Measuring the Emergence of Specific Abilities in Young Children with Autism Spectrum Disorders: The Example of Early Hyperlexic Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Analyses Strategy
3. Results
3.1. Description of the ASD Sample Derived from the Hyperlexic Trait Score
3.2. Cross-Sectional Results
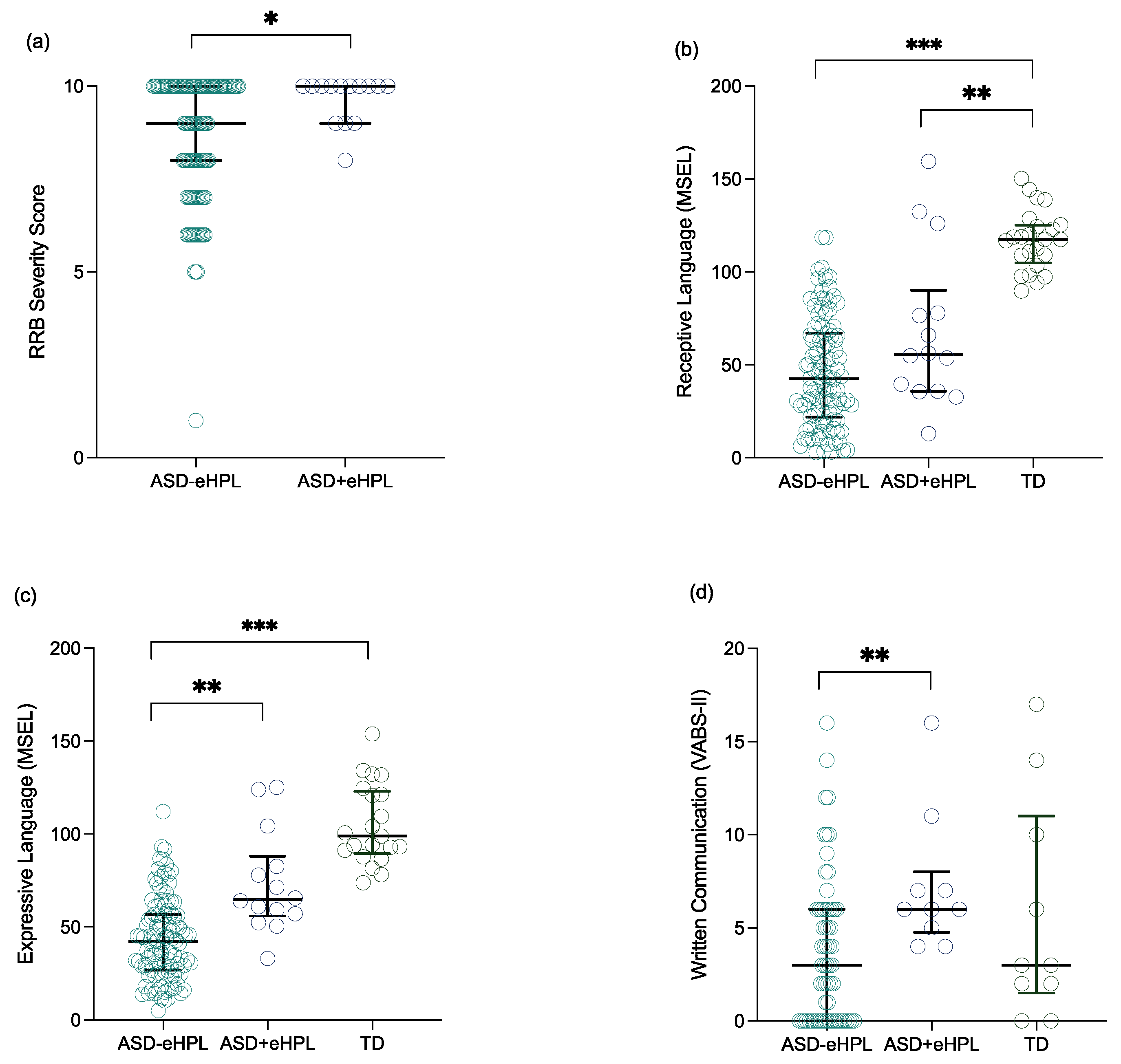
3.2.1. Group Differences
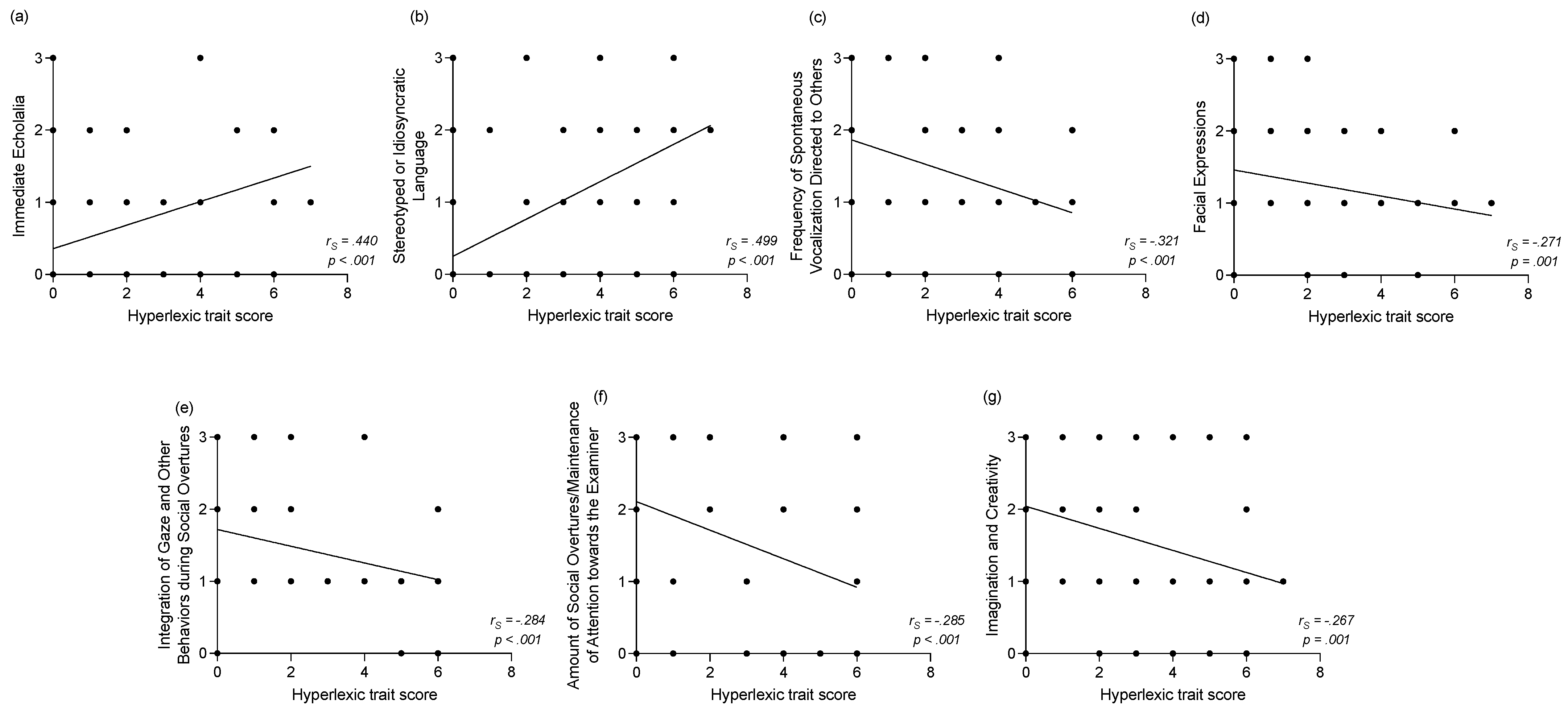
3.2.2. Correlations between the Hyperlexic Trait Score and Clinical Assessments at Baseline
3.3. Longitudinal Results
3.3.1. Correlations between the Hyperlexic Traits Score and Change over Time
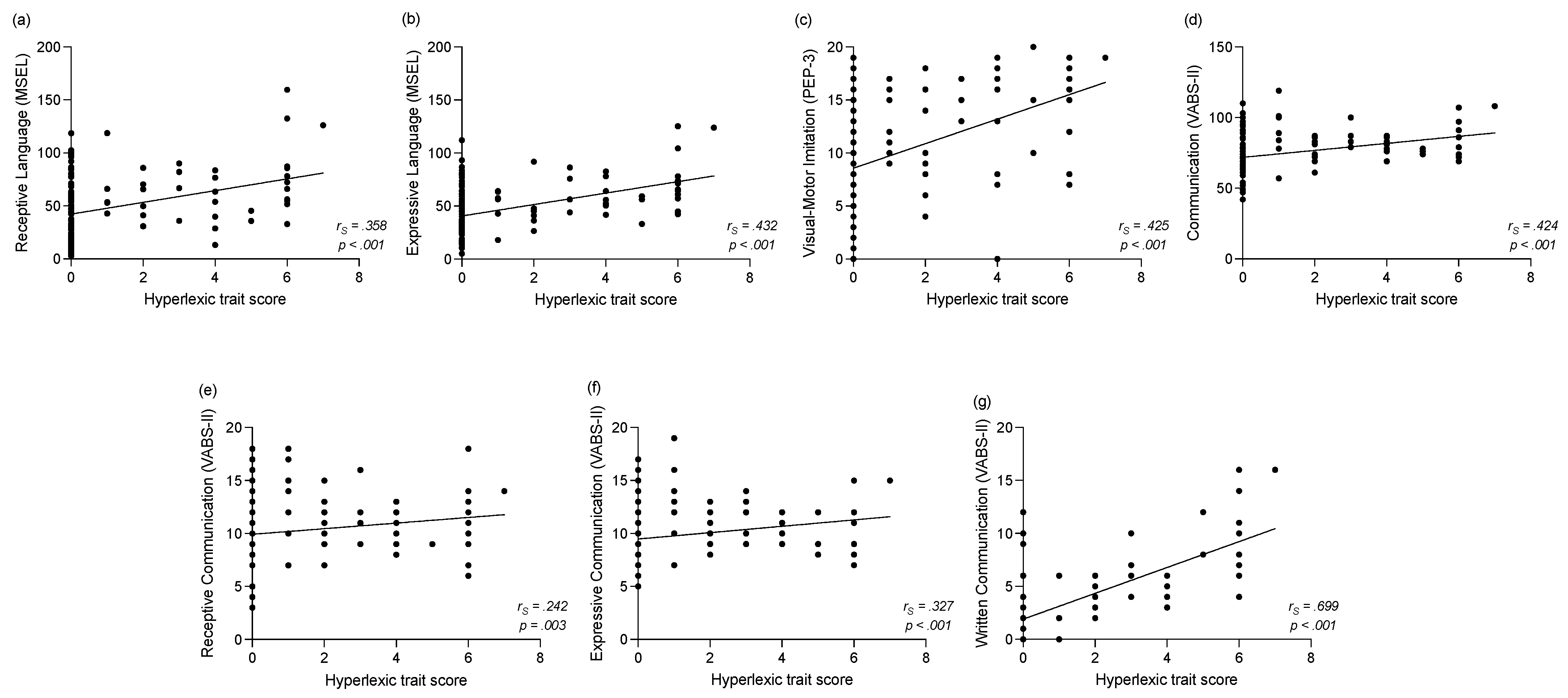
3.3.2. Correlations between the Hyperlexic Traits Score and Clinical Assessments One Year Later
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- Honey, E.; Rodgers, J.; McConachie, H. Measurement of restricted and repetitive behaviour in children with autism spectrum disorder: Selecting a questionnaire or interview. Res. Autism Spectr. Disord. 2012, 6, 757–776. [Google Scholar] [CrossRef]
- Bishop, S.L.; Richler, J.; Cain, A.C.; Lord, C. Predictors of perceived negative impact in mothers of children with autism spectrum disorder. Am. J. Ment. Retard. 2007, 112, 450. [Google Scholar] [CrossRef]
- Lecavalier, L.; Leone, S.; Wiltz, J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. J. Intellect. Disabil. Res. 2006, 50, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S.; Bodfish, J.W.; Piven, J. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms: Evidence for three subtypes of repetitive behavior in autism. J. Child Psychol. Psychiatry 2008, 49, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Pierce, K.; Courchesne, E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol. Psychiatry 2001, 49, 655–664. [Google Scholar] [CrossRef]
- Harrop, C. Evidence-based, parent-mediated interventions for young children with autism spectrum disorder: The case of restricted and repetitive behaviors. Autism 2015, 19, 662–672. [Google Scholar] [CrossRef]
- Bonnel, A.; Mottron, L.; Peretz, I.; Trudel, M.; Gallun, E.; Bonnel, A.-M. Enhanced Pitch Sensitivity in Individuals with Autism: A Signal Detection Analysis. J. Cogn. Neurosci. 2003, 15, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Heavey, L.; Pring, L.; Hermelin, B. A date to remember: The nature of memory in savant calendrical calculators. Psychol. Med. 1999, 29, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.; Eames, K. Contrasting Styles of Drawing in Gifted Individuals with Autism. Autism 1999, 3, 397–409. [Google Scholar] [CrossRef]
