Polish Adaptation of the Social Communication Questionnaire (SCQ) and Female Autism Phenotype: An Investigation of Potentially Sex-Biased Items in the Screening Assessment and Their Impact on Scores
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Author’ Information
References
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- World Health Organization. International Classification of Diseases for Mortality and Morbidity Statistics, 11th ed.; World Health Organization: Geneva, Switzerland, 2018; Available online: https://icd.who.int/browse11/l-m/en (accessed on 29 March 2021).
- Fusar-Poli, L.; Brondino, N.; Politi, P.; Aguglia, E. Missed Diagnoses and Misdiagnoses of Adults with Autism Spectrum Disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hull, L.; Petrides, K.V.; Mandy, W. The Female Autism Phenotype and Camouflaging: A Narrative Review. Rev. J. Autism Dev. Disord. 2020, 7, 306–317. [Google Scholar] [CrossRef]
- Happé, F.G.; Mansour, H.; Barrett, P.; Brown, T.; Abbott, P.; Charlton, R.A. Demographic and Cognitive Profile of Individuals Seeking a Diagnosis of Autism Spectrum Disorder in Adulthood. J. Autism Dev. Disord. 2016, 46, 3469–3480. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Wilson, C.E.; Robertson, D.M.; Ecker, C.; Daly, E.M.; Hammond, N.; Galanopoulos, A.; Dud, I.; Murphy, D.G.; McAlonan, G.M. Autism Spectrum Disorder in Adults: Diagnosis, Management, and Health Services Development. Neuropsychiatry Dis. Treat. 2016, 12, 1669–1686. [Google Scholar] [CrossRef]
- Rynkiewicz, A.; Łucka, I. Autism spectrum disorder (ASD) in girls. Co-occurring psychopathology. Sex differences in clinical manifestation. Psychiatry Pol. 2018, 52, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Fombonne, E. Epidemiology of Pervasive Developmental Disorders. Pediatr. Res. 2009, 65, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Steer, C.; Golding, J. Social and Demographic Factors That Influence the Diagnosis of Autistic Spectrum Disorders. Soc. Psychiatry Psychiatry Epidemiol. 2011, 46, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Rynkiewicz, A.; Janas-Kozik, M.; Słopień, A. Dziewczęta i kobiety z autyzmem. Girls and Women with Autism. Psychiatry Pol. 2019, 53, 737–752. [Google Scholar] [CrossRef]
- Rynkiewicz, A.; Lassalle, A.; King, B.H.; Smith, R.; Mazur, A.; Podgórska-Bednarz, J.; Słopień, A.; Tabarkiewicz, J. Females and Autism. In Oxford Bibliographies in Childhood Studies; Montgomery, H., Ed.; Oxford University Press: New York, NY, USA, 2018. [Google Scholar]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Tillmann, J.; Ashwood, K.; Absoud, M.; Bölte, S.; Bonnet-Brilhault, F.; Buitelaar, J.K.; Calderoni, S.; Calvo, R.; Canal-Bedia, R.; Canitano, R.; et al. Evaluating Sex and Age Differences in ADI-R and ADOS Scores in a Large European Multi-Site Sample of Individuals with Autism Spectrum Disorder. J. Autism Dev. Disord. 2018, 48, 2490–2505. [Google Scholar] [CrossRef]
- Hirvikoski, T.; Mittendorfer-Rutz, E.; Boman, M.; Larsson, H.; Lichtenstein, P.; Bölte, S. Premature Mortality in Autism Spectrum Disorder. Br. J. Psychiatry 2016, 208, 232–238. [Google Scholar] [CrossRef]
- Autism Community Priorities for Suicide Prevention. An International Society for Autism Research (INSAR) Policy Brief 2021. Available online: https://cdn.ymaws.com/www.autism-insar.org/resource/resmgr/files/policybriefs/2021-insar_policy_brief.pdf (accessed on 7 May 2021).
