 2018, 8584653. [Google Scholar] [CrossRef]
- Liu, M.; Fan, S.; Xu, Y.; Cui, L. Non-invasive brain stimulation for fatigue in multiple sclerosis patients: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2019, 36, 101375. [Google Scholar] [CrossRef]
- Centonze, D.; Petta, F.; Versace, V.; Rossi, S.; Torelli, F.; Prosperetti, C.; Marfia, G.; Bernardi, G.; Koch, G.; Miano, R.; et al. Effects of motor cortex rTMS on lower urinary tract dysfunction in multiple sclerosis. Mult. Scler. 2007, 13, 269–271. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.-V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS ONE 2013, 8, e55774. [Google Scholar] [CrossRef]
- Stevanovic, I.; Ninkovic, M.; Mancic, B.; Milivojevic, M.; Stojanovic, I.; Ilic, T.; Vujovic, M.; Djukic, M. Compensatory Neuroprotective Response of Thioredoxin Reductase against Oxidative-Nitrosative Stress Induced by Experimental Autoimmune Encephalomyelitis in Rats: Modulation by Theta Burst Stimulation. Molecules 2020, 25, 3922. [Google Scholar] [CrossRef]
- Medina-Fernandez, F.J.; Escribano, B.M.; Agüera, E.; Aguilar-Luque, M.; Feijoo, M.; Luque, E.; Garcia-Maceira, F.I.; Pascual-Leone, A.; Drucker-Colin, R.; Tunez, I.; et al. Effects of transcranial magnetic stimulation on oxidative stress in experimental autoimmune encephalomyelitis. Free Radic. Res. 2017, 51, 460–469. [Google Scholar] [CrossRef]
- Dragic, M.; Zeljkovic, M.; Stevanovic, I.; Ilic, T.; Ilic, N.; Nedeljkovic, N.; Ninkovic, M. Theta burst stimulation ameliorates symptoms of experimental autoimmune encephalomyelitis and attenuates reactive gliosis. Brain Res. Bull. 2020, 162, 208–217. [Google Scholar] [CrossRef]
- Stevanovic, I.; Mancic, B.; Ilic, T.; Milosavljevic, P.; Lavrnja, I.; Stojanovic, I.; Ninkovic, M. Theta burst stimulation influence the expression of BDNF in the spinal cord on the experimental autoimmune encephalomyelitis. Folia Neuropathol. 2019, 57, 129–145. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Lavrnja, I.; Bozic, I.; Savic, D.; Bjelobaba, I.; Pekovic, S.; Sévigny, J.; Nedeljkovic, N.; Laketa, D. Down-regulation of NTPDase2 and ADP-sensitive P2 Purinoceptors Correlate with Severity of Symptoms during Experimental Autoimmune Encephalomyelitis. Front. Cell. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Mancic, B.; Stevanovic, I.; Ilic, T.V.; Djuric, A.; Stojanovic, I.; Milanovic, S.; Ninkovic, M. Transcranial theta-burst stimulation alters GLT-1 and vGluT1 expression in rat cerebellar cortex. Neurochem. Int. 2016, 100, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Chen, R.S.; Rothwell, J.C.; Wen, H.Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin. Neurophysiol. 2007, 118, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Dragić, M.; Zarić, M.; Mitrović, N.; Nedeljković, N.; Grković, I. Application of Gray Level Co-Occurrence Matrix Analysis as a New Method for Enzyme Histochemistry Quantification. Microsc. Microanal. 2019, 25, 690–698. [Google Scholar] [CrossRef]
- Dunn, K.W.; Kamocka, M.M.; McDonald, J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Physiol. 2011, 300, C723–C742. [Google Scholar] [CrossRef]
- Lin, C.-C.; Edelson, B.T. New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. J. Immunol. 2017, 198, 4553–4560. [Google Scholar] [CrossRef]