- Selfe, L. A Single Case Study of an Autistic Child with Exceptional Drawing Ability. In The Child’s Representation of the World; Butterworth, G., Ed.; Springer US: Boston, MA, USA, 1977; pp. 31–48. ISBN 978-1-4684-2351-8. [Google Scholar]
- Grigorenko, E.L.; Klin, A.; Pauls, D.L.; Senft, R.; Hooper, C.; Volkmar, F. A descriptive study of hyperlexia in a clinically referred sample of children with developmental delays. J. Autism Dev. Disord. 2002, 32, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Nation, K. Reading skills in hyperlexia: A developmental perspective. Psychol. Bull. 1999, 125, 338–355. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, N.; Hermelin, B. Two autistic savant readers. J. Autism Dev. Disord. 1994, 24, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Silberberg, N.E.; Silberberg, M.C. Hyperlexia—Specific Word Recognition Skills in Young Children. Except. Child. 1967, 34, 41–42. [Google Scholar] [CrossRef]
- Ostrolenk, A.; D’Arc, B.F.; Jelenic, P.; Samson, F.; Mottron, L. Hyperlexia: Systematic review, neurocognitive modelling, and outcome. Neurosci. Biobehav. Rev. 2017, 79, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Grigorenko, E.L.; Klin, A.; Volkmar, F. Annotation: Hyperlexia: Disability or superability? J. Child Psychol. Psychiatry 2003, 44, 1079–1091. [Google Scholar] [CrossRef]
- Cossu, G.; Rossini, F.; Marshall, J. When reading is acquired but phonemic awareness is not: A study of literacy in Down’s syndrome. Cognition 1993, 46, 129–138. [Google Scholar] [CrossRef]
- Temple, C.M. Developmental and acquired dyslexias. Cortex 2006, 42, 898–910. [Google Scholar] [CrossRef]
- Zhang, S.; Joshi, R.M. Profile of hyperlexia: Reconciling conflicts through a systematic review and meta-analysis. J. Neurolinguistics 2019, 49, 1–28. [Google Scholar] [CrossRef]
- Baumer, N.; Spence, S.J. Evaluation and Management of the Child with Autism Spectrum Disorder. Contin. Lifelong Learn. Neurol. 2018, 24, 248–275. [Google Scholar] [CrossRef]
- Klin, A.; Danovitch, J.H.; Merz, A.B.; Volkmar, F.R. Circumscribed Interests in Higher Functioning Individuals with Autism Spectrum Disorders: An Exploratory Study. Res. Pract. Pers. Sev. Disabil. 2007, 32, 89–100. [Google Scholar] [CrossRef]