- Yeargin-Allsopp, M.; Rice, C.; Karapurkar, T.; Doernberg, N.; Boyle, C.; Murphy, C. Prevalence of Autism in a US Metropolitan Area. JAMA 2003, 289, 49–55. [Google Scholar] [CrossRef] [PubMed]
- King, B.H.; Rynkiewicz, A.; Janas- Kozik, M.; Tyszkiewicz-Nwafor, M. Medications to Treat Co-Occurring Psychiatric Conditions in Autism Spectrum Disorder. In The Oxford Handbook of Autism and Co-Occurring Psychiatric Conditions; White, S.W., Madox, B.B., Mazefsky, C.A., Eds.; Oxford University Press: New York, NY, USA, 2020; pp. 371–386. [Google Scholar]
- White, S.W.; Maddox, B.B.; Mazefsky, C.A. The Oxford Handbook of Autism and Co- Occurring Psychiatric Conditions; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Parish-Morris, J.; Liberman, M.Y.; Cieri, C.; Herrington, J.D.; Yerys, B.E.; Bateman, L.; Donaher, J.; Ferguson, E.; Pandey, J.; Schultz, R.T. Linguistic Camouflage in Girls with Autism Spectrum Disorder. Mol. Autism 2017, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Ormond, S.; Brownlow, C.; Garnett, M.S.; Rynkiewicz, A.; Attwood, T. Profiling autism symptomatology: An exploration of the Q-ASC parental report scale in capturing sex differences in autism. J. Autism Dev. Disord. 2018, 48, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Rynkiewicz, A.; Schuller, B.; Marchi, E.; Piana, S.; Camurri, A.; Lassalle, A.; Baron-Cohen, S. An investigation of the ‘female camouflage effect’ in autism using a computerized ADOS-2 and a test of sex/gender differences. Mol. Autism 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Bailey, A.; Lord, C. SCQ: Social Communication Questionnaire. Manual; Western Psychological Services: Los Angeles, CA, USA, 2010. [Google Scholar]
- Lai, M.-C.; Baron-Cohen, S. Identifying the Lost Generation of Adults with Autism Spectrum Conditions. Lancet Psychiatry 2015, 2, 1013–1027. [Google Scholar] [CrossRef]
- Baron-Cohen, S. ASD vs. ASC: Is one small letter important? In Proceedings of the IMFAR 2015 14th Annual International Meeting for Autism Research. International Society for Autism Research (INSAR), Salt Lake City, UT, USA, 13–16 May 2015. [Google Scholar]
- Rutter, M.; Le Couteur, A.; Lord, C. Autism Diagnostic Interview-Revised (ADI-R); Western Psychological Services: Los Angeles, CA, USA, 2003. [Google Scholar]
- Lord, C.; Rutter, M.; Di Lavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule—Second Edition (ADOS-2). Man. Part I Modul. 2012, 1, 4. [Google Scholar]
- Barnard- Brak, L.; Richman, D.; Almekdash, M. How Many Girls Are We Missing in ASD? An Examination from Clinic-Community-Based Sample. Adv. Autism 2019, 5, 214–224. [Google Scholar] [CrossRef]
- Lai, M.C.; Szatmari, P. Sex and Gender Impacts on the Behavioural Presentation and Recognition of Autism. Curr. Opin. Psychiatry 2020, 33, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zener, D. Journey to Diagnosis for Women with Autism. Adv. Autism 2019, 5, 2–13. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research, 10th ed.; World Health Organization: Geneva, Switzerland, 2007; Available online: https://icd.who.int/browse10/2019/en (accessed on 29 March 2021).
