The Effects of 4 Weeks of Chiropractic Spinal Adjustments on Motor Function in People with Stroke: A Randomized Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Design and Setting
2.2. Study Participants
2.3. Interventions
2.3.1. Chiropractic Intervention
2.3.2. Sham Chiropractic Intervention
2.3.3. Physical Therapy Intervention
2.4. Outcome Measures
2.4.1. Fugl-Meyer Assessment Scale
2.4.2. Stroke Specific Quality of Life Scale
2.4.3. Timed up and Go Test
2.4.4. Modified Rankin Scale
2.4.5. Five-Repetition Sit-to-Stand Test
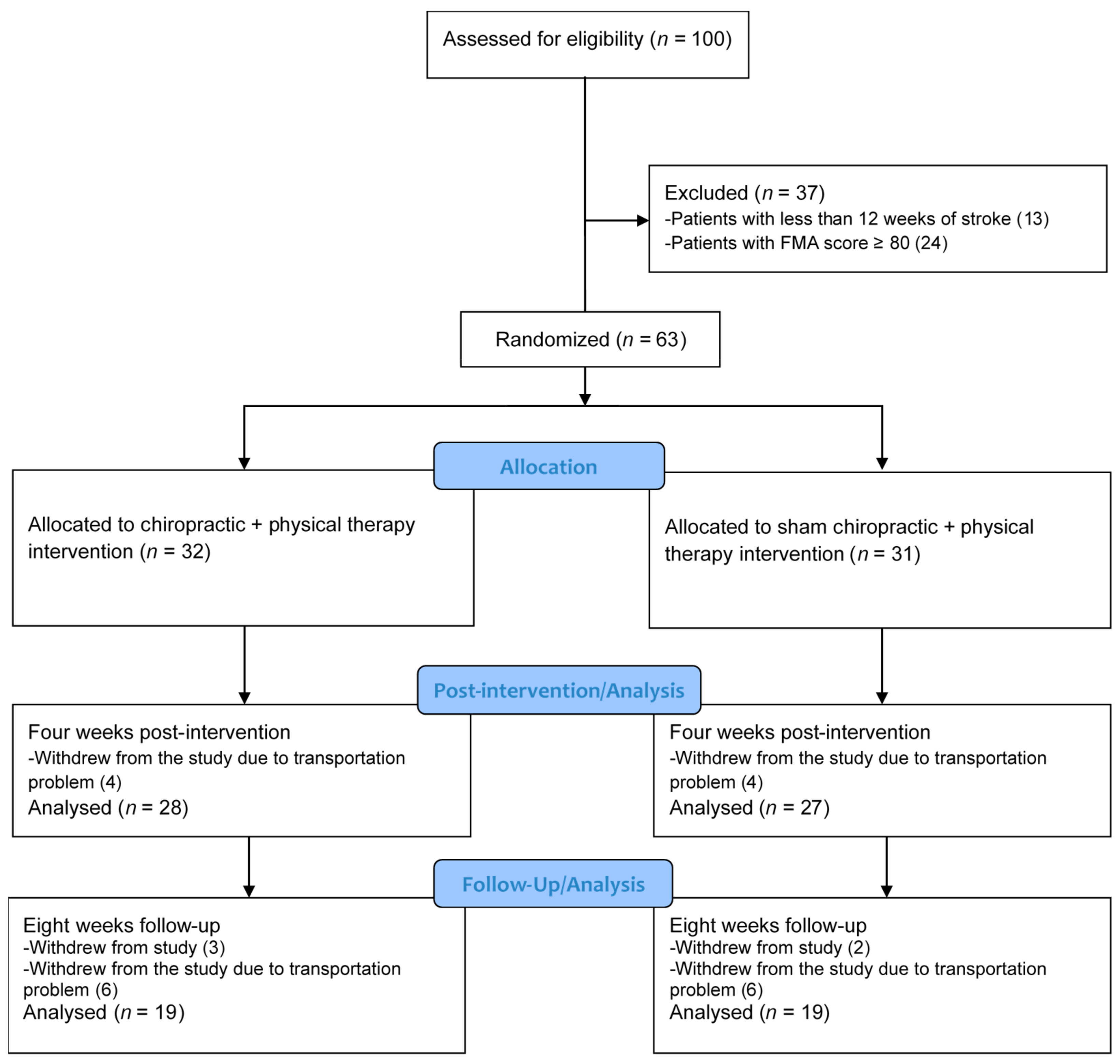
2.5. Randomization and Blinding
2.6. Statistical Analysis
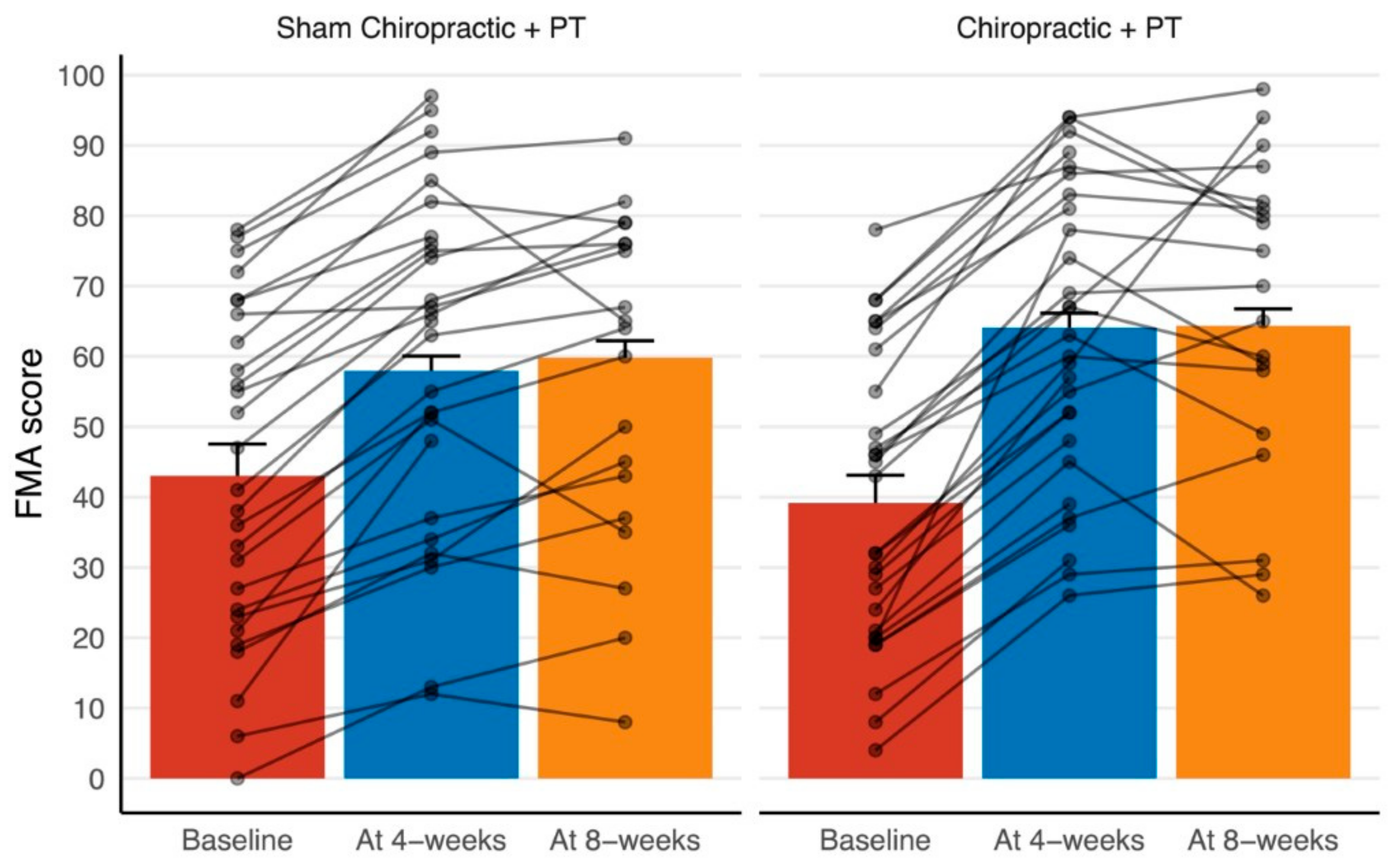
3. Results
3.1. Between-Group Differences
3.2. Within-Group Differences
3.3. Participant Blinding
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Bittencourt, M.S. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Krishnamurthi, R.V.; Barker-Collo, S.; Forouzanfar, M.H.; Naghavi, M.; Connor, M.; Lawes, C.M.; Moran, A.E.; Anderson, L.M.; Roth, G.A.; et al. The Global Burden of Ischemic Stroke: Findings of the GBD 2010 Study. Glob. Heart 2014, 9, 107–112. [Google Scholar] [CrossRef]
- Clarke, D.J.; Forster, A. Improving post-stroke recovery: The role of the multidisciplinary health care team. J. Multidiscip. Health 2015, 8, 433–442. [Google Scholar] [CrossRef]
- Krakauer, J.W. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef]
- Greisberger, A.; Aviv, H.; Garbade, S.F.; Diermayr, G. Clinical relevance of the effects of reach-to-grasp training using trunk restraint in individuals with hemiparesis poststroke: A systematic review. J. Rehabil. Med. 2016, 48, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Wist, S.; Clivaz, J.; Sattelmayer, M. Muscle strengthening for hemiparesis after stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2016, 59, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Kwan, J.; Sandercock, P. In-Hospital Care Pathways for, Stroke. Stroke 2005, 36, 1348–1349. [Google Scholar] [CrossRef]
- Taylor, H.H.; Holt, K.; Murphy, B. Exploring the Neuromodulatory Effects of the Vertebral Subluxation and Chiropractic Care. Chiropr. J. Aust. 2010, 40, 37. [Google Scholar]
- Navid, M.S.; Niazi, I.K.; Lelic, D.; Nedergaard, R.B.; Holt, K.; Amjad, I.; Drewes, A.M.; Haavik, H. Investigating the Effects of Chiropractic Spinal Manipulation on EEG in Stroke Patients. Brain Sci. 2020, 10, 253. [Google Scholar] [CrossRef]
