Parkinson’s Disease in Romania: A Scoping Review
Abstract
1. Introduction
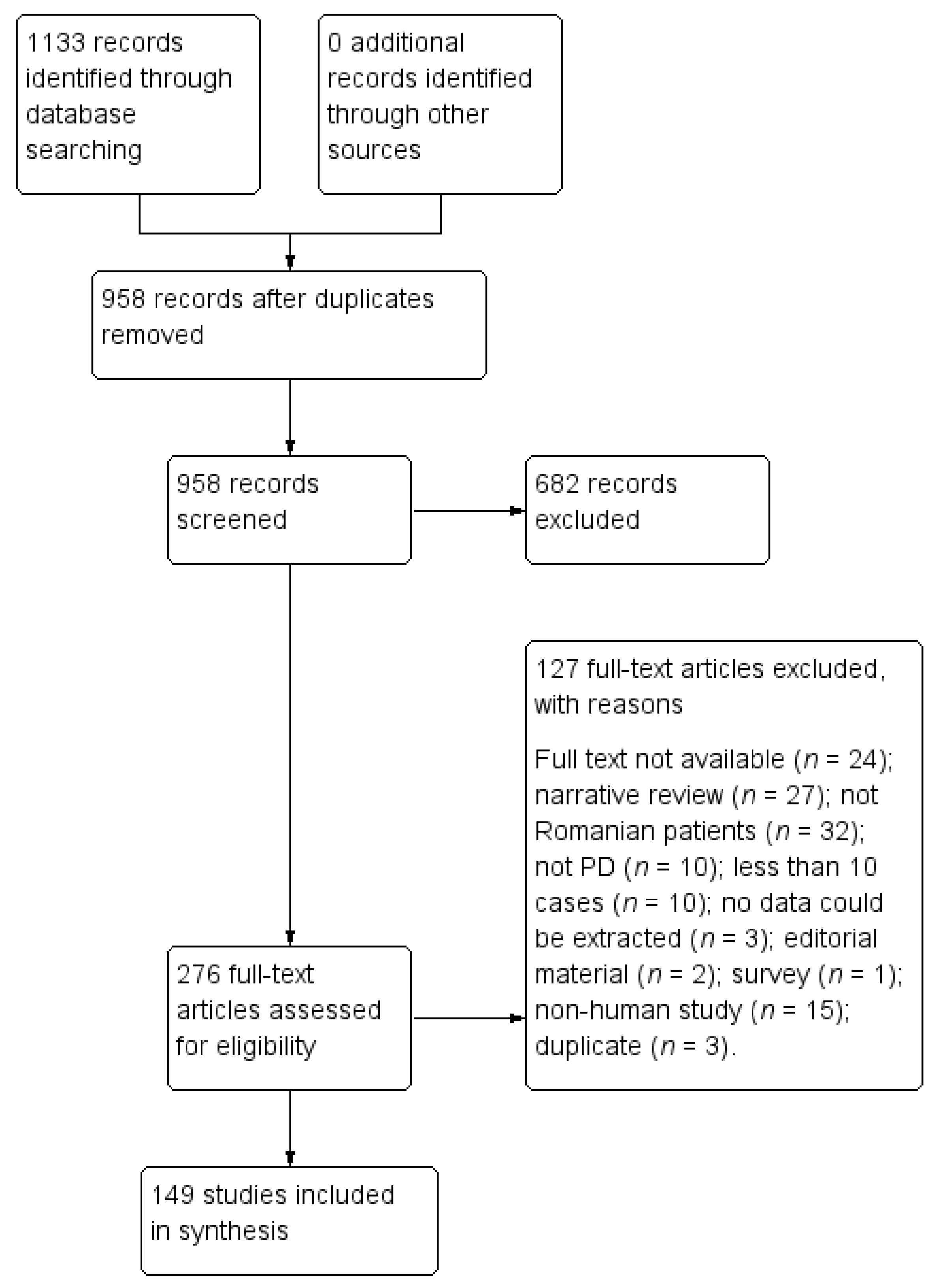
2. Materials and Methods
- What data are published on the epidemiology of PD in Romania?
- What clinical aspects have been investigated in Romanian PD patients?
- What are the interventions introduced in Romania to reduce the burden of PD and improve the patient’s care and quality of life?