- Matczak, A.; Piotrowska, A.; Ciarkowska, W. WISC-R-Wechsler Intelligence Scale for Children-Revised, 3rd ed.; Laboratory of Psychological Tests of the Polish Psychological Association: Warsaw, Poland, 2008. [Google Scholar]
- Brzezinski, J.; Gaul, M.; Hornowska, E.; Jaworowska, A.; Machowski, A.; Zakrzewska, M. WAIS-R (PL)- Wechsler Adult Intelligence Scale- Revised Version, 2nd ed.; Laboratory of Psychological Tests of the Polish Psychological Association: Warsaw, Poland, 2004. [Google Scholar]
- Western Psychological Services (WPS). Available online: https://www.wpspublish.com/ (accessed on 29 March 2021).
- Kaat, A.J.; Shui, A.M.; Ghods, S.S.; Farmer, C.A.; Esler, A.N.; Thurm, A.; Georgiades, S.; Kanne, S.M.; Lord, C.; Kim, Y.S.; et al. Sex Differences in Scores on Standardized Measures of Autism Symptoms: A Multisite Integrative Data Analysis. J. Child Psychol. Psychiatry 2021, 62, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cho, S.-C.; Cho, I.H.; Kim, B.-N.; Kim, J.-W.; Shin, M.-S.; Chung, U.-S.; Park, T.-W.; Son, J.-W.; Yoo, H.J. Sex Differences in Children with Autism Spectrum Disorders Compared with Their Unaffected Siblings and Typically Developing Children. Res. Autism Spectr. Disord. 2012, 6, 861–870. [Google Scholar] [CrossRef]
- Lai, M.-C.; Lombardo, M.V.; Auyeung, B.; Chakrabarti, B.; Baron-Cohen, S. Sex/Gender Differences and Autism: Setting the Scene for Future Research. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Wood-Downie, H.; Wong, B.; Kovshoff, H.; Cortese, S.; Hadwin, J.A. Research Review: A Systematic Review and Meta-Analysis of Sex/Gender Differences in Social Interaction and Communication in Autistic and Nonautistic Children and Adolescents. J. Child Psychol. Psychiatry 2020, in press. [Google Scholar] [CrossRef]