- Lanter, E.; Freeman, D.; Dove, S. Procedural and Conceptual Print-Related Achievements in Young Children with Autism Spectrum Disorders. Focus Autism Dev. Disabil. 2013, 28, 14–25. [Google Scholar] [CrossRef]
- Meilleur, A.-A.S.; Jelenic, P.; Mottron, L. Prevalence of Clinically and Empirically Defined Talents and Strengths in Autism. J. Autism Dev. Disord. 2015, 45, 1354–1367. [Google Scholar] [CrossRef]
- Healy, J.M.; Aram, D.M.; Horwitz, S.J.; Kessler, J.W. A study of hyperlexia. Brain Lang. 1982, 17, 1–23. [Google Scholar] [CrossRef]
- Jacques, C.; Courchesne, V.; Meilleur, A.-A.S.; Mineau, S.; Ferguson, S.; Cousineau, D.; Labbe, A.; Dawson, M.; Mottron, L. What interests young autistic children? An exploratory study of object exploration and repetitive behavior. PLoS ONE 2018, 13, e0209251. [Google Scholar] [CrossRef]
- Nakano, T.; Tanaka, K.; Endo, Y.; Yamane, Y.; Yamamoto, T.; Nakano, Y.; Ohta, H.; Kato, N.; Kitazawa, S. Atypical gaze patterns in children and adults with autism spectrum disorders dissociated from developmental changes in gaze behaviour. Proc. R. Soc. B Boil. Sci. 2010, 277, 2935–2943. [Google Scholar] [CrossRef] [PubMed]
- Gliga, T.; Bedford, R.; Charman, T.; Johnson, M.H.; Baron-Cohen, S.; Bolton, P.; Cheung, C.; Davies, K.; Liew, M.; Fernandes, J.; et al. Enhanced Visual Search in Infancy Predicts Emerging Autism Symptoms. Curr. Biol. 2015, 25, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.M.; Macomber, D.; Naples, A.J.; Babitz, T.; Volkmar, F.; Grigorenko, E.L. Hyperlexia in Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2007, 37, 760–774. [Google Scholar] [CrossRef]
- Castles, A.; Rastle, K.; Nation, K. Ending the Reading Wars: Reading Acquisition from Novice to Expert. Psychol. Sci. Public Interes. 2018, 19, 5–51. [Google Scholar] [CrossRef]
- Knight, E.; Blacher, J.; Eisenhower, A. Predicting reading comprehension in young children with autism spectrum disorder. Sch. Psychol. 2019, 34, 168–177. [Google Scholar] [CrossRef]
- Westerveld, M.F.; Paynter, J.; Trembath, D.; Webster, A.A.; Hodge, A.M.; Roberts, J. The Emergent Literacy Skills of Preschool Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 424–438. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.; Luk, G.; Quintin, E.-M. Early Word Reading of Preschoolers with ASD, Both with and Without Hyperlexia, Compared to Typically Developing Preschoolers. J. Autism Dev. Disord. 2020, 51, 1598–1612. [Google Scholar] [CrossRef]