- Which are the diagnostic tests used in PD patients in Romania?
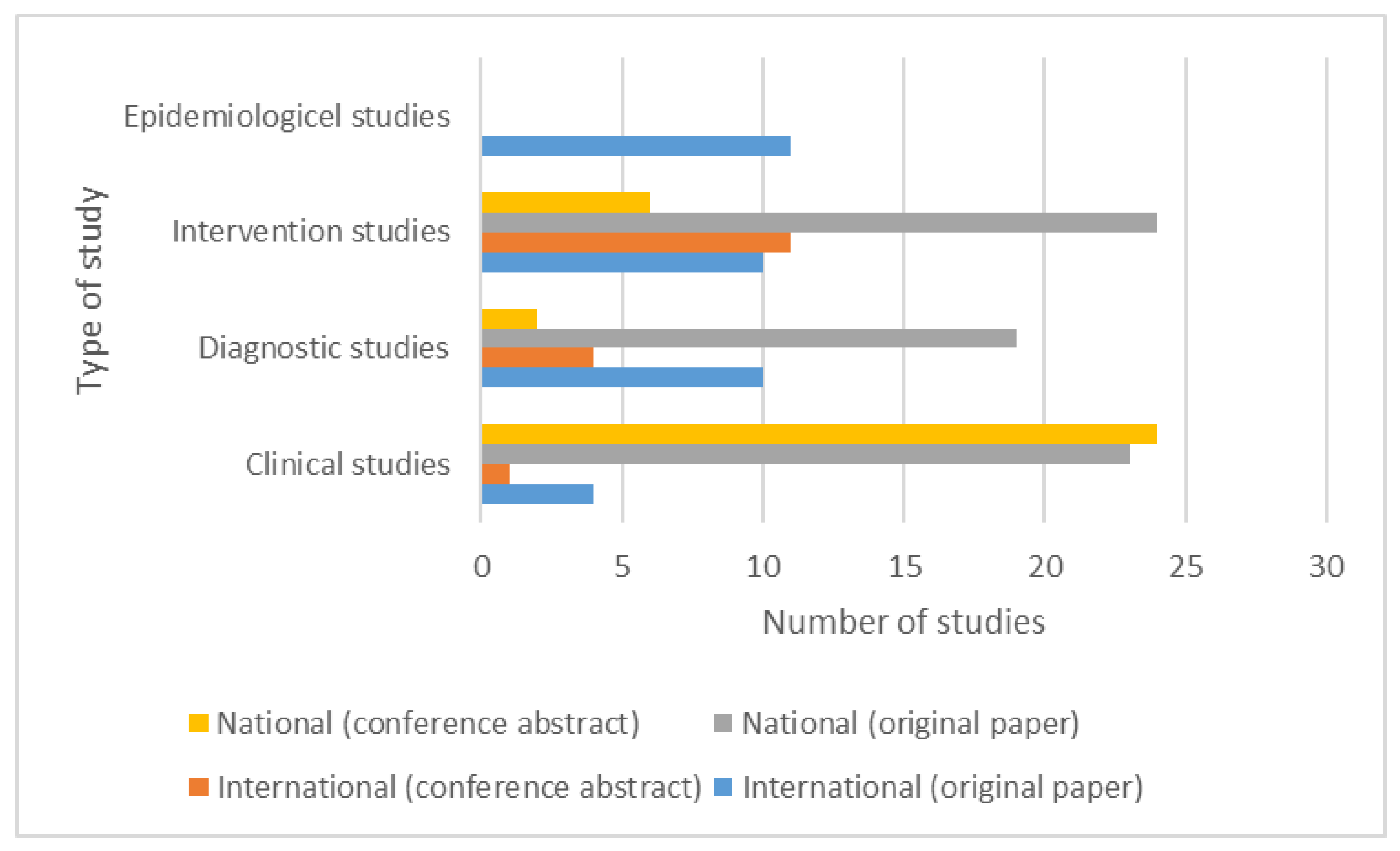
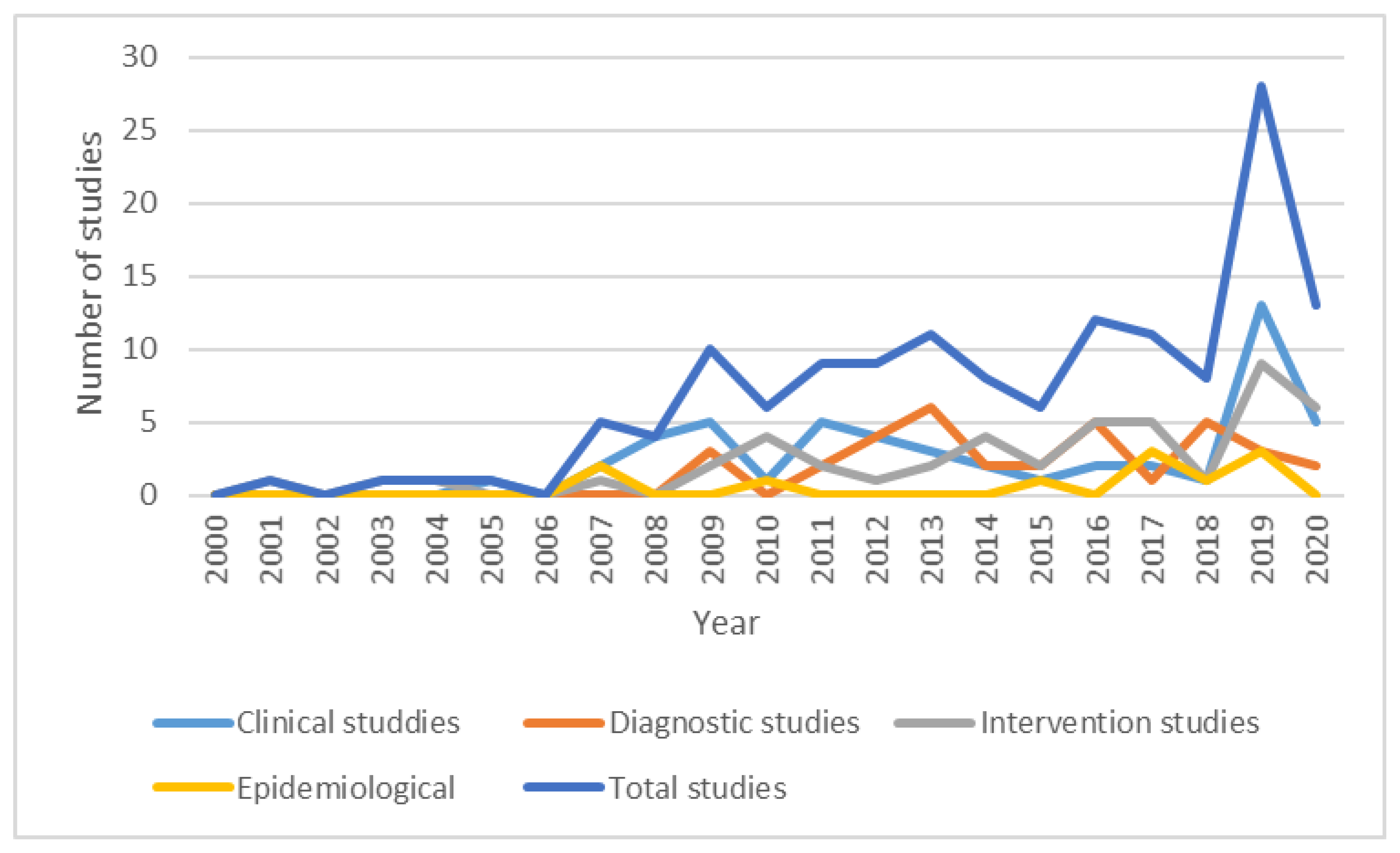
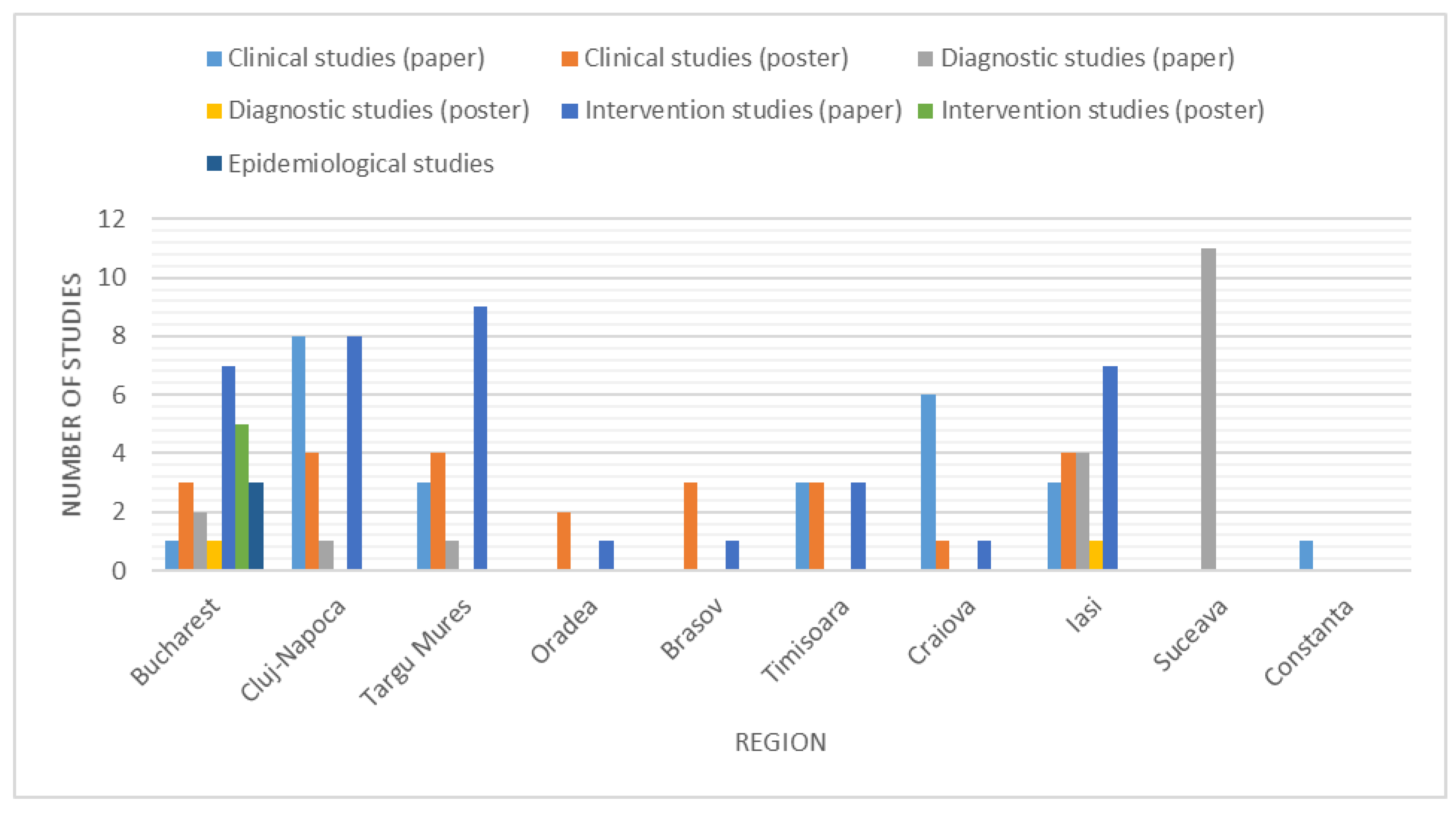
3. Results
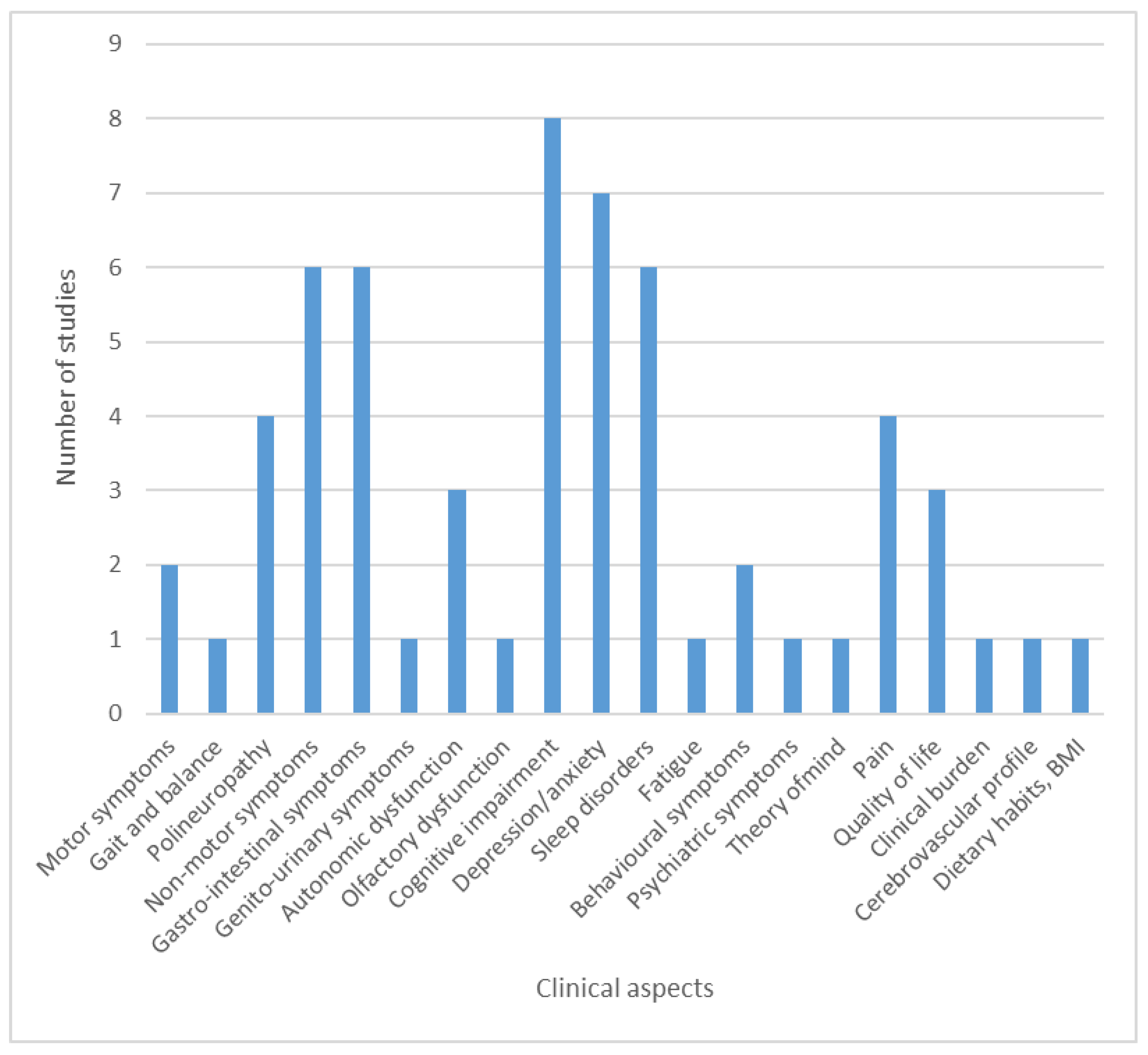
3.1. Studies Investigating Clinical Aspects of Parkinson’s Disease
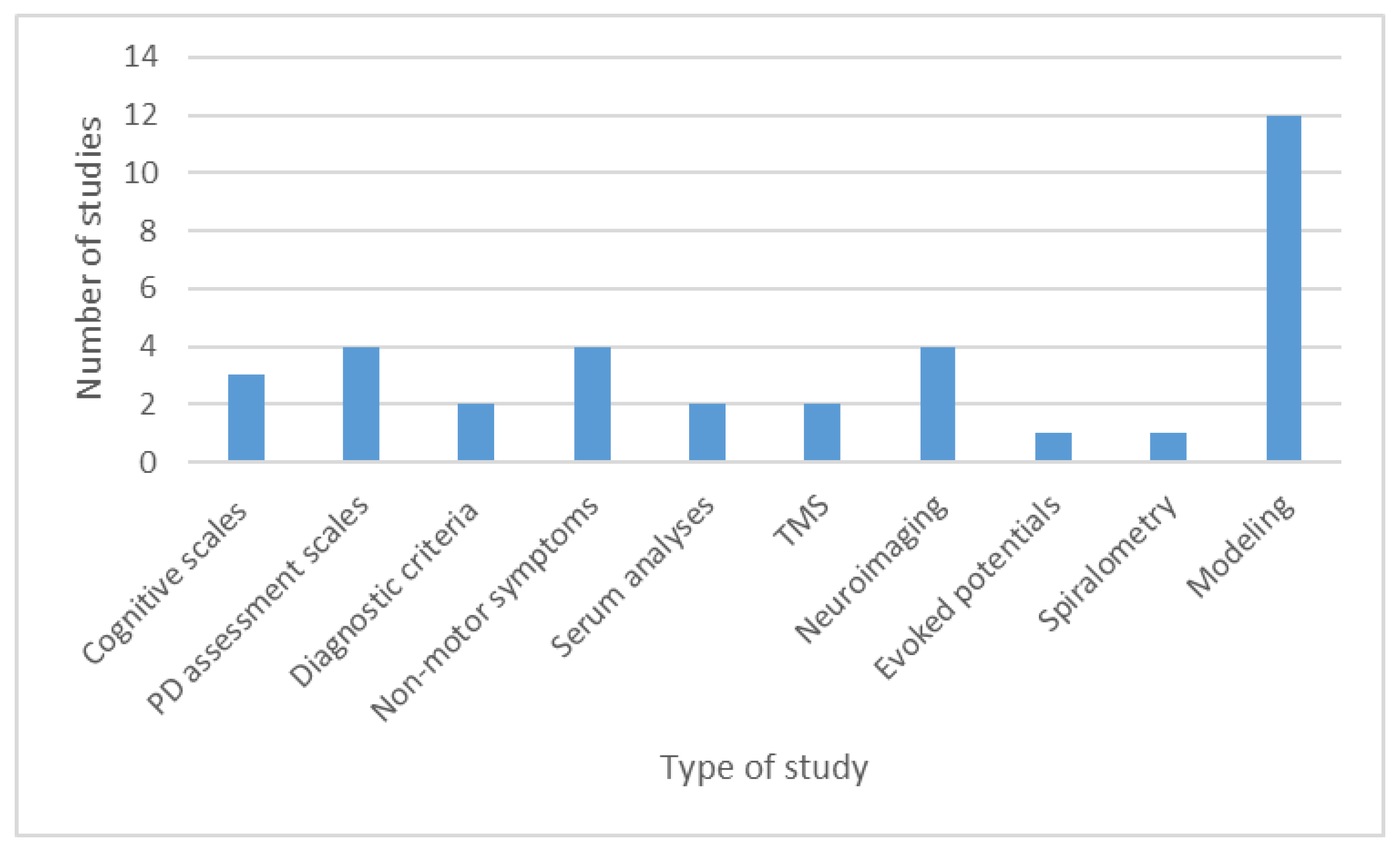
3.2. Studies on Diagnostic Tests in Parkinson’s Disease
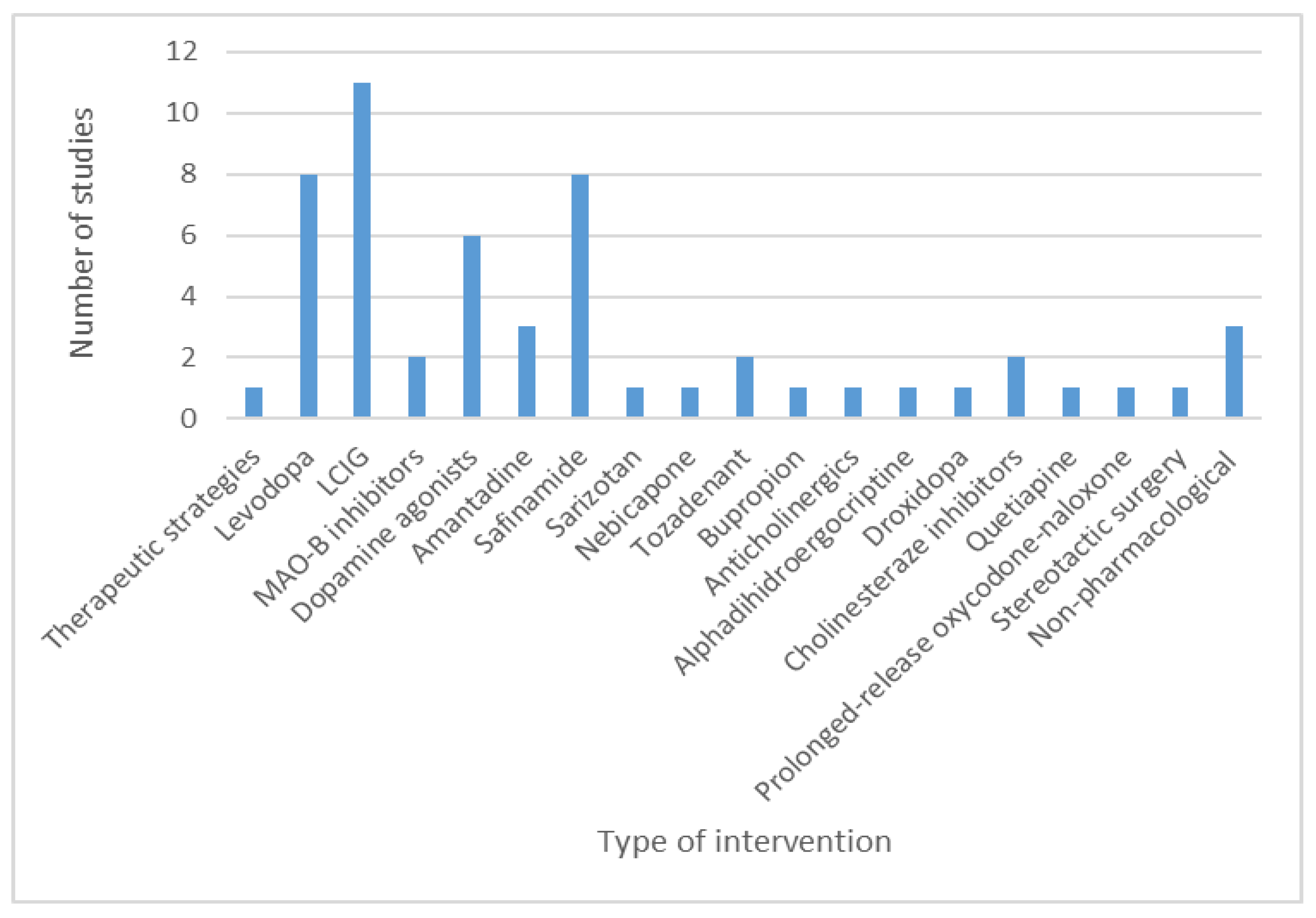