- Seltzer, M.M.; Krauss, M.W.; Shattuck, P.T.; Orsmond, G.; Swe, A.; Lord, C. The symptoms of autism spectrum disorders in adolescence and adulthood. J. Autism Dev. Disord. 2003, 33, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, L.; Brondino, N.; Rocchetti, M.; Panisi, C.; Provenzani, U.; Damiani, S.; Politi, P. Diagnosing ASD in Adults Without ID: Accuracy of the ADOS-2 and the ADI-R. J. Autism Dev. Disord. 2017, 47, 3370–3379. [Google Scholar] [CrossRef] [PubMed]
- Western Psychological Services (WPS). Available online: https://pages.wpspublish.com/telepractice-101 (accessed on 27 April 2021).
- Frazier, T.W.; Youngstrom, E.A.; Sinclair, L.; Kubu, C.S.; Law, P.; Rezai, A.; Constantino, J.N.; Eng, C. Autism Spectrum Disorders as a Qualitatively Distinct Category From Typical Behavior in a Large, Clinically Ascertained Sample. Assessment 2010, 17, 308–320. [Google Scholar] [CrossRef]
- Evans, S.; Boan, A.; Bradley, C.; Carpenter, L. Sex/Gender Differences in Screening for Autism Spectrum Disorder: Implications for Evidence-Based Assessment. J. Clin. Child Adolesc. Psychol. 2018, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]

| Participants with ASD (N = 90) | |||
|---|---|---|---|
| Sex | Females (N = 30) | Males (N = 60) | |
| IQ | Mean (SD) | 108 (12.27) | 102.62 (12.53) |
| Age | Mean (SD) | 15.93 (11.09) | 11.4 (5.85) |
| Comorbidity | With comorbidity | 19 (63.3%) | 44 (73.3%) |
| Without comorbidity | 11 (36.7%) | 16 (26.7%) | |