- Simonoff, E.; Pickles, A.; Charman, T.; Chandler, S.; Loucas, T.; Baird, G. Psychiatric Disorders in Children with Autism Spectrum Disorders: Prevalence, Comorbidity, and Associated Factors in a Population-Derived Sample. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 921–929. [Google Scholar] [CrossRef]
- Joshi, G.; Petty, C.; Wozniak, J.; Henin, A.; Fried, R.; Galdo, M.; Kotarski, M.; Walls, S.; Biederman, J. The Heavy Burden of Psychiatric Comorbidity in Youth with Autism Spectrum Disorders: A Large Comparative Study of a Psychiatrically Referred Population. J. Autism Dev. Disord. 2010, 40, 1361–1370. [Google Scholar] [CrossRef]
- Burd, L.; Kerbeshian, J.; Fisher, W. Inquiry into the Incidence of Hyperlexia in a Statewide Population of Children with Pervasive Developmental Disorder. Psychol. Rep. 1985, 57, 236–238. [Google Scholar] [CrossRef]
- Burd, L.; Kerbeshian, J. Familial pervasive development disorder, Tourette disorder and hyperlexia. Neurosci. Biobehav. Rev. 1988, 12, 233–234. [Google Scholar] [CrossRef]
- Fisher, W.; Burd, L.; Kerbeshian, J. Markers for improvement in children with pervasive developmental disorders. J. Intellect. Disabil. Res. 1988, 32, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.E. On hermetic reading abilities. J. Autism Dev. Disord. 1987, 17, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Charman, T.; Pickles, A.; Simonoff, E.; Chandler, S.; Loucas, T.; Baird, G. IQ in children with autism spectrum disorders: Data from the Special Needs and Autism Project (SNAP). Psychol. Med. 2011, 41, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Eigsti, I.-M.; de Marchena, A.; Schuh, J.M.; Kelley, E. Language acquisition in autism spectrum disorders: A developmental review. Res. Autism Spectr. Disord. 2011, 5, 681–691. [Google Scholar] [CrossRef]
- Hill, E.L. Evaluating the theory of executive dysfunction in autism. Dev. Rev. 2004, 24, 189–233. [Google Scholar] [CrossRef]
- Volkmar, F.R.; Paul, R.; Rogers, S.J.; Pelphrey, K.A.; Bauminger-Zviely, N. School-Age Children With ASD. In Handbook of Autism and Pervasive Developmental Disorders, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Treffert, D.A. Hyperlexia III: Separating ’autistic-like’ behaviors from autistic disorder; assessing children who read early or speak late. Wis. Med. J. 2011, 110, 281–287. [Google Scholar]