- Waterstone, T.S.; Niazi, I.K.; Navid, M.S.; Amjad, I.; Shafique, M.; Holt, K.; Haavik, H.; Samani, A. Functional Connectivity Analysis on Resting-State Electroencephalography Signals Following Chiropractic Spinal Manipulation in Stroke Patients. Brain Sci. 2020, 10, 644. [Google Scholar] [CrossRef]
- Holt, K.; Niazi, I.K.; Nedergaard, R.W.; Duehr, J.; Amjad, I.; Shafique, M.; Anwar, M.N.; Ndetan, H.; Turker, K.S.; Haavik, H. The effects of a single session of chiropractic care on strength, cortical drive, and spinal excitability in stroke patients. Sci. Rep. 2019, 9, 2673. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Niazi, I.K.; Holt, K.; Nedergaard, R.W.; Duehr, J.; Allen, K.; Marshall, P.; Türker, K.S.; Hartvigsen, J.; Haavik, H. The effects of a single session of spinal manipulation on strength and cortical drive in athletes. Eur. J. Appl. Physiol. 2018, 118, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Haavik, H.; Murphy, B. The role of spinal manipulation in addressing disordered sensorimotor integration and altered motor control. J. Electromyogr. Kinesiol. 2012, 22, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Kingett, M.; Holt, K.; Niazi, I.K.; Nedergaard, R.W.; Lee, M.; Haavik, H. Increased Voluntary Activation of the Elbow Flexors Following a Single Session of Spinal Manipulation in a Subclinical Neck Pain Population. Brain Sci. 2019, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Australian Spinal Research Foundation. The Vertebral Subluxation: Conceptual Definition for Research and Practice; Australian Spinal Research Foundation: Melbourne, Australia, 2017. [Google Scholar]
- Hart, J. Analysis and Adjustment of Vertebral Subluxation as a Separate and Distinct Identity for the Chiropractic Profession: A Commentary. J. Chiropr. Humanit. 2016, 23, 46–52. [Google Scholar] [CrossRef]
- The Rubicon Group. Definition and Position Statement on the Chiropractic Subluxation; The Rubicon Group: Oak Brook, IL, USA, 2017. [Google Scholar]
- Rosner, A.L. Chiropractic Identity: A Neurological, Professional, and Political Assessment. J. Chiropr. Humanit. 2016, 23, 35–45. [Google Scholar] [CrossRef][Green Version]
- Suter, E.; McMorland, G. Decrease in elbow flexor inhibition after cervical spine manipulation in patients with chronic neck pain. Clin. Biomech. 2002, 17, 541–544. [Google Scholar] [CrossRef]
- Suter, E.; McMorland, G.; Herzog, W.; Bray, R. Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain. J. Manip. Physiol. Ther. 1999, 22, 149–153. [Google Scholar] [CrossRef]
- Haavik, H.; Niazi, I.K.; Jochumsen, M.; Sherwin, D.; Flavel, S.; Türker, K.S. Impact of Spinal Manipulation on Cortical Drive to Upper and Lower Limb Muscles. Brain Sci. 2016, 7, 2. [Google Scholar] [CrossRef]