- Lin, W.; Yue, Y.; Stone, S. Role of nuclear factor κB in multiple sclerosis and experimental autoimmune encephalomyelitis. Neural Regen. Res. 2018, 13, 1507–1515. [Google Scholar] [CrossRef]
- van Loo, G.; De Lorenzi, R.; Schmidt, H.; Huth, M.; Mildner, A.; Schmidt-Supprian, M.; Lassmann, H.; Prinz, M.R.; Pasparakis, M. Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat. Immunol. 2006, 7, 954–961. [Google Scholar] [CrossRef]
- Ozenci, V.; Kouwenhoven, M.; Huang, Y.M.; Xiao, B.; Kivisäkk, P.; Fredrikson, S.; Link, H. Multiple sclerosis: Levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-beta-1b treatment. Scand. J. Immunol. 1999, 49, 554–561. [Google Scholar] [CrossRef]
- Burnstock, G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology 2016, 104, 4–17. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Lavrnja, I.; Bozic, I.; Milosevic, A.; Bjelobaba, I.; Savic, D.; Sévigny, J.; Pekovic, S.; Nedeljkovic, N.; Laketa, D. Induction of NTPDase1/CD39 by Reactive Microglia and Macrophages Is Associated With the Functional State During EAE. Front. Neurosci. 2019, 13, 410. [Google Scholar] [CrossRef]
- Bijelić, D.D.; Milićević, K.D.; Lazarević, M.N.; Miljković, D.M.; Pristov, J.J.B.; Savić, D.Z.; Petković, B.B.; Andjus, P.R.; Momčilović, M.B.; Nikolić, L.M. Central nervous system-infiltrated immune cells induce calcium increase in astrocytes via astroglial purinergic signaling. J. Neurosci. Res. 2020, 98, 2317–2332. [Google Scholar] [CrossRef]
- Safarzadeh, E.; Jadidi-Niaragh, F.; Motallebnezhad, M.; Yousefi, M. The role of adenosine and adenosine receptors in the immunopathogenesis of multiple sclerosis. Inflamm. Res. 2016, 65, 511–520. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, G.; Guo, J.; Kong, F.; Chen, S.; Wang, Z. Pro-inflammatory Effect of Downregulated CD73 Expression in EAE Astrocytes. Front. Cell. Neurosci. 2019, 13, 233. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef]
- Haskó, G.; Cronstein, B. Regulation of inflammation by adenosine. Front. Immunol. 2013, 4, 85. [Google Scholar] [CrossRef]
- Morandi, F.; Horenstein, A.L.; Rizzo, R.; Malavasi, F. The Role of Extracellular Adenosine Generation in the Development of Autoimmune Diseases. Mediat. Inflamm. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Cunha, R.A. Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Purinergic Signal. 2005, 1, 111–134. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Hinz, S.; Navarro, G.; Franco, R.; Müller, C.E.; Fuxe, K. Understanding the Role of Adenosine A2AR Heteroreceptor Complexes in Neurodegeneration and Neuroinflammation. Front. Neurosci. 2018, 12, 43. [Google Scholar] [CrossRef]
- George, M.S.; Wassermann, E.M.; Williams, W.A.; Callahan, A.; Ketter, T.A.; Basser, P.; Hallett, M.; Post, R.M. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. NeuroReport 1995, 6, 1853–1856. [Google Scholar] [CrossRef]
- Siebner, H.R.; Mentschel, C.; Auer, C.; Conrad, B. Repetitive transcranial magnetic stimulation has a beneficial effect on bradykinesia in Parkinson’s disease. Neuroreport 1999, 10, 589–594. [Google Scholar] [CrossRef]
- Downar, J.; Daskalakis, Z.J. New Targets for rTMS in Depression: A Review of Convergent Evidence. Brain Stimul. 2013, 6, 231–240. [Google Scholar] [CrossRef]
- Okada, K.; Matsunaga, K.; Yuhi, T.; Kuroda, E.; Yamashita, U.; Tsuji, S. The long-term high-frequency repetitive transcranial magnetic stimulation does not induce mRNA expression of inflammatory mediators in the rat central nervous system. Brain Res. 2002, 957, 37–41. [Google Scholar] [CrossRef]