3.3. Studies on Pharmacological and Non-Pharmacological Interventions in Parkinson’s Disease
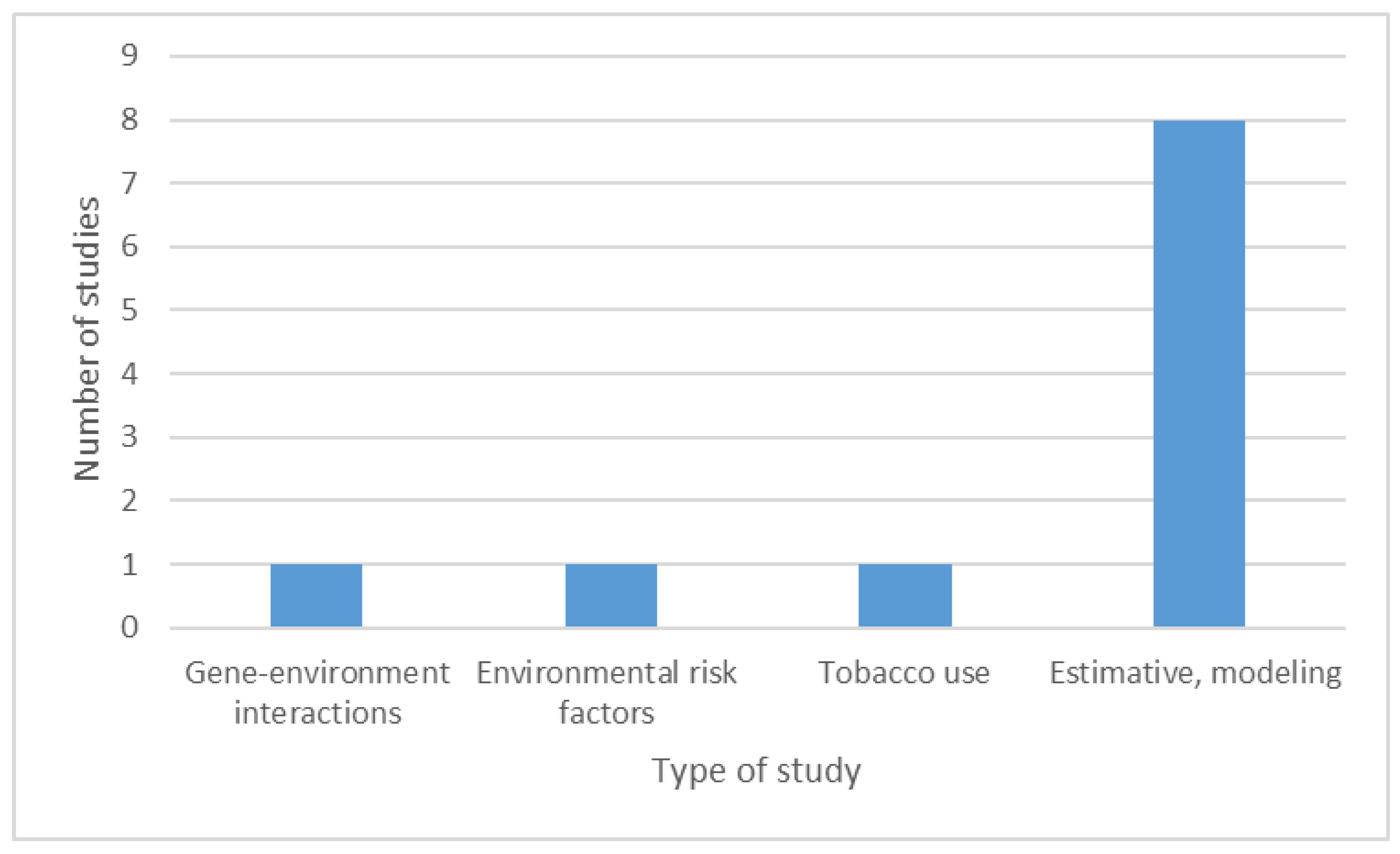
3.4. Epidemiological Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Rosca, E.C.; Tudor, R.; Cornea, A.; Simu, M. Parkinson’s Disease in Romania: A Scoping Review Protocol. Brain Sci. 2021, 11, 251. [Google Scholar] [CrossRef]
- Available online: http://www.asociatia-antiparkinson.ro/ (accessed on 25 January 2021).