- Haavik, H.; Özyurt, M.G.; Niazi, I.K.; Holt, K.; Nedergaard, R.W.; Yilmaz, G.; Türker, K.S. Chiropractic Manipulation Increases Maximal Bite Force in Healthy Individuals. Brain Sci. 2018, 8, 76. [Google Scholar] [CrossRef]
- Haavik, H.; Murphy, B.A.; Kruger, J. Effect of Spinal Manipulation on Pelvic Floor Functional Changes in Pregnant and Nonpregnant Women: A Preliminary Study. J. Manip. Physiol. Ther. 2016, 39, 339–347. [Google Scholar] [CrossRef]
- Haavik Taylor, H.; Murphy, B. The effects of spinal manipulation on central integration of dual somatosensory input observed following motor training: A crossover study. J. Manip. Physiol. Ther. 2010, 33, 261–272. [Google Scholar] [CrossRef]
- Pickar, J.; Bolton, P. Spinal manipulative therapy and somatosensory activation. J. Electromyogr. Kinesiol. 2012, 22, 785–794. [Google Scholar] [CrossRef]
- Haavik-Taylor, H.; Murphy, B. Cervical spine manipulation alters sensorimotor integration: A somatosensory evoked potential study. Clin. Neurophysiol. 2007, 118, 391–402. [Google Scholar] [CrossRef]
- Brown, K.E.; Neva, J.L.; Feldman, S.J.; Staines, W.R.; Boyd, L.A. Sensorimotor integration in chronic stroke: Baseline differences and response to sensory training. Restor. Neurol. Neurosci. 2018, 36, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Niazi, I.K.; Türker, K.S.; Flavel, S.; Kinget, M.; Duehr, J.; Haavik, H. Changes in H-reflex and V-waves following spinal manipulation. Exp. Brain Res. 2015, 233, 1165–1173. [Google Scholar] [CrossRef]
- Rehme, A.K.; Eickhoff, S.B.; Rottschy, C.; Fink, G.R.; Grefkes, C. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage 2012, 59, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Wu, P.; Zeng, F.; Li, Y.-X.; Yu, B.-L.; Qiu, L.-H.; Qin, W.; Li, J.; Zhou, Y.-M. Changes of resting cerebral activities in subacute ischemic stroke patients. Neural Regen. Res. 2015, 10, 760–765. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R. Post-stroke hemiplegia assessment of physical properties. Scand. J. Rehabil. Med. Suppl. 1980, 7, 85–93. [Google Scholar] [PubMed]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Holt, K.; Russell, D.; Cooperstein, R.; Young, M.; Sherson, M.; Haavik, H. Interexaminer reliability of a multidimensional battery of tests used to assess for vertebral subluxations. Chiropr. J. Aust. 2018, 46, 1. [Google Scholar]