- Sasso, V.; Bisicchia, E.; Latini, L.; Ghiglieri, V.; Cacace, F.; Carola, V.; Molinari, M.; Viscomi, M.T. Repetitive transcranial magnetic stimulation reduces remote apoptotic cell death and inflammation after focal brain injury. J. Neuroinflamm. 2016, 13, 150. [Google Scholar] [CrossRef]
- Clarke, D.; Beros, J.; Bates, K.A.; Harvey, A.R.; Tang, A.D.; Rodger, J. Low intensity repetitive magnetic stimulation reduces expression of genes related to inflammation and calcium signalling in cultured mouse cortical astrocytes. Brain Stimul. 2021, 14, 183–191. [Google Scholar] [CrossRef]
- Mills, J.H.; Thompson, L.F.; Mueller, C.; Waickman, A.T.; Jalkanen, S.; Niemela, J.; Airas, L.; Bynoe, M.S. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2008, 105, 9325–9330. [Google Scholar] [CrossRef]
- Mills, J.H.; Alabanza, L.M.; Mahamed, D.A.; Bynoe, M.S. Extracellular adenosine signaling induces CX3CL1 expression in the brain to promote experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2012, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, W.; Guo, J.; Kong, F.; Zhou, S.; Chen, S.; Wang, Z.; Zang, D. Adenosine binds predominantly to adenosine receptor A1 subtype in astrocytes and mediates an immunosuppressive effect. Brain Res. 2018, 1700, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.H.; Fan, S.; Maguire, C.A.; Perry, S.; Hardiek, K.; Ramkumar, V.; Gelbard, H.A.; Dewhurst, S.; Maggirwar, S.B. Activation of adenosine A2A receptor protects sympathetic neurons against nerve growth factor withdrawal. J. Neurosci. Res. 2004, 77, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Jablonka, S.; Holtmann, B.; Orel, N.; Rajagopal, R.; Chao, M.V.; Sendtner, M. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc. Natl. Acad. Sci. USA 2007, 104, 17210–17215. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Bak, H.; Seo, J.; Han, S.M.; Lee, S.H.; Han, S.-H.; Kwon, K.J.; Ryu, J.H.; Cheong, J.H.; Ko, K.H.; et al. Oroxylin A Induces BDNF Expression on Cortical Neurons through Adenosine A2A Receptor Stimulation: A Possible Role in Neuroprotection. Biomol. Ther. 2012, 20, 27–35. [Google Scholar] [CrossRef]
- Kopec, B.M.; Kiptoo, P.; Zhao, L.; Rosa-Molinar, E.; Siahaan, T.J. Noninvasive Brain Delivery and Efficacy of BDNF to Stimulate Neuroregeneration and Suppression of Disease Relapse in EAE Mice. Mol. Pharm. 2019, 17, 404–416. [Google Scholar] [CrossRef]
- Costenla, A.R.; Diógenes, M.J.; Canas, P.M.; Rodrigues, R.J.; Nogueira, C.; Maroco, J.; Agostinho, P.M.; Ribeiro, J.A.; Cunha, R.A.; De Mendonça, A. Enhanced role of adenosine A2A receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur. J. Neurosci. 2011, 34, 12–21. [Google Scholar] [CrossRef]

| Antibody | Source and Type | Used Dilution | Manufacturer |
|---|---|---|---|
| Iba-1 | Goat, polyclonal | 1:400 | Abcam ab5076, RRID:AB_2224402 |
| CD73, rNu-9L(I4,I5) | Rabbit, polyclonal | 1:300 | Ectonucleotidases-ab.com |
| GFAP | Rabbit, polyclonal | 1:500 | DAKO, Agilent Z0334, RRID:AB_10013382 |
| IL-10 | Goat, polyclonal | 1:100 | Santa Cruz Biotechnology, sc-1783, RRID: AB_2125115 |
| NF-kB | Rabbit, polyclonal | 1:100 | Santa Cruz Biotechnology, sc-109, RRID: AB_632039 |
| IL-1β/IL-1F2 | Goat, polyclonal | 1:100 | R&D Systems, AF-501-NA, RRID: AB_ 354508 |