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Godfrey, C.; McInerney, P.; Munn, Z.; Tricco, A.C.; Khalil, H. Chapter 11: Scoping Reviews (2020 Version). Available online: https://wiki.jbi.global/display/MANUAL/JBI+Manual+for+Evidence+Synthesis (accessed on 16 February 2021).
- Scherer, R.W.; Meerpohl, J.J.; Pfeifer, N.; Schmucker, C.; Schwarzer, G.; von Elm, E. Full publication of results initially presented in abstracts. Cochrane Database Syst. Rev. 2018, 11, MR000005. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Abbade, L.P.F.; Nwosu, I.; Jin, Y.; Leenus, A.; Maaz, M.; Wang, M.; Bhatt, M.; Zielinski, L.; Sanger, N.; et al. A scoping review of comparisons between abstracts and full reports in primary biomedical research. BMC Med. Res. Methodol. 2017, 17, 181. [Google Scholar] [CrossRef]
- Szocs, I.; Hojda, A.; Szalma, I.; Szasz, J.A.; Szatmari, S.Z. Possible correlation between mental dysfunction and mator performance in Parkinson’s disease patients. Eur. J. Neurol. 2005, 12, 109. [Google Scholar]
- Popescu, B.O.; Bajenaru, O.; Obretin, D.; Muresanu, D.F. PDQ 39 correlates with “on” time in patient diaries in fluctuating Parkinson’s disease patients. Eur. J. Neurol. 2007, 14, 205. [Google Scholar]
- Reisz, D.; Simu, M.A.; Chirileanu, D.R.; Tamasan, S. Depression and pseudo-depression in Parkinson’s disease. Eur. J. Neurol. 2007, 14, 206. [Google Scholar]
- Mihancea, P.; Brisc, C.M.; Havasi, N.; Brisc, C. The non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15, 122. [Google Scholar]
- Reisz, D.; Simu, M.A.; Chirileanu, D.R.; Carstina, A.; Bursa, A. Particularities of anxiety, depression and sleep disorder in Parkinson’s disease. Eur. J. Neurol. 2008, 15, 130. [Google Scholar]
- Stoian, A.; Barsan, A.O.; Szasz, J.A.; Szatmari, S.; Marusteri, M.; Mihai, A.; Incze, T.; Stoian, M.; Schiopu, A. Repression in Parkinson’s disease. Eur. J. Neurol. 2008, 15, 134. [Google Scholar]
- Szocs, I.; Szatmari, S.; Szasz, J.A. Motor fluctuations, depressive symptoms and excessive sleepiness influencing quality of life of Parkinson’s disease patients. Eur. J. Neurol. 2008, 15, 135. [Google Scholar]
- Martinez-Martin, P.; Falup-Pecurariu, C.; Rodriguez-Blazquez, C.; van Hilten, B.; Odin, P.; Chaudhuri, K.R.; Grp, E. Gender differences in non-motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, S66–S67. [Google Scholar] [CrossRef]
- Muntean, L.; Perju-Dumbrava, L.; Tohanean, N.; Perju-Dumbrava, L. Pain and quality of life in Parkinson’s disease patients. Eur. J. Neurol. 2009, 16, 542. [Google Scholar]
- Pirscoveanu, D.; Tudorica, V.; Zaharia, C.; Matcau, D.; Stanca, D.; Trifan, F. One year follow-up study on cognitive performances in patients with Parkinson’s disease. Rom. J. Neurol. Rev. Romana Neurol. 2009, 8, 84–87. [Google Scholar]
- Sandulescu, M.C.; Zaharia, C.; Tudorica, V. Study on gait and balance in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, S50. [Google Scholar] [CrossRef]
- Tudorica, V.; Zaharia, C.; Pirscoveanu, D.; Stanca, D.; Alexandru, O.; Albu, C. Study on anxiety in patients with Parkinson’s disease. Rom. J. Neurol. Rev. Romana Neurol. 2009, 8, 46–49. [Google Scholar]
- Căpuşan, C.; Şerban, A.M.; Cosman, D. The relation between cognitive impairment and clinical presentation in early stages of Parkinson’s disease. Hum. Vet. Med. 2011, 3, 213–219. [Google Scholar]
- Căpuşan, C.; Cosman, D.; Rusu, I. The deficit of executive functions in early stages of Parkinson’s disease. Hum. Vet. Med. 2011, 3, 171–177. [Google Scholar]
- Georgescu, D.; Georgescu, C.; Simu, M.; Georgescu, L.A. Understanding dyspepsia in patients with Parkinson’s disease. J. Neurol. 2011, 258, 89. [Google Scholar]
- Muntean, M.L.; Perju-Dumbrava, L. Night-time sleep disturbances and their impact on the quality of life of parkinson’s disease patients. Eur. J. Neurol. 2011, 18, 494. [Google Scholar]
- Tudorica, V.; Zaharia, C.; Pirscoveanu, D.; Pirici, D. Study on factors correlated with pain in patients with parkinson’s disease. Eur. J. Neurol. 2011, 18, 204. [Google Scholar]
- Martinez-Martin, P.; Pecurariu, C.F.; Odin, P.; Van Hilten, J.J.; Antonini, A.; Rojo-Abuin, J.M.; Borges, V.; Trenkwalder, C.; Aarsland, D.; Brooks, D.J.; et al. Gender-related differences in the burden of non-motor symptoms in Parkinson’s disease. J. Neurol. 2012, 259, 1639–1647. [Google Scholar] [CrossRef]
- Muntean, M.L.; Perju-Dumbrava, L. The impact of non-motor symptoms on the quality of life of Parkinson’s disease patients. Eur. J. Neurol. 2012, 19, 685. [Google Scholar]
- Susin, A. Daily living and quality of life in Parkinson’s disease. Philobiblon 2012, 17, 247–257. [Google Scholar]
- Tohanean, N.; Dumbrava, L.P. Olfactory dysfunction in Parkinson’ disease diagnosis. Rom. J. Neurol. Rev. Romana Neurol. 2012, 11, 108–114. [Google Scholar]
- Dumitru, M. Sleep problems in patients with Parkinson’s disease in a hospital setting from Romania. Sleep Med. 2013, 14, e111. [Google Scholar] [CrossRef]
- Dumitru, M.M.; Botezatu, C.; Andrei, R.; Popescu, C.D.; Chirita, V.; Chirita, R. Study on the incidence of depression and apathy in a group of patients diagnosed with parkinson’s disease. Eur. Psychiatry 2013, 28, 1333. [Google Scholar] [CrossRef]
- Dumitru, M.M.; Chirita, R.; Chirita, V. Depression and apathy in patients with Parkinson’s disease in a hospital in Romania. Eur. Neuropsychopharmacol. 2013, 23, S322. [Google Scholar] [CrossRef]
- Perju-Dumbravă, L.; Muntean, M.L.; Mureşanu, D.F. Cerebrovascular profile assessment in Parkinson’s disease patients. CNS Neurol. Disord. Drug Targets 2014, 13, 712–717. [Google Scholar] [CrossRef]
- Vasile, T.M.; Roceanu, A.M.; Antochi, F.; Bajenaru, O.A. Prevalence of non-motor symptoms in Parkinson’s disease—An observational study. Rom. J. Neurol. Rev. Romana Neurol. 2014, 13, 125–132. [Google Scholar]
- Baetu, C.; Buraga, I.; Buraga, M.; Petre, V. Polineuropathy and B12 deficiency in levodopa/carbidopa intestinal gel etiology and management -our experience. J. Neurol. Sci. 2015, 357, E255–E256. [Google Scholar] [CrossRef]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: Is there hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef]
- Jurcau, A.; Simion, A. Autonomic dysfunctions and sleep disturbances identify Parkinson’s disease patients at risk for developing dementia. Eur. J. Neurol. 2016, 23, 529. [Google Scholar]
- Diaconu, S.; Bucur, B.; Urdea, A.; Farcas, A.; Moarcas, M.; Pecurariu, C.F. Fatigue assessment and risk factors in Parkinson’s disease. Eur. J. Neurol. 2017, 24, 296. [Google Scholar]
- Kramberger, M.G.; Auestad, B.; Garcia-Ptacek, S.; Abdelnour, C.; Olmo, J.G.; Walker, Z.; Lemstra, A.W.; Londos, E.; Blanc, F.; Bonanni, L.; et al. Long-Term Cognitive Decline in Dementia with Lewy Bodies in a Large Multicenter, International Cohort. J. Alzheimer Dis. 2017, 57, 787–795. [Google Scholar] [CrossRef]
- Davidescu, E.I.; Tanasoiu, E.R. Neurocognitive and mood disorders in Parkinson’s disease. Eur. Psychiatry 2018, 48, S343. [Google Scholar]
- Tohanean, N.; Crisan, C.; Perju-Dumbrava, L. Distribution and correlation of psychiatric symptoms in early stages of parkinson’s disease. Rom. J. Neurol. Rev. Romana Neurol. 2018, 17, 78–83. [Google Scholar] [CrossRef]
- Criciotoiu, O.; Stanca, D.I.; Bondari, S.; Malin, R.D.; Ciolofan, M.S.; Schenker, M.; Stepan, M.D.; Romanescu, F.M.; Georgescu, O.S.; Dragomir, L.P.; et al. Correlation between the age, motor subtypes and the necessity of advanced therapy in Parkinson disease. Rev. Chim. 2019, 70, 2128–2131. [Google Scholar] [CrossRef]
- Criciotoiu, O.; Stanca, D.I.; Glavan, D.G.; Bondari, S.; Malin, R.D.; Ciolofan, M.S.; Bunescu, M.G.; Romanescu, F.M.; Schenker, M.; Georgescu, O.S.; et al. The relations between non-motor symptoms and motor symptoms in Parkinson disease. Rev. Chim. 2019, 70, 2652–2655. [Google Scholar] [CrossRef]