| Type of comorbidity | Mood (affective) disorders: depression, BD | 3 (15.8%) | 4 (9.1%) |
| Neurotic, stress-related, and somatoform disorders: anxiety, OCD, phobias, agarophobia, panic disorder | 10 (52.6%) | 13 (29.5%) | |
| Disorders with onset occuring in childchood or adolescence: ADHD, ODD, tic disorder | 2 (10.5%) | 17 (38.6%) | |
| Psychological development: dyslexia, dyspraxia | 1 (5.3%) | 7 (15.9%) | |
| Syndromes associated with psychological disturbance: ED | 2 (10.5%) | 0 (0.0%) | |
| Others: adult personality or substance-related disorders | 1 (5.3%) | 3 (6.8%) | |
| Item | Sex | Current (n) | Lifetime (n) | p | |
|---|---|---|---|---|---|
| No | Yes | ||||
| 11. Has she/he ever had any interests that preoccupy her/him and might seem odd to other people(e.g., traffic lights, drain pipes, or time tables)? | Female | No | 17 | 1 | 1.000 |
| Yes | 0 | 12 | |||
| Male | No | 23 | 2 | 1.000 | |
| Yes | 2 | 33 | |||
| 12. Has she/he ever seemed to be more interested in parts of a toy or an object (e.g., spinning the wheels of a car), rather than using an object as it was intended? | Female | No | 22 | 1 | 1.000 |
| Yes | 2 | 5 | |||
| Male | No | 36 | 2 | 0.039 | |
| Yes | 10 | 11 | |||
| 13. Has she/he ever had any special interests that were unusual in their intensity but otherwise appropriate for her/his age and peer group (e.g., trains, dinosaurs)? | Female | No | 17 | 0 | 1.000 |
| Yes | 1 | 12 | |||
| Male | No | 19 | 4 | 0.754 | |
| Yes | 6 | 31 | |||
| 14. Has she/he ever seemed to be unusually interested in the sight, feel, sound, taste, or smell of things or people? | Female | No | 7 | 1 | 0.625 |
| Yes | 3 | 19 | |||