- Franchini, M.; De Wilde, H.W.; Glaser, B.; Gentaz, E.; Eliez, S.; Schaer, M. Brief Report: A Preference for Biological Motion Predicts a Reduction in Symptom Severity 1 Year Later in Preschoolers with Autism Spectrum Disorders. Front. Psychiatry 2016, 7. [Google Scholar] [CrossRef]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule—Generic: A Standard Measure of Social and Communication Deficits Associated with the Spectrum of Autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S.L. Autism Diagnostic Observation Schedule, 2nd ed.; (ADOS-2); Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Schopler, E.; Lansing, M.D.; Reichler, R.J.; Marcus, L.M. Psychoeducational Profile: TEACCH Individualized Assessment for Children with Autism Spectrum Disorders, 3rd ed.; Pro-Ed, Inc.: Berlin, Germany, 2005. [Google Scholar]
- Sandini, C.; Zöller, D.; Scariati, E.; Padula, M.C.; Schneider, M.; Schaer, M.; Van De Ville, D.; Eliez, S. Development of Structural Covariance from Childhood to Adolescence: A Longitudinal Study in 22q11.2DS. Front. Neurosci. 2018, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Gotham, K.; Pickles, A.; Lord, C. Standardizing ADOS Scores for a Measure of Severity in Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 693–705. [Google Scholar] [CrossRef]
- Hus, V.; Gotham, K.; Lord, C. Standardizing ADOS Domain Scores: Separating Severity of Social Affect and Restricted and Repetitive Behaviors. J. Autism Dev. Disord. 2014, 44, 2400–2412. [Google Scholar] [CrossRef]
- Mullen, E.M. Mullen Scales of Early Learning; AGS, Ed.; American Guidance Service: Circle Pines, MN, USA, 1995. [Google Scholar]
- Sparrow, S.S.; Balla, D.A.; Cicchetti, D.V. Vineland II: Vineland Adaptive Behavior Scales; American Guidance Service: Circle Pines, MN, USA, 2005. [Google Scholar]
- Happé, F.; Vital, P. What aspects of autism predispose to talent? Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1369–1375. [Google Scholar] [CrossRef]
- Itzchak, E.-B.; Aviva, B.; Zachor, D.A. Are special abilities in autism spectrum disorder associated with a distinct clinical presentation? Res. Autism Spectr. Disord. 2013, 7, 1122–1128. [Google Scholar] [CrossRef]