- Criciotoiu, O.; Stanca, I.D.; Glavan, D.G.; Latea, R.M.; Mita, A.; Calborean, V.; Gheorman, V.; Udristoiu, I.; Dijmarescu, A.L.; Davitoiu, D.V.; et al. The influence of digestive dysfunctions on quality of life in Parkinson disease. Rev. Chim. 2019, 70, 1667–1670. [Google Scholar] [CrossRef]
- Cuciureanu, D.I.; Croitoru, C.G.; Constantinescu, V.; Bolohan, L.; Cuciureanu, T. Neuropsychiatric changes in parkinson’s disease patients: A prospective observational two years study. Rom. J. Neurol. Rev. Romana Neurol. 2019, 18, 161–166. [Google Scholar]
- Cuciureanu, D.I.; Cuciureanu, T.; Bolohan, L.; Cuciureanu, A. Psychiatric morbidity in Parkinson’s disease in northeast region of Romania. J. Parkinson Dis. 2019, 9, 110. [Google Scholar]
- Fasano, A.; Fung, V.S.C.; Lopiano, L.; Elibol, B.; Smolentseva, I.G.; Seppi, K.; Takáts, A.; Onuk, K.; Parra, J.C.; Bergmann, L.; et al. Characterizing advanced Parkinson’s disease: OBSERVE-PD observational study results of 2615 patients. BMC Neurol. 2019, 19, 50. [Google Scholar] [CrossRef]
- Irene, R. Genitourinary Dysfunction Prevalence in Parkinson Disease Patients. ARS Med. Tomitana 2019, 25, 6–10. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Rizos, A.M.; Wetmore, J.B.; Antonini, A.; Odin, P.; Pal, S.; Sophia, R.; Carroll, C.; Martino, D.; Falup-Pecurariu, C.; et al. Relationship of Nocturnal Sleep Dysfunction and Pain Subtypes in Parkinson’s Disease. Mov. Disord. Clin. Pr. 2019, 6, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.; Luca, C.; Andritoi, D.; Fuior, R.; Zaharia, D.; Onu, I.; Corciova, C. Autonomic dysfunction and peripheral nerve involvement in patients with Parkinson’s disease. Balneo Res. J. 2019, 10, 55–61. [Google Scholar] [CrossRef]
- Romosan, A.M.; Dehelean, L.; Romosan, R.S.; Andor, M.; Bredicean, A.C.; Simu, M.A. Affective theory of mind in parkinson’s disease: The effect of cognitive performance. Neuropsychiatr. Dis. Treat. 2019, 15, 2521–2535. [Google Scholar] [CrossRef]
- Szász, J.A.; Constantin, V.A.; Orbán-Kis, K.; Rácz, A.; Arianabancu, L.; Georgescu, D.; Szederjesi, J.; Mihály, I.; Fárr, A.M.; Kelemen, K.; et al. Profile of patients with advanced parkinson’s disease suitable for device-aided therapies: Restrospective data of a large cohort of romanian patients. Neuropsychiatr. Dis. Treat. 2019, 15, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Vanta, O.M.; Pintea, S.; Perju-Dumbrava, L. The impact of associated large-fiber peripheral neuropathy on health-related quality of life in Parkinson’s disease—Results from a romanian cohort. Rom. J. Neurol. Rev. Romana Neurol. 2019, 18, 167–173. [Google Scholar]
- Vanta, O.M.; Tohanean, N.; Pintea, S.; Perju-Dumbrava, L. Large-Fiber Neuropathy in Parkinson’s Disease: Clinical, Biological, and Electroneurographic Assessment of a Romanian Cohort. J. Clin. Med. 2019, 8, 1533. [Google Scholar] [CrossRef] [PubMed]
- Ciopleias, B.; Al-Masri, N.; Torok, T.; Diaconu, S.; Gioroc, R.; Zosin, R.; Falup-Pecurariu, C.G. Autonomic dysfunctions in males with Parkinson’s disease: Case control study. Eur. J. Neurol. 2020, 27, 534. [Google Scholar]
- Diaconu, S.; Maceasa, A.; Ciopleias, B.; Zosin, R.; Falup-Pecurariu, C. Nutrient patterns in Parkinson’s disease: A case-control study. Mov. Disord. 2020, 35, S558. [Google Scholar]
- Szász, J.A.; Szatmari, S.; Constantin, V.; Mihaly, I.; Racz, A.; Torok, I.; Nagy, E.; Kelemen, K.; Forro, T.; Baroti, B.; et al. The importance of evaluation of gastrointestinal symptoms in advanced Parkinson’s disease. Orv. Hetil. 2020, 161, 1681–1687. [Google Scholar] [CrossRef]
- Muntean, M.L.; Tohanean, N.; Perju-Dumbrava, L. Pain and night time sleep disturbances assessed by bedside questionnaires in Parkinson’s disease patients. Eur. J. Neurol. 2010, 17, 396. [Google Scholar]
- Szasz, J.; Constantin, V.; Szatmari, S.; Bancu, L.; Georgescu, D.; Szederjesi, J.; Ciorba, M.; Racz, A.; Mihaly, I.; Orban, K. Possible correlations between symptoms suggesting gastroparesis and dyskinesias in advanced Parkinson’s disease. Mov. Disord. 2020, 35, S353–S354. [Google Scholar]
- Martinez-Martin, P.; Falup-Pecurariu, C.; Rodriguez-Blazquez, C.; van Hilten, B.; Odin, P.; Tluk, S.; Naidu, Y.; Chaudhuri, K.R.; Grp, E. Parkinson’s disease staging based of the non-motor symptoms scale. Parkinsonism Relat. Disord. 2009, 15, S66. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Rodriguez-Blazquez, C.; Abe, K.; Bhattacharyya, K.B.; Bloem, B.R.; Carod-Artal, F.J.; Prakash, R.; Esselink, R.A.J.; Falup-Pecurariu, C.; Gallardo, M.; et al. International study on the psychometric attributes of the Non-Motor Symptoms Scale in Parkinson disease. Neurology 2009, 73, 1584–1591. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Rodriguez-Blazquez, C.; Carod-Artal, J.; Falup-Pecurariu, C.; Serrano-Duenas, M. Measuring cognitive deterioration in Parkinson’s disease with the SCOPA-COG. Results of an international study. Eur. J. Neurol. 2009, 16, 521. [Google Scholar]
- Kraus, P.H.; Hoffmann, A.; Nirnberger, G.; Ipatov, M.; Schlegelmilch, I. Spiralometry is a valuable technique for control of kinetic tremor in large multicentre studies. Mov. Disord. 2011, 26, S133. [Google Scholar]
- Martinez-Martin, P.; Falup-Pecurariu, C.; Rodriguez-Blazquez, C.; Serrano-Dueñas, M.; Carod Artal, F.J.; Rojo Abuin, J.M.; Aarsland, D. Dementia associated with Parkinson’s disease: Applying the Movement Disorder Society Task Force criteria. Parkinsonism Relat. Disord. 2011, 17, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Geman, O.; Zamfir, C. Using Wavelet for Early Detection of Pathological Tremor. In Proceedings of the 20th European Signal Processing Conference, EUSIPCO 2012, Bucharest, Romania, 27–31 August 2012; pp. 1723–1727. [Google Scholar]
- Popa, L.; Bolbocean, O.; Ignat, B.; Popescu, C.D. Transcranial magnetic stimulation assessment of functional electrical stimulation effect on Parkinson’s disease patients. Eur. J. Neurol. 2012, 19, 679. [Google Scholar]
- Popa, L.; Constantinescu, A.; Popescu, C.D. Differences of cortical excitability between parkinson’s disease patients and healthy subjects. A comparative TMS study. Rom. J. Neurol. Rev. Romana Neurol. 2012, 11, 38–43. [Google Scholar]
- Ungurean, I.; Gaitan, N.C. Speech analysis for medical predictions based on Cell Broadband Engine. In Proceedings of the 20th European Signal Processing Conference, EUSIPCO 2012, Bucharest, Romania, 27–31 August 2012; pp. 1733–1736. [Google Scholar]
- Alexa, D.; Alexa, L.; Popa, L.; Nicolae Paduraru, D.; Ignat, B.; Constantinescu, A.; Baltag, D.; Rotar, A.; Dinu Popescu, C. Brainstem auditory evoked potentials in Parkinson’s disease. Rom. J. Neurol. Rev. Romana Neurol. 2013, 12, 198–201. [Google Scholar]
- Geman, O. Nonlinear dynamics, artificial neural networks and neuro-fuzzy classifier for automatic assessing of tremor severity. In Proceedings of the 4th IEEE International Conference on E-Health and Bioengineering, EHB 2013, Iasi, Romania, 21–23 November 2013. [Google Scholar]
- Geman, O.; Costin, H. Parkinson’s disease prediction based on multistate markov models. Int. J. Comput. Commun. Control 2013, 8, 525–537. [Google Scholar]
- Geman, O.; Turcu, C.O.; Graur, A. Parkinson’s disease Assessment using Fuzzy Expert System and Nonlinear Dynamics. Adv. Electr. Comput. Eng. 2013, 13, 41–46. [Google Scholar] [CrossRef]
- Fazakas, Z.; Tilinca, M.; Nemes-Nagy, E.; Tripon, R.; Kovacs, Z.; Şuş, I.; Kocsis, K.K.; Sǎmǎrghitan, V.B. Arylsulphatase a—A possible important determinant in the pathophysiology of metabolic and neuropsychiatric diseases. Ann. Rom. Soc. Cell Biol. 2013, 18, 80–84. [Google Scholar]
- Pohoaţǎ, S.; Graur, A. HDTV system for parkinson’s disease diagnosis. Adv. Electr. Comput. Eng. 2013, 13, 91–96. [Google Scholar] [CrossRef]
- Geman, O.; Costin, H. Automatic assessing of tremor severity using nonlinear dynamics, artificial neural networks and neuro-fuzzy classifier. Adv. Electr. Comput. Eng. 2014, 14, 133–138. [Google Scholar] [CrossRef]