- Triano, J.J.; Budgell, B.; Bagnulo, A.; Roffey, B.; Bergmann, T.; Cooperstein, R.; Gleberzon, B.; Good, C.; Perron, J.; Tepe, R. Review of methods used by chiropractors to determine the site for applying manipulation. Chiropr. Man. Ther. 2013, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Cooperstein, R.; Gleberzon, B. Technique Systems in Chiropractic; Churchill Livingstone: London, UK, 2004. [Google Scholar]
- Hancock, M.J.; Maher, C.G.; Latimer, J.; McAuley, J.H. Selecting an appropriate placebo for a trial of spinal manipulative therapy. Aust. J. Physiother. 2006, 52, 135–138. [Google Scholar] [CrossRef]
- Rosner, A.L. Evidence-based medicine: Revisiting the pyramid of priorities. J. Bodyw. Mov. Ther. 2012, 16, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Saeed, H.; Saleem, Z.; Rathore, H.A.; Rasool, F.; Tahir, E.; Bhatti, T.; Khalid, J.; Bhatti, I.; Tariq, A. A cross-sectional assessment of knowledge, attitudes and self-perceived effectiveness of complementary and alternative medicine among pharmacy and non-pharmacy university students. BMC Complement. Altern. Med. 2019, 19, 95. [Google Scholar] [CrossRef] [PubMed]
- Pollock, A.; George, B.S.; Fenton, M.; Firkins, L. Top 10 Research Priorities Relating to Life after Stroke—Consensus from Stroke Survivors, Caregivers, and Health Professionals. Int. J. Stroke 2012, 9, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.; Moreland, J.; Swanson, L.R.; Stratford, P.W.; Gowland, C. Reliability of the Fugl-Meyer Assessment for Testing Motor Performance in Patients Following Stroke. Phys. Ther. 1993, 73, 447–454. [Google Scholar] [CrossRef]
- Duncan, P.W.; Propst, M.; Nelson, S.G. Reliability of the Fugl-Meyer Assessment of Sensorimotor Recovery Following Cerebrovascular Accident. Phys. Ther. 1983, 63, 1606–1610. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabil. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Kwakkel, G.; Lannin, N.A.; Borschmann, K.; English, C.; Ali, M.; Churilov, L.; Saposnik, G.; Winstein, C.; Van Wegen, E.E.; Wolf, S.L.; et al. Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2017, 12, 451–461. [Google Scholar] [CrossRef]
- Duncan, P.W.; Goldstein, L.B.; Horner, R.D.; Landsman, P.B.; Samsa, G.P.; Matchar, D. Similar motor recovery of upper and lower extremities after stroke. Stroke 1994, 25, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.S.; Weinberger, M.; Harris, L.E.; Clark, D.O.; Biller, J. Development of a Stroke-Specific Quality of Life Scale. Stroke 1999, 30, 1362–1369. [Google Scholar] [CrossRef]
- Hsueh, I.-P.; Jeng, J.-S.; Lee, Y.; Sheu, C.-F.; Hsieh, C.-L. Construct Validity of the Stroke-Specific Quality of Life Questionnaire in Ischemic Stroke Patients. Arch. Phys. Med. Rehabil. 2011, 92, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [PubMed]