| Male | No | 11 | 2 | 1.000 | |
| Yes | 14 | 44 | |||
| Item | Age | Current (n) | Lifetime (n) | p | |
|---|---|---|---|---|---|
| No | Yes | ||||
| 11. Has she/he ever had any interests that preoccupy her/him and might seem odd to other people (e.g., traffic lights, drain pipes, or time tables)? | Child | No | 27 | 1 | 1.000 |
| Yes | 2 | 26 | |||
| Adolescent | No | 8 | 1 | 1.000 | |
| Yes | 0 | 10 | |||
| Adult | No | 5 | 1 | 1.000 | |
| Yes | 0 | 9 | |||
| 12. Has she/he ever seemed to be more interested in parts of a toy or an object (e.g., spinning the wheels of a car), rather than using an object as it was intended? | Child | No | 37 | 2 | 0.289 |
| Yes | 6 | 11 | |||
| Adolescent | No | 14 | 1 | 1.000 | |
| Yes | 2 | 2 | |||
| Adult | No | 7 | 0 | 0.125 | |
| Yes | 4 | 3 | |||
| 13. Has she/he ever had any special interests that were unusual in their intensity but otherwise appropriate for her/his age and peer group (e.g., trains, dinosaurs) ? | Child | No | 26 | 3 | 0.727 |
| Yes | 5 | 22 | |||
| Adolescent | No | 5 | 1 | 1.000 | |
| Yes | 2 | 11 | |||
| Adult | No | 5 | 0 | 1.000 | |
| Yes | 0 | 10 | |||
| 14. Has she/he ever seemed to be unusually interested in the sight, feel, sound, taste, or smell of things or people? | Child | No | 7 | 1 | 0.375 |
| Yes | 4 | 42 | |||
| Adolescent | No | 6 | 2 | 1.000 | |
| Yes | 1 | 10 | |||
| Adult | No | 5 | 0 | 1.000 | |
| Yes | 1 | 9 | |||
| Females (n = 30) | Males (n = 60) | Z | p | η2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Rank | M | Me | SD | Mean Rank | M | Me | SD | ||||
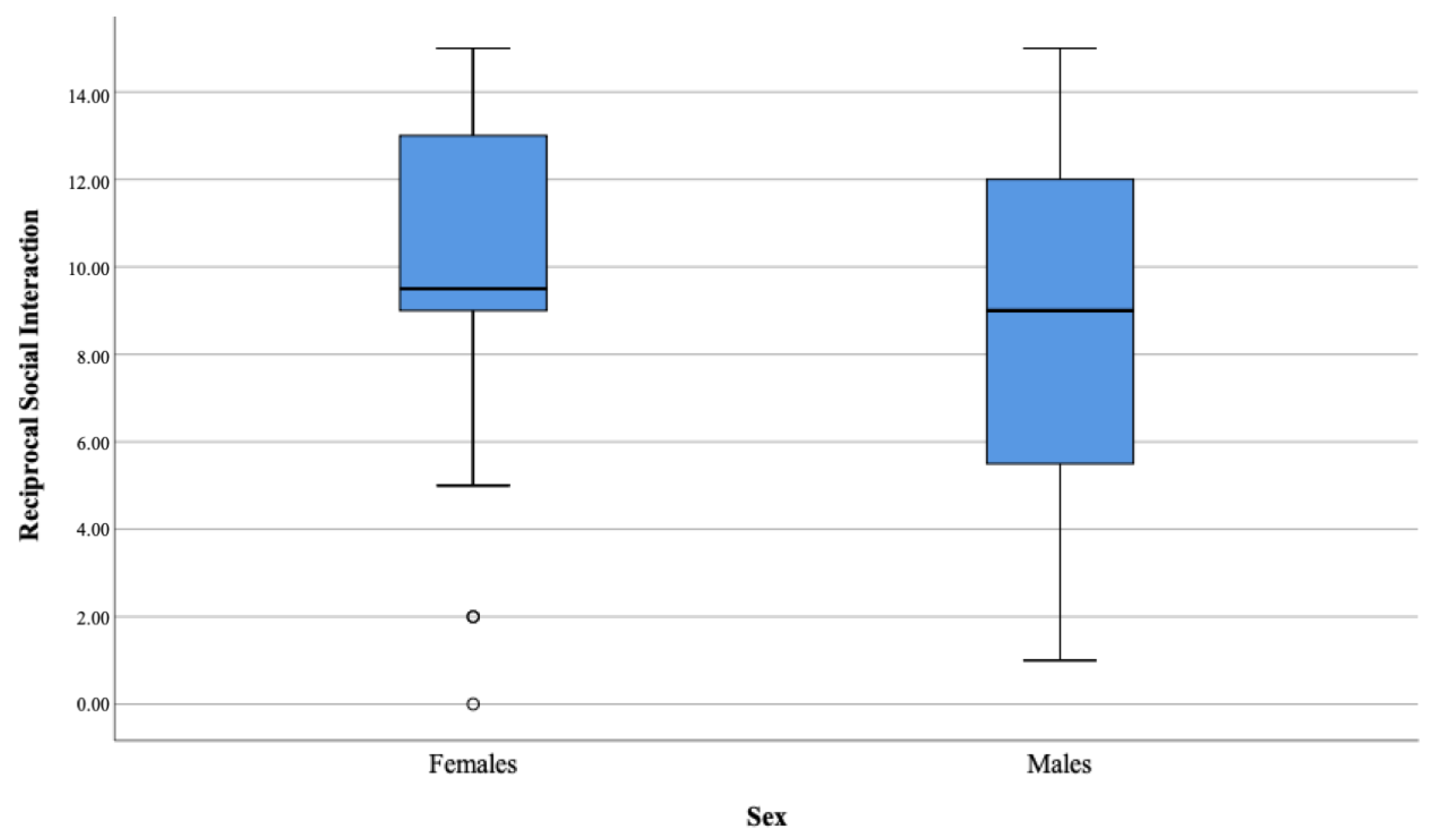