- Geman, O.; Sanei, S.; Chiuchisan, I.; Graur, A.; Prochazka, A.; Vysata, O. Towards an inclusive Parkinson’s screening system. In Proceedings of the 18th International Conference on System Theory, Control and Computing, ICSTCC 2014, Sinaia, Romania, 17–19 October 2014; pp. 470–475. [Google Scholar]
- Chaudhuri, K.R.; Rizos, A.; Trenkwalder, C.; Rascol, O.; Pal, S.; Martino, D.; Carroll, C.; Paviour, D.; Falup-Pecurariu, C.; Kessel, B.; et al. King’s Parkinson’s disease pain scale, the first scale for pain in PD: An international validation. Mov. Disord. 2015, 30, 1623–1631. [Google Scholar] [CrossRef]
- Onu, M.; Badea, L.; Roceanu, A.; Tivarus, M.; Bajenaru, O. Increased connectivity between sensorimotor and attentional areas in Parkinson’s disease. Neuroradiology 2015, 57, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Biundo, R.; Weis, L.; Bostantjopoulou, S.; Stefanova, E.; Falup-Pecurariu, C.; Kramberger, M.G.; Geurtsen, G.J.; Antonini, A.; Weintraub, D.; Aarsland, D. MMSE and MoCA in Parkinson’s disease and dementia with Lewy bodies: A multicenter 1-year follow-up study. J. Neural Transm. 2016, 123, 431–438. [Google Scholar] [CrossRef]
- Fiorenzato, E.; Weis, L.; Falup-Pecurariu, C.; Diaconu, S.; Siri, C.; Reali, E.; Pezzoli, G.; Bisiacchi, P.; Antonini, A.; Biundo, R. Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE) performance in progressive supranuclear palsy and multiple system atrophy. J. Neural Transm. 2016, 123, 1435–1442. [Google Scholar] [CrossRef]
- Geman, O.; Chiuchisan, I.; Covasa, M.; Eftaxias, K.; Sanei, S.; Madeira, J.G.F.; Boloy, R.A.M. Joint EEG—EMG signal processing for identification of the mental tasks in patients with neurological diseases. In Proceedings of the 24th European Signal Processing Conference, EUSIPCO 2016, Budapest, Hungary, 28 August–2 September 2016; pp. 1598–1602. [Google Scholar]
- Toderean, R.; Geman, O.; Chiuchisan, I.; Lazar, A.M. A Comparison between Healthy and Neurological Disorders Patients using Nonlinear Dynamic Tools. In Proceedings of the 9th International Conference and Exposition on Electrical and Power Engineering (EPE), Iasi, Romania, 20–22 October 2016; pp. 299–303. [Google Scholar]
- Tohănean, N.; Perju-Dumbravă, L. The contribution of transcranial sonographic examination to the diagnosis of Parkinson’s disease. Hum. Vet. Med. 2016, 8, 5–9. [Google Scholar]
- Badea, L.; Onu, M.; Wu, T.; Roceanu, A.; Bajenaru, O. Exploring the reproducibility of functional connectivity alterations in Parkinson’s disease. PLoS ONE 2017, 12, e0197121. [Google Scholar] [CrossRef]
- Bajenaru, O.L.; Ene, A.; Ribigan, A.; Terecoasa, E.; Tiu, C.; Bajenaru, O.A. Study of the role of vitamin d and insulin resistance in patients with parkinson’s disease. Parkinsonism Relat. Disord. 2018, 46, E71. [Google Scholar] [CrossRef]
- Geman, O.; Chiuchisan, I. Response Surface Model Prediction of Deep Brain Stimulation Applied in Parkinson’s Disease Tremor. In Proceedings of the 10th International Conference and Expositions on Electrical And Power Engineering, EPE 2018, Iasi, Romania, 18–19 October 2018; pp. 703–707. [Google Scholar]
- Martinez-Martin, P.; Rizos, A.M.; Wetmore, J.; Antonini, A.; Odin, P.; Pal, S.; Sophia, R.; Carroll, C.; Martino, D.; Falup-Pecurariu, C.; et al. First comprehensive tool for screening pain in Parkinson’s disease: The King’s Parkinson’s Disease Pain Questionnaire. Eur. J. Neurol. 2018, 25, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Stocchi, F.; Radicati, F.G.; Chaudhuri, K.R.; Johansson, A.; Padmakumar, C.; Falup-Pecurariu, C.; Martinez-Martin, P. The Parkinson’s Disease Composite Scale: Results of the first validation study. Eur. J. Neurol. 2018, 25, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Toderean Aldea, R.; Geman, O.; Chiuchisan, I.; Balas, V.E.; Beiu, V. Novel method for neurodegenerative disorders screening patients using hurst coefficients on EEG delta rhythm. In Proceedings of the 7th International Workshop on Soft Computing Applications, SOFA 2016, Arad, Romania, 24–26 August 2018; pp. 349–358. [Google Scholar]
- Balestrino, R.; Hurtado-Gonzalez, C.A.; Stocchi, F.; Radicati, F.G.; Chaudhuri, K.R.; Rodriguez-Blazquez, C.; Martinez-Martin, P.; Adarmes, A.D.; Méndez-del-Barrio, C.; Ariadne, V.; et al. Applications of the European Parkinson’s Disease Association sponsored Parkinson’s Disease Composite Scale (PDCS). NPJ Parkinson Dis. 2019, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Cuciureanu, D.I.; Popescu, R.M.; Cuciureanu, T.; Constantinescu, V.A.; Hodorog, D.N.; Szalontay, A.S. Dopaminergic centers neurodegeneration biochemical and radiologic approach. Rev. Chim. 2019, 70, 1835–1838. [Google Scholar] [CrossRef]
- Martinez-Martin, P.; Radicati, F.G.; Rodriguez Blazquez, C.; Wetmore, J.; Kovacs, N.; Ray Chaudhuri, K.; Stocchi, F.; Vuletic, V.; Falup-Pecurariu, C.; Diaconu, Ş.; et al. Extensive validation study of the Parkinson’s Disease Composite Scale. Eur. J. Neurol. 2019, 26, 1281–1288. [Google Scholar] [CrossRef]
- Aldred, J.; Anca-Herschkovitsch, M.; Antonini, A.; Bajenaru, O.; Bergmann, L.; Bourgeois, P.; Cubo, E.; Davis, T.L.; Iansek, R.; Kovacs, N.; et al. Application of the ‘5-2-1’ screening criteria in advanced Parkinson’s disease: Interim analysis of DUOGLOBE. Neurodegener. Dis. Manag. 2020, 10, 309–323. [Google Scholar] [CrossRef]
- Geroin, C.; Artusi, C.A.; Gandolfi, M.; Zanolin, E.; Ceravolo, R.; Capecci, M.; Andrenelli, E.; Ceravolo, M.G.; Bonanni, L.; Onofrj, M.; et al. Does the Degree of Trunk Bending Predict Patient Disability, Motor Impairment, Falls, and Back Pain in Parkinson’s Disease? Front. Neurol. 2020, 11, 207. [Google Scholar] [CrossRef]
- Arseni, C.; Iacob, M.; Simionescu, M. Stereotaxia in extrapyramidal diseases. Neurologia 1973, 18, 525–538. [Google Scholar]
- Pendefunda, G.; Pollingher, B.; Stefanache, F. The treatment of Parkinson’s syndrome with levodopa and amantadine. Neurologia 1973, 18, 559–568. [Google Scholar]
- Pendefunda, G.; Pollingher, B.; Stefanache, F. The treatment with l dopa and amantadine in Parkinson’s disease. Ther. Hung. 1975, 23, 12–16. [Google Scholar]
- Cinca, I.; Bulandra, R.; Serbanesco, A.; Ninosu, N. Current medical therapy of Parkinsonian syndromes. Rev. Roum. Neurol. 1973, 10, 257–285. [Google Scholar]
- Minea, D.; Varga, I.; Falup-Pěcurariu, C.; De Mey, C.; Retzow, A.; Althaus, M. Influence of the dopamine agonist α-dihydroergocryptine on the pharmacokinetics of levodopa in patients with Parkinson’s disease. Clin. Neuropharmacol. 2001, 24, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Baltag, D.; Ignat, B.; Manole, O.Z. Secondary effects of chronic treatment with levodopa in Parkinson disease. Rev. Med. Chir. A Soc. Med. Nat. Iasi 2003, 107, 131–135. [Google Scholar]
- Olanow, C.W.; Damier, P.; Goetz, C.G.; Mueller, T.; Nutt, J.; Rascol, O.; Serbanescu, A.; Deckers, F.; Russ, H. Multicenter, Open-Label, Trial of Sarizotan in Parkinson Disease Patients with Levodopa-Induced Dyskinesias (the SPLENDID Study). Clin. Neuropharmacol. 2004, 27, 58–62. [Google Scholar] [CrossRef]
- Ferreira, J.J.; Almeida, L.; Cunha, L.; Ticmeanu, M.; Rosa, M.M.; Januário, C.; Mitu, C.E.; Coelho, M.; Correia-Guedes, L.; Morgadinho, A.; et al. Effects of nebicapone on levodopa pharmacokinetics, catechol-O- methyltransferase activity, and motor fluctuations in patients with Parkinson disease. Clin. Neuropharmacol. 2008, 31, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Borgohain, R.; Szasz, J.; Bhatt, M.; Rossetti, S.; Lucini, V.; Anand, R.; Study, I. Safinamide as an adjunct to levodopa significantly improved motor fluctuations in Parkinson’s disease: A phase III, randomized, double-blind, placebo-controlled study. Parkinsonism Relat. Disord. 2009, 15, S115. [Google Scholar]
- Szasz, J.; Borgohain, R.; Bhatt, M.; Rossetti, S.; Lucini, V.; Anand, R.; Study, I. Improvements in symptom severity and daily living with safinamide in Parkinson’s disease: A phase III, randomized, double-blind, placebo-controlled study. Parkinsonism Relat. Disord. 2009, 15, S118. [Google Scholar] [CrossRef]
- Bhatt, M.; Chirileanu, D.; Meshram, C.; Stanzione, P.; Forrest, E.C.; Lucini, V.; Study, I. Effect of Safinamide on Dyskinesia in Patients with Mid- to Late-Stage Parkinson’s Disease: Data from a Post Hoc Analysis. Mov. Disord. 2010, 25, S676. [Google Scholar]
- Kaufmann, H.; Mathias, C.; Low, P.; Biaggioni, I.; Freeman, R. Experience with droxidopa (NortheraTM) in a phase III multinational, placebo-controlled, parallel group, induction-design study to assess clinical benefit and safety in subjects with neurogenic orthostatic hypotension. Clin. Auton. Res. 2010, 20, 295. [Google Scholar]
- Meshram, C.; Bhatt, M.; Chirileanu, D.; Stanzione, P.; Lucini, V.; Rossetti, S.; Anand, R.; Study, I. Safinamide improves motor function without worsening troublesome dyskinesia as add-on therapy in L-DOPA-treated patients with mid- to late-stage Parkinson’s disease (PD). Eur. J. Neurol. 2010, 17, 99. [Google Scholar]
- Vasile, D.; Vasiliu, O. Management of Cognitive Symptoms in Dementia Associated with Parkinson’s Disease. In Proceedings of the World Medical Conference, Malta, 15–17 December 2010; pp. 284–286. [Google Scholar]
- Borgohain, R.; Szasz, J.; Stanzione, P.; Meshram, C.; Bhatt, M.; Stocchi, F.; Lucini, V.; Anand, R.; Study, I. Results from the first 2-year, controlled study to assess safinamide as add-on to l-dopa in patients with parkinson’s disease and motor fluctuations. Eur. J. Neurol. 2011, 18, 20. [Google Scholar]
- Meshram, C.; Borgohain, R.; Bhatt, M.; Chirileanu, D.; Stocchi, F.; Lucini, V.; Giuliani, R.; Anand, R.; Study, I. Two-year, placebo-controlled safety and tolerability data for safinamide as add-on to l-dopa in patients with parkinson’s disease. Eur. J. Neurol. 2011, 18, 232–233. [Google Scholar]
- Vasile, D.; Vasiliu, O.; Mangalagiu, A.G.; Blandu, M.; Nanca, A. Comparative efficacy of cholinesterase inhibitors in dementia associated with Parkinson’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 174. [Google Scholar] [CrossRef]
- Popa, L.; Constantinescu, A.; Mureşanu, D.F.; Irimie, A.; Bǎlǎnescu, N.R.; Popescu, C.D. Clinical improvement and cortical adaptations after functional electrical stimulation in parkinson’s disease patients. CNS Neurol. Disord. Drug Targets 2013, 12, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Vasile, D.; Macovei, R.A.; Vasiliu, O. Efficacy and tolerability of bupropion in major depressive disorder associated with Parkinson’s disease. Eur. Neuropsychopharmacol. 2013, 23, S322. [Google Scholar] [CrossRef]
- Baetu, C.; Buraga, I. Levodopa/carbidopa intestinal gel pump treatment in patients with advanced Parkinson’s disease—From Romanian experience. Mov. Disord. 2014, 29, S227. [Google Scholar]
- Borgohain, R.; Szasz, J.; Stanzione, P.; Meshram, C.; Bhatt, M.; Chirilineau, D.; Stocchi, F.; Lucini, V.; Giuliani, R.; Forrest, E.; et al. Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov. Disord. 2014, 29, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Borgohain, R.; Szasz, J.; Stanzione, P.; Meshram, C.; Bhatt, M.H.; Chirilineau, D.; Stocchi, F.; Lucini, V.; Giuliani, R.; Forrest, E.; et al. Two-Year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov. Disord. 2014, 29, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.A.; Olanow, C.W.; Kieburtz, K.D.; Pourcher, E.; Docu-Axelerad, A.; Lew, M.; Kozyolkin, O.; Neale, A.; Resburg, C.; Meya, U.; et al. Tozadenant (SYN115) in patients with Parkinson’s disease who have motor fluctuations on levodopa: A phase 2b, double-blind, randomised trial. Lancet Neurol. 2014, 13, 767–776. [Google Scholar] [CrossRef]
- Baetu, C.; Buraga, I.; Buraga, M.; Mihailescu, G.; Petre, V. Complications related to levodopa/carbidopa intestinal gel treatment colentina hospital experience. J. Neurol. Sci. 2015, 357, E256. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Chaudhuri, K.R.; Martinez-Martin, P.; Rascol, O.; Ehret, R.; Vališ, M.; Sátori, M.; Krygowska-Wajs, A.; Marti, M.J.; Reimer, K.; et al. Prolonged-release oxycodone-naloxone for treatment of severe pain in patients with Parkinson’s disease (PANDA): A double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2015, 14, 1161–1170. [Google Scholar] [CrossRef]
- Băjenaru, O.; Ene, A.; Popescu, B.O.; Szász, J.A.; Sabău, M.; Mureşan, D.F.; Perju-Dumbrava, L.; Popescu, C.D.; Constantinescu, A.; Buraga, I.; et al. The effect of levodopa–carbidopa intestinal gel infusion long-term therapy on motor complications in advanced Parkinson’s disease: A multicenter Romanian experience. J. Neural Transm. 2016, 123, 407–414. [Google Scholar] [CrossRef]
- Crăciun, E.C.; Dronca, E.; Leach, N.V. Antioxidant enzymes activity in subjects with Parkinson’s disease under L-DOPA therapy. Hum. Vet. Med. 2016, 8, 124–127. [Google Scholar]
- Dogaru, G.; Muresan, D.; Stanescu, I.; Motricala, M.; Akos, M. The therapeutic benefits of natural therapeutic factors in Baile Tusnad for the rehabilitation of patients with Parkinson’s disease. Balneo Res. J. 2016, 7, 116–124. [Google Scholar] [CrossRef]
- Kenney, C.; Kieburtz, K.; Olanow, W.; Bandak, S. Regional differences in the results of a phase 2b study of tozadenant in Parkinson’s disease patients with motor fluctuations. Mov. Disord. 2016, 31 (Suppl. S2), S693. [Google Scholar]
- Rizos, A.; Sauerbier, A.; Antonini, A.; Weintraub, D.; Martinez-Martin, P.; Kessel, B.; Henriksen, T.; Falup-Pecurariu, C.; Silverdale, M.; Durner, G.; et al. A European multicentre survey of impulse control behaviours in Parkinson’s disease patients treated with short- and long-acting dopamine agonists. Eur. J. Neurol. 2016, 23, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Poewe, W.; Chaudhuri, K.R.; Jech, R.; Pickut, B.; Pirtošek, Z.; Szasz, J.; Valldeoriola, F.; Winkler, C.; Bergmann, L.; et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s: Final results of the GLORIA registry. Parkinsonism Relat. Disord. 2017, 45, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Antonini, A.; Poewe, W.; Standaert, D.; Odin, P.; Zamudio, J.; Bergmann, L. A long-term study on effectiveness of levodopa-carbidopa intestinal gel treatment in advanced Parkinson’s disease patients. Mov. Disord. 2017, 32 (Suppl. S2), 952. [Google Scholar]
- Muller, T.; Tolosa, E.; Badea, L.; Asgharnejad, M.; Grieger, F.; Markowitz, M.; Nonfondaz, X.; Bauer, L.; Timmermann, L. An observational study of rotigotine transdermal patch and other currently prescribed therapies in patients with Parkinson’s disease. Eur. J. Neurol. 2017, 24, 394–395. Available online: https://apps.webofknowledge.com/full_record.do?product=WOS&search_mode=GeneralSearch&qid=12&SID=5FoVfRjUX9FVKjgvKMl&page=1&doc=1 (accessed on 26 May 2021). [CrossRef] [PubMed]
- Szász, J.A.; Constantin, V.; Fazakas, P.A.; Blényesi, E.; Grieb, L.G.; Balla, A.; Sárig, M.; Szegedi, K.; Bartha, E.N.; Szatmári, S. The role of selective monoamine oxidase B inhibitors in the therapeutic strategy of Parkinson’s disease in the neurology clinics of Tirgu Mures County Emergency Clinical Hospital. Orv. Hetil. 2017, 158, 2023–2028. [Google Scholar] [CrossRef]
- Müller, T.; Tolosa, E.; Badea, L.; Asgharnejad, M.; Grieger, F.; Markowitz, M.; Nondonfaz, X.; Bauer, L.; Timmermann, L. An observational study of rotigotine transdermal patch and other currently prescribed therapies in patients with Parkinson’s disease. J. Neural Transm. 2018, 125, 953–963. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-85042524722&doi=10.1007%2fs00702-018-1860-x&origin=inward&txGid=e695ab90c3ab4d9a10e2b625ad350dd5&featureToggles=FEATURE_NEW_MAIN_SECTION:1,FEATURE_NEW_SOURCE_INFO:1,FEATURE_NEW_REAXYS_SECTION:1,FEATURE_NEW_SCIVAL_TOPICS:1,FEATURE_VIEWS_COUNT:1 (accessed on 26 May 2021). [CrossRef]
- Breda, X.M.; Fodor, D.M.; Verdes, R.; Tartamus, D.; Muresan, D.A.; Stanescu, I.C.; Capusan, C.; Dumbrava Perju, L. The Mozart effect combined with specific kinetic treatment in patients with Parkinson’s disease. Balneo Res. J. 2019, 10, 294–299. [Google Scholar] [CrossRef]