| A2AR | Rabbit, polyclonal | 1:300 | Abcam, ab3461, RRID: AB_303823 |
| A1R | Rabbit, polyclonal | 1:200 | Novus Biologicals, NB300-549, RRID: AB_10002337 |
| Anti-mouse IgG Alexa Fluor 488 | Donkey, polyclonal | 1:400 | Invitrogen A21202, RRID:AB_141607 |
| Anti-goat IgG Alexa Fluor 488 | Donkey, polyclonal | 1:400 | Invitrogen A-11055, RRID:AB_142672 |
| Anti-rabbit IgG Alexa Fluor 555 | Donkey, polyclonal | 1:400 | Invitrogen A-21428, RRID:AB_141784 |
| Anti-mouse IgG Alexa Fluor 647 | Donkey, polyclonal | 1:400 | Thermo Fisher Scientific A-31571, RRID:AB_162542 |
| Analysis Performed | ANOVA Results | p Values |
|---|---|---|
| PCC GFAP–CD73 | F(2, 28) = 0.7792 | p = 0.4736 |
| PCC IBA1–CD73 | F(2, 31) = 33.48 | p < 0.0001 |
| MCC1 GFAP–CD73 | F(2, 28) = 3.228 | p = 0.0648 |
| MCC2 CD73-GFAP | F(2, 28) = 4.975 | p < 0.05 |
| MCC1 IBA1–CD73 | F(2, 27) = 5.482 | p < 0.05 |
| MCC2 CD73–IBA1 | F(2, 27) = 17.05 | p < 0.0001 |
| PCC A1R–CD73 | F(2, 30) = 22.19 | p < 0.0001 |
| PCC IBA1–A1R | F(2, 28) = 9.155 | p < 0.01 |
| MCC1 GFAP–A1R | F(2, 27) = 24.45 | p < 0.0001 |
| MCC2 A1R-GFAP | F(2, 27) = 9.217 | p < 0.01 |
| MCC1 IBA1–A1R | F(2, 28) = 9.502 | p < 0.01 |
| MCC2 A1R–IBA1 | F(2, 28) = 5.458 | p < 0.05 |
| PCC GFAP–A2AR | F(2, 33) = 12.74 | p < 0.001 |
| PCC IBA1–A2AR | F(2, 32) = 25.23 | p < 0.0001 |
| MCC1 GFAP–A2AR | F(2, 26) = 20.86 | p < 0.0001 |
| MCC2 A2AR-GFAP | F(2, 27) = 8.629 | p < 0.01 |
| MCC1 IBA1–A2AR | F(2, 29) = 10.93 | p < 0.001 |
| MCC2 A2AR–IBA1 | F(2, 29) = 31.472 | p < 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragić, M.; Zeljković, M.; Stevanović, I.; Adžić, M.; Stekić, A.; Mihajlović, K.; Grković, I.; Ilić, N.; Ilić, T.V.; Nedeljković, N.; et al. Downregulation of CD73/A2AR-Mediated Adenosine Signaling as a Potential Mechanism of Neuroprotective Effects of Theta-Burst Transcranial Magnetic Stimulation in Acute Experimental Autoimmune Encephalomyelitis. Brain Sci. 2021, 11, 736. https://doi.org/10.3390/brainsci11060736
Dragić M, Zeljković M, Stevanović I, Adžić M, Stekić A, Mihajlović K, Grković I, Ilić N, Ilić TV, Nedeljković N, et al. Downregulation of CD73/A2AR-Mediated Adenosine Signaling as a Potential Mechanism of Neuroprotective Effects of Theta-Burst Transcranial Magnetic Stimulation in Acute Experimental Autoimmune Encephalomyelitis. Brain Sciences. 2021; 11(6):736. https://doi.org/10.3390/brainsci11060736
Chicago/Turabian StyleDragić, Milorad, Milica Zeljković, Ivana Stevanović, Marija Adžić, Andjela Stekić, Katarina Mihajlović, Ivana Grković, Nela Ilić, Tihomir V. Ilić, Nadežda Nedeljković, and et al. 2021. "Downregulation of CD73/A2AR-Mediated Adenosine Signaling as a Potential Mechanism of Neuroprotective Effects of Theta-Burst Transcranial Magnetic Stimulation in Acute Experimental Autoimmune Encephalomyelitis" Brain Sciences 11, no. 6: 736. https://doi.org/10.3390/brainsci11060736
APA StyleDragić, M., Zeljković, M., Stevanović, I., Adžić, M., Stekić, A., Mihajlović, K., Grković, I., Ilić, N., Ilić, T. V., Nedeljković, N., & Ninković, M. (2021). Downregulation of CD73/A2AR-Mediated Adenosine Signaling as a Potential Mechanism of Neuroprotective Effects of Theta-Burst Transcranial Magnetic Stimulation in Acute Experimental Autoimmune Encephalomyelitis. Brain Sciences, 11(6), 736. https://doi.org/10.3390/brainsci11060736