| Reciprocal Social Interaction | 49.88 | 9.67 | 9.50 | 3.80 | 43.31 | 8.55 | 9.00 | 4.24 | −1.13 | 0.258 | 0.01 |
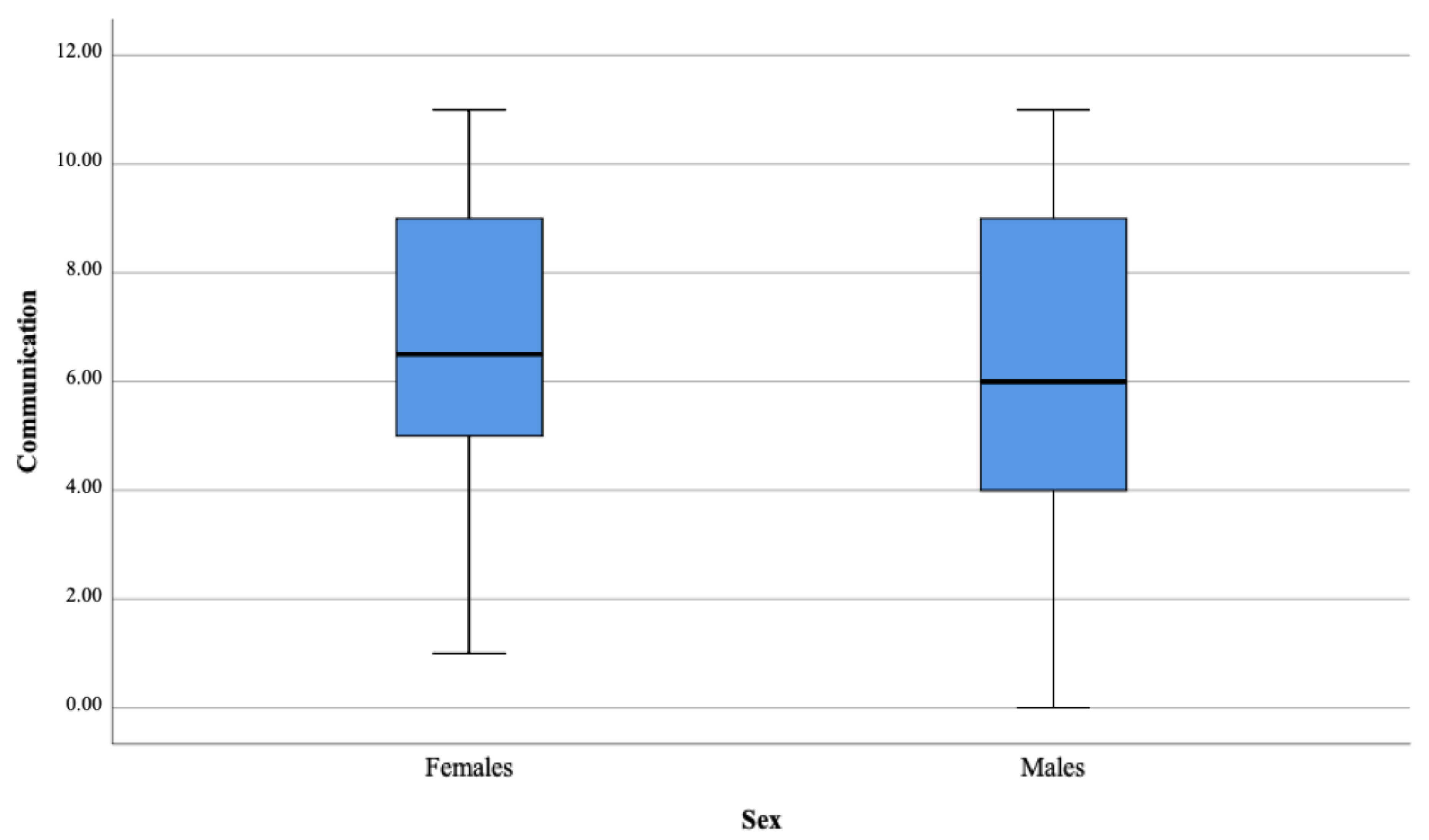
| Communication | 49.73 | 6.73 | 6.50 | 2.75 | 43.38 | 6.07 | 6.00 | 2.85 | −1.10 | 0.273 | 0.01 |
| Restricted, Repetitive, and Stereotyped Patterns of Behavior | 40.62 | 3.57 | 4.00 | 2.54 | 47.94 | 4.28 | 4.00 | 2.31 | −1.26 | 0.206 | 0.02 |
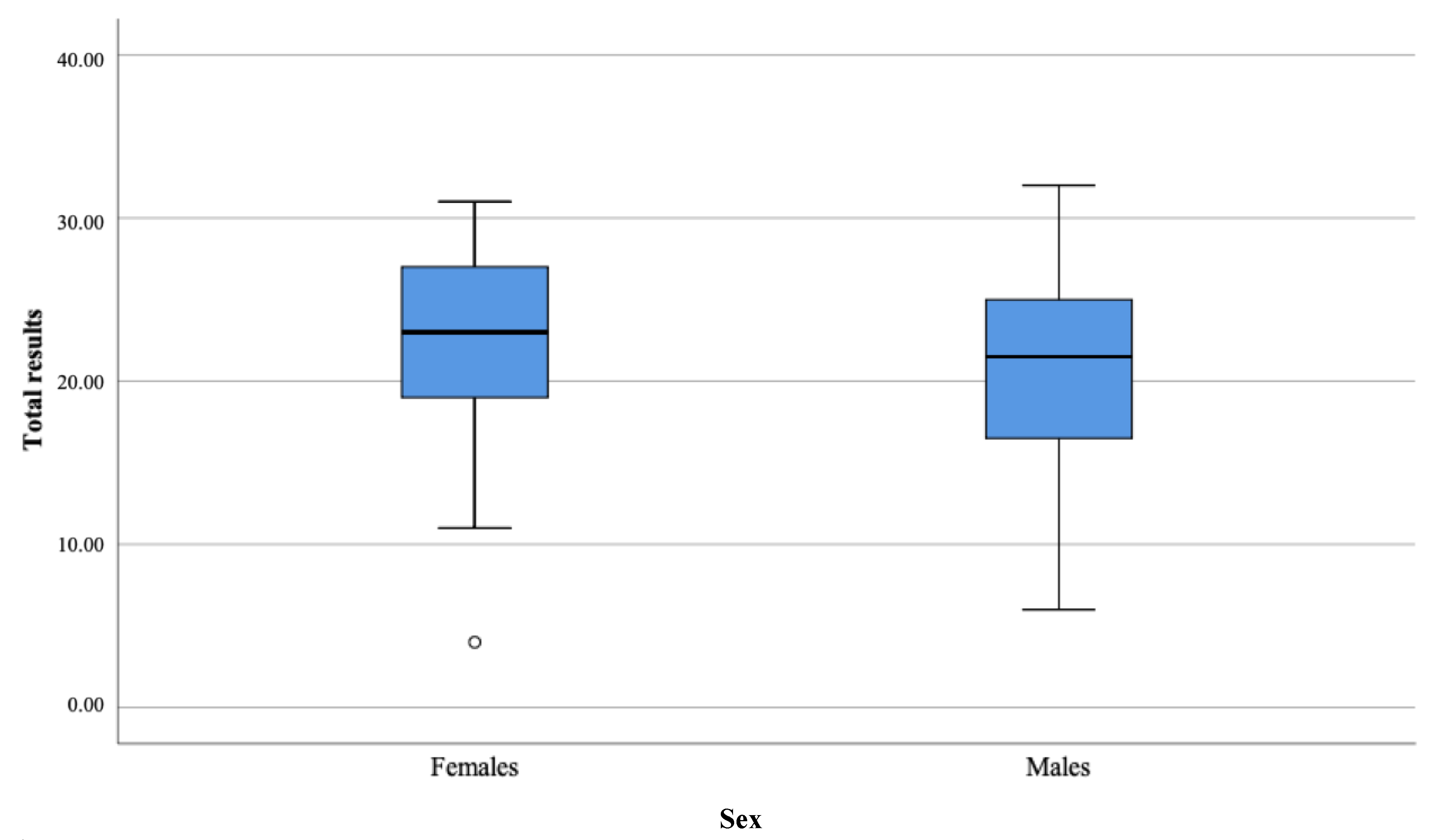
| Total | 50.20 | 21.97 | 23.00 | 6.10 | 43.15 | 20.63 | 21.50 | 5.71 | −1.21 | 0.227 | 0.02 |
| Age | Parents/Carers’ Comments and Annotations | Category |
|---|---|---|
| 7 y.o. | She is interested only in cat breeds identification | Animals |
| 9 y.o. | She collects makeup products | Fashion and beauty |
| 17 y.o. | She is obssessed about horses and spends long hours researching all about horses on the internet | Animals |
| 8 y.o. | She is an avid reader, and she always takes her book with her when she leaves the house | Literature |
| 15 y.o. | She insists on going to the same play several times | Theatre and dance |
| 6 y.o. | She insists on reading books only about fairies, collects only fairy dolls, and goes out only dressed as a fairy | Fairytales |
| 19 y.o. | All she wants to learn is Italian, she repeats new phrases all the time, and she already speaks two other foreign languages fluently | Languages |
| 13 y.o. | She knows everything about Gustav Klimt’s art | Arts |
| 32 y.o | She is interested only in autism in females, writes a blog about it, attends meetings as a self-advocate, talks only about this topic if you let her | Human behaviour |