- Kim, S.H.; Lord, C. Restricted and repetitive behaviors in toddlers and preschoolers with autism spectrum disorders based on the Autism Diagnostic Observation Schedule (ADOS). Autism Res. 2010, 3, 162–173. [Google Scholar] [CrossRef]
- Cardoso-Martins, C.; Gonçalves, D.T.; De Magalhães, C.G. What are the Mechanisms Behind Exceptional Word Reading Ability in Hyperlexia?: Evidence from a 4-Year-Old Hyperlexic Boy’s Invented Spellings. J. Autism Dev. Disord. 2013, 43, 3001–3003. [Google Scholar] [CrossRef]
- Golysheva, M. A Review on Echolalia in Childhood Autism. In Proceedings of the Internation Conference on “Humanities and Social Sciences: Novations, Problems, Prospects” (HSSNPP 2019); Atlantis Press: Novosibirsk, Russia, 2019. [Google Scholar]
- Lin, C.-S. Early language learning profiles of young children with autism: Hyperlexia and its subtypes. Res. Autism Spectr. Disord. 2013, 8, 168–177. [Google Scholar] [CrossRef]
- Stiegler, L.N. Examining the Echolalia Literature: Where Do Speech-Language Pathologists Stand? Am. J. Speech Lang. Pathol. 2015, 24, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Howlin, P. Echolalic and Spontaneous Phrase Speech in Autistic Children. J. Child Psychol. Psychiatry 1982, 23, 281–293. [Google Scholar] [CrossRef]
- Stone, W.L.; Ousley, O.Y.; Yoder, P.J.; Hogan, K.L.; Hepburn, S.L. Nonverbal Communication in Two- and Three-Year-Old Children with Autism. J. Autism Dev. Disord. 1997, 27, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, D.A.; Hoskyn, M.; Birmingham, E. Facial Expression Production in Autism: A Meta-Analysis. Autism Res. 2018, 11, 1586–1601. [Google Scholar] [CrossRef]
- Colonnesi, C.; Zijlstra, B.J.; Van Der Zande, A.; Bögels, S.M. Coordination of gaze, facial expressions and vocalizations of early infant communication with mother and father. Infant Behav. Dev. 2012, 35, 523–532. [Google Scholar] [CrossRef]
- Honey, E.; Leekam, S.; Turner, M.; McConachie, H. Repetitive Behaviour and Play in Typically Developing Children and Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2007, 37, 1107–1115. [Google Scholar] [CrossRef]
- Jarrold, C. A Review of Research into Pretend Play in Autism. Autism 2003, 7, 379–390. [Google Scholar] [CrossRef]
- Lanter, E.; Watson, L.R.; Erickson, K.A.; Freeman, D. Emergent Literacy in Children with Autism: An Exploration of Developmental and Contextual Dynamic Processes. Lang. Speech Hear. Serv. Sch. 2012, 43, 308–324. [Google Scholar] [CrossRef]