- Criciotoiu, O.; Stanca, D.I.; Glavan, D.G.; Mirea, C.S.; Mita, A.; Calborean, V.; Gheorman, V.; Udristoiu, I.; Dijmarescu, A.L.; Davitoiu, D.V.; et al. Statistical analysis between the routes of administration of L-DOPA and digestive dysfunctions in Parkinson disease. Rev. Chim. 2019, 70, 1403–1405. [Google Scholar] [CrossRef]
- Fasano, A.; Parra, J.C.; Gurevich, T.; Jech, R.; Kovacs, N.; Per, S.; Szasz, J.; Bergmann, L.; Johnson, A.; Sanchez-Solino, O.; et al. Utilization of monotherapy and combination therapies in advanced Parkinson’s disease patients during levodopa-carbidopa intestinal gel treatment from the cosmos study. J. Neurol. Sci. 2019, 405, 104857. [Google Scholar] [CrossRef]
- Nemes, B.O.; Pirlog, R.; Tartamus, D.; Capusan, C.; Fodor, D.M. The role of dance therapy in the rehabilitation of Parkinson disease patients. Balneo Res. J. 2019, 10, 300–304. [Google Scholar] [CrossRef]
- Petrescu, B.; Vasile, D.; Vasiliu, O.; Mangalagiu, A.; Candea, C.; Riga, D.; Riga, S.; Mitrache, A. Management of psychosis with quetiapine in Alzheimer type dementia and Parkinson type dementia. Eur. Neuropsychopharmacol. 2019, 29, S134–S135. [Google Scholar] [CrossRef]
- Szász, J.A.; Orbán-Kis, K.; Constantin, V.A.; Péter, C.; Bíró, I.; Mihály, I.; Szegedi, K.; Balla, A.; Szatmári, S. Therapeutic strategies in the early stages of Parkinson’s disease: A cross-sectional evaluation of 15 years’ experience with a large cohort of Romanian patients. Neuropsychiatr. Dis. Treat. 2019, 15, 831–838. [Google Scholar] [CrossRef]
- Szász, J.A.; Szatmári, S.; Constantin, V.; Mihály, I.; Rácz, A.; Domokos, L.C.; Vajda, T.; Orbán-Kis, K. Characteristics of levodopa treatment in advanced Parkinson’s disease in the experiences of the neurology clinics of Târgu Mureș, Romania. Orv. Hetil. 2019, 160, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Szász, J.A.; Viorelia, C.; Mihály, I.; Biró, I.; Péter, C.; Orbán-Kis, K.; Szatmári, S. Dopamine agonists in Parkinson’s disease therapy—15 years of experience of the Neurological Clinics from Tîrgu Mureș. A cross-sectional study. Ideggyogy Sz 2019, 72, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Constantin, V.A.; Szász, J.A.; Orbán-Kis, K.; Rosca, E.C.; Popovici, M.; Cornea, A.; Bancu, L.A.; Ciorba, M.; Mihály, I.; Nagy, E.; et al. Levodopa-carbidopa intestinal gel infusion therapy discontinuation: A ten-year retrospective analysis of 204 treated patients. Neuropsychiatr. Dis. Treat. 2020, 16, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Popa, L.C.; Leucuta, D.C.; Tohanean, N.; Popa, S.L.; Perju-Dumbrava, L. Intrajejunal vs oral levodopa-carbidopa therapy in Parkinson disease: A retrospective cohort study. Medicine 2020, 99, e23249. [Google Scholar] [CrossRef]
- Popa, L.C.; Leucuta, D.C.; Tohanean, N.; Popa, S.L.; Perju-Dumbrava, L. Levodopa-carbidopa intestinal gel therapy in parkinson’s disease: Procedure complications. Rom. J. Neurol. Rev. Romana Neurol. 2020, 19, 84–88. [Google Scholar] [CrossRef]
- Rizos, A.; Sauerbier, A.; Falup-Pecurariu, C.; Odin, P.; Antonini, A.; Martinez-Martin, P.; Kessel, B.; Henriksen, T.; Silverdale, M.; Durner, G.; et al. Tolerability of non-ergot oral and transdermal dopamine agonists in younger and older Parkinson’s disease patients: An European multicentre survey. J. Neural Transm. 2020, 127, 875–879. [Google Scholar] [CrossRef]
- Szasz, J.; Simu, M.; Perju-Dumbrava, L.; Antonini, A.; Bergmann, L.; Popescu, D.; Bajenaru, O.A. Efficacy, safety and patient’s quality of life of long-term treatment with levodopa-carbidopa intestinal gel in advanced parkinson’s disease in romania: Results from gloria observational study. Rom. J. Neurol. Rev. Romana Neurol. 2020, 19, 27–35. [Google Scholar] [CrossRef]
- Fasano, A.; Parra, J.C.; Gurevich, T.; Jech, R.; Kovacs, N.; Svenningsson, P.; Szasz, J.; Bergmann, L.; Sanchez-Solino, O.; Vela-Desojo, L. Improvements in motor symptoms in patients with advanced Parkinson’s disease on long-term LCIG monotherapy or combination therapy: An analysis of the COSMOS observational study. Eur. J. Neurol. 2020, 27, 662–663. [Google Scholar]
- De Palma, G.; Dick, F.D.; Calzetti, S.; Scott, N.W.; Prescott, G.J.; Osborne, A.; Haites, N.; Mozzoni, P.; Negrotti, A.; Scaglioni, A.; et al. A case-control study of Parkinson’s disease and tobacco use: Gene-tobacco interactions. Mov. Disord. 2010, 25, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Dick, F.D.; De Palma, G.; Ahmadi, A.; Osborne, A.; Scott, N.W.; Prescott, G.J.; Bennett, J.; Semple, S.; Dick, S.; Mozzoni, P.; et al. Gene-environment interactions in parkinsonism and Parkinson’s disease: The Geoparkinson study. Occup. Environ. Med. 2007, 64, 673–680. [Google Scholar] [CrossRef]
- Dick, F.D.; De Palma, G.; Ahmadi, A.; Scott, N.W.; Prescott, G.J.; Bennett, J.; Semple, S.; Dick, S.; Counsell, C.; Mozzoni, P.; et al. Environmental risk factors for Parkinson’s disease and parkinsonism: The Geoparkinson study. Occup. Environ. Med. 2007, 64, 666–672. [Google Scholar] [CrossRef]
- Naghavi, M.; Wang, H.; Lozano, R.; Davis, A.; Liang, X.; Zhou, M.; Vollset, S.E.; Abbasoglu Ozgoren, A.; Abdalla, S.; Abd-Allah, F.; et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Hay, S.I.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1260–1344. [Google Scholar] [CrossRef]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Ärnlöv, J.; Afshin, A.; et al. Global, regional, and national age-sex specifc mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Vos, T.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Brodszky, V.; Beretzky, Z.; Baji, P.; Rencz, F.; Péntek, M.; Rotar, A.; Tachkov, K.; Mayer, S.; Simon, J.; Niewada, M.; et al. Cost-of-illness studies in nine Central and Eastern European countries. Eur. J. Health Econ. 2019, 20, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Canevelli, M.; Bruno, G.; Valletta, M.; Fabbrini, A.; Vanacore, N.; Berardelli, A.; Fabbrini, G. Parkinson’s disease among migrants in Europe: Estimating the magnitude of an emerging phenomenon. J. Neurol. 2019, 266, 1120–1126. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef] [PubMed]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, S0140-6736(21)00218-X, Epub ahead of print. [Google Scholar] [CrossRef]
- Schneider, S.A.; Alcalay, R.N. Precision medicine in Parkinson’s disease: Emerging treatments for genetic Parkinson’s disease. J. Neurol. 2020, 267, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Kaiyrzhanov, R.; Rizig, M.; Aitkulova, A.; Zharkinbekova, N.; Shashkin, C.; Kaishibayeva, G.; Karimova, A.; Khaibullin, T.; Sadykova, D.; Ganieva, M.; et al. Parkinson’s Disease in Central Asian and Transcaucasian Countries: A Review of Epidemiology, Genetics, Clinical Characteristics, and Access to Care. Parkinsons Dis. 2019, 2019, 2905739. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Peters, M.D.J.; Tricco, A.C.; Pollock, D.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Munn, Z. Conducting high quality scoping reviews-challenges and solutions. J. Clin. Epidemiol. 2021, 130, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.; Palmer, S.; Craig, J.C.; Strippoli, G.F. A guide to reading and using systematic reviews of qualitative research. Nephrol. Dial. Transpl. 2016, 31, 897–903. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosca, E.C.; Tudor, R.; Cornea, A.; Simu, M. Parkinson’s Disease in Romania: A Scoping Review. Brain Sci. 2021, 11, 709. https://doi.org/10.3390/brainsci11060709
Rosca EC, Tudor R, Cornea A, Simu M. Parkinson’s Disease in Romania: A Scoping Review. Brain Sciences. 2021; 11(6):709. https://doi.org/10.3390/brainsci11060709
Chicago/Turabian StyleRosca, Elena Cecilia, Raluca Tudor, Amalia Cornea, and Mihaela Simu. 2021. "Parkinson’s Disease in Romania: A Scoping Review" Brain Sciences 11, no. 6: 709. https://doi.org/10.3390/brainsci11060709
APA StyleRosca, E. C., Tudor, R., Cornea, A., & Simu, M. (2021). Parkinson’s Disease in Romania: A Scoping Review. Brain Sciences, 11(6), 709. https://doi.org/10.3390/brainsci11060709