| 9 y.o | She only talks about dog’s behaviour, how to train dogs, knows the addresses of every dog training center and pet shop in the area, and insists on going there in any spare moment | Animal behaviour |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rynkiewicz, A.; Szura, M.; Bernaciak, D.; Kozak, A.; Karwowska, M. Polish Adaptation of the Social Communication Questionnaire (SCQ) and Female Autism Phenotype: An Investigation of Potentially Sex-Biased Items in the Screening Assessment and Their Impact on Scores. Brain Sci. 2021, 11, 682. https://doi.org/10.3390/brainsci11060682
Rynkiewicz A, Szura M, Bernaciak D, Kozak A, Karwowska M. Polish Adaptation of the Social Communication Questionnaire (SCQ) and Female Autism Phenotype: An Investigation of Potentially Sex-Biased Items in the Screening Assessment and Their Impact on Scores. Brain Sciences. 2021; 11(6):682. https://doi.org/10.3390/brainsci11060682
Chicago/Turabian StyleRynkiewicz, Agnieszka, Magdalena Szura, Daria Bernaciak, Anna Kozak, and Magdalena Karwowska. 2021. "Polish Adaptation of the Social Communication Questionnaire (SCQ) and Female Autism Phenotype: An Investigation of Potentially Sex-Biased Items in the Screening Assessment and Their Impact on Scores" Brain Sciences 11, no. 6: 682. https://doi.org/10.3390/brainsci11060682
APA StyleRynkiewicz, A., Szura, M., Bernaciak, D., Kozak, A., & Karwowska, M. (2021). Polish Adaptation of the Social Communication Questionnaire (SCQ) and Female Autism Phenotype: An Investigation of Potentially Sex-Biased Items in the Screening Assessment and Their Impact on Scores. Brain Sciences, 11(6), 682. https://doi.org/10.3390/brainsci11060682