- Carlsson, L.H.; Norrelgen, F.; Kjellmer, L.; Westerlund, J.; Gillberg, C.; Fernell, E. Coexisting Disorders and Problems in Preschool Children with Autism Spectrum Disorders. Sci. World J. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Hudry, K.; Leadbitter, K.; Temple, K.; Slonims, V.; McConachie, H.; Aldred, C.; Howlin, P.; Charman, T. Preschoolers with autism show greater impairment in receptive compared with expressive language abilities. Int. J. Lang. Commun. Disord. 2010, 45, 681–690. [Google Scholar] [CrossRef]
- Loucas, T.; Charman, T.; Pickles, A.; Simonoff, E.; Chandler, S.; Meldrum, D.; Baird, G. Autistic symptomatology and language ability in autism spectrum disorder and specific language impairment. J. Child Psychol. Psychiatry 2008, 49, 1184–1192. [Google Scholar] [CrossRef]
- Paul, R.; Chawarska, K.; Cicchetti, D.; Volkmar, F. Language outcomes of toddlers with autism spectrum disorders: A two year follow-up. Autism Res. 2008, 1, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Westerveld, M.F.; Paynter, J.; Adams, D. Brief Report: Associations Between Autism Characteristics, Written and Spoken Communication Skills, and Social Interaction Skills in Preschool-Age Children on the Autism Spectrum. J. Autism Dev. Disord. 2021, 1–6. [Google Scholar] [CrossRef]
- Caron, M.-J.; Mottron, L.; Berthiaume, C.; Dawson, M. Cognitive mechanisms, specificity and neural underpinnings of visuospatial peaks in autism. Brain 2006, 129, 1789–1802. [Google Scholar] [CrossRef]
- Mottron, L.; Bouvet, L.; Bonnel, A.; Samson, F.; Burack, J.A.; Dawson, M.; Heaton, P. Veridical mapping in the development of exceptional autistic abilities. Neurosci. Biobehav. Rev. 2013, 37, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Mottron, L.; Dawson, M.; Soulières, I. Enhanced perception in savant syndrome: Patterns, structure and creativity. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1385–1391. [Google Scholar] [CrossRef]
- Mottron, L.; Dawson, M.; Soulières, I.; Hubert, B.; Burack, J. Enhanced Perceptual Functioning in Autism: An Update, and Eight Principles of Autistic Perception. J. Autism Dev. Disord. 2006, 36, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Samson, F.; Mottron, L.; Soulières, I.; Zeffiro, T.A. Enhanced visual functioning in autism: An ALE meta-analysis. Hum. Brain Mapp. 2012, 33, 1553–1581. [Google Scholar] [CrossRef]