- Ng, S.S.; Hui-Chan, C.W. The Timed Up & Go Test: Its Reliability and Association with Lower-Limb Impairments and Locomotor Capacities in People with Chronic Stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1641–1647. [Google Scholar] [CrossRef]
- Quinn, T.J.; Dawson, J.; Walters, M.R.; Lees, K.R. Functional Outcome Measures in Contemporary Stroke Trials. Int. J. Stroke 2009, 4, 200–205. [Google Scholar] [CrossRef]
- Csuka, M.; Mccarty, D.J. Simple method for measurement of lower extremity muscle strength. Am. J. Med. 1985, 78, 77–81. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Ouellette, M.M.; Lebrasseur, N.K.; Bean, J.F.; Phillips, E.; Stein, J.; Frontera, W.R.; Fielding, R.A. High-Intensity Resistance Training Improves Muscle Strength, Self-Reported Function, and Disability in Long-Term Stroke Survivors. Stroke 2004, 35, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Belgen, B.; Beninato, M.; Sullivan, P.E.; Narielwalla, K. The Association of Balance Capacity and Falls Self-Efficacy with History of Falling in Community-Dwelling People with Chronic Stroke. Arch. Phys. Med. Rehabil. 2006, 87, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Lebrasseur, N.K.; Sayers, S.P.; Ouellette, M.M.; Fielding, R.A. Muscle Impairments and Behavioral Factors Mediate Functional Limitations and Disability Following Stroke. Phys. Ther. 2006, 86, 1342–1350. [Google Scholar] [CrossRef]
- Saghaei, M.; Saghaei, S. Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials. J. Biomed. Sci. Eng. 2011, 04, 734–739. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823. [Google Scholar]
- Koller, M. robustlmm: An R Package for Robust Estimation of Linear Mixed-Effects Models. J. Stat. Softw. 2016, 75, 1–24. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Twisk, J.; Bosman, L.; Hoekstra, T.; Rijnhart, J.; Welten, M.; Heymans, M. Different ways to estimate treatment effects in randomised controlled trials. Contemp. Clin. Trials Commun. 2018, 10, 80–85. [Google Scholar]
- De Boer, M.R.; Waterlander, W.E.; Kuijper, L.D.J.; Steenhuis, I.H.M.; Twisk, J.W.R. Testing for baseline differences in randomized controlled trials: An unhealthy research behavior that is hard to eradicate. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 1–8. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Katz, N.P.; Paillard, F.C.; Ekman, E. Determining the clinical importance of treatment benefits for interventions for painful orthopedic conditions. J. Orthop. Surg. Res. 2015, 10, 