- Ingersoll, B. The Social Role of Imitation in Autism: Implications for the Treatment of Imitation Deficits. Infants Young Child. 2008, 21, 107–119. [Google Scholar] [CrossRef]
- Williams, J.H.G.; Whiten, A.; Singh, T. A Systematic Review of Action Imitation in Autistic Spectrum Disorder. J. Autism Dev. Disord. 2004, 34, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Mottron, L. Should we change targets and methods of early intervention in autism, in favor of a strengths-based education? Eur. Child Adolesc. Psychiatry 2017, 26, 815–825. [Google Scholar] [CrossRef] [PubMed]
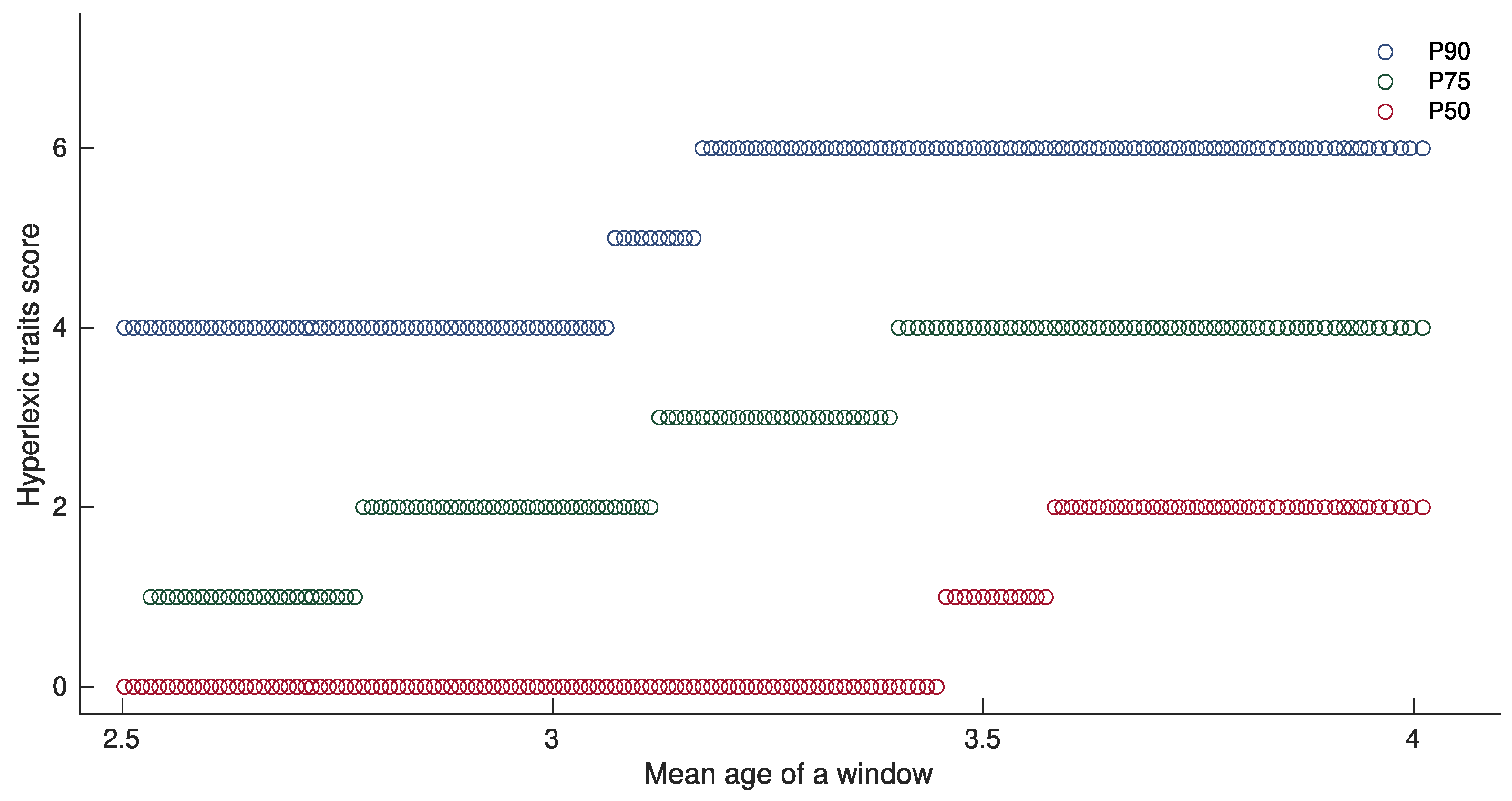

| ASD (n = 155; 20♀) | TD (n = 30; 9♀) | p | ||
|---|---|---|---|---|
| Age | 2.95 ± 0.72 | 2.87 ± 0.79 | 0.808 | |
| ADOS | SA | 6.82 ± 2.18 | 1.03 ± 0.183 | <0.001 |
| RRB | 8.74 ± 1.60 | 2.13 ± 1.925 | <0.001 | |
| Total | 7.66 ± 1.95 | 1.00 ± 0.00 | <0.001 | |
| MSEL | Early learning composite | 60.14 ± 22.74 | 110.89 ± 14.50 | <0.001 |
| VABS-II | Communication skills | 74.45 ± 14.43 | 105.62 ± 12.07 | <0.001 |
| Daily living skills | 81.41 ± 13.11 | 102.48 ± 7.55 | <0.001 | |
| Socialization skills | 77.41 ± 9.80 | 102.28 ± 7.55 | <0.001 | |
| Motor skills | 86.98 ± 11.68 | 97.62 ± 7.70 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solazzo, S.; Kojovic, N.; Robain, F.; Schaer, M. Measuring the Emergence of Specific Abilities in Young Children with Autism Spectrum Disorders: The Example of Early Hyperlexic Traits. Brain Sci. 2021, 11, 692. https://doi.org/10.3390/brainsci11060692
Solazzo S, Kojovic N, Robain F, Schaer M. Measuring the Emergence of Specific Abilities in Young Children with Autism Spectrum Disorders: The Example of Early Hyperlexic Traits. Brain Sciences. 2021; 11(6):692. https://doi.org/10.3390/brainsci11060692
Chicago/Turabian StyleSolazzo, Stefania, Nada Kojovic, François Robain, and Marie Schaer. 2021. "Measuring the Emergence of Specific Abilities in Young Children with Autism Spectrum Disorders: The Example of Early Hyperlexic Traits" Brain Sciences 11, no. 6: 692. https://doi.org/10.3390/brainsci11060692
APA StyleSolazzo, S., Kojovic, N., Robain, F., & Schaer, M. (2021). Measuring the Emergence of Specific Abilities in Young Children with Autism Spectrum Disorders: The Example of Early Hyperlexic Traits. Brain Sciences, 11(6), 692. https://doi.org/10.3390/brainsci11060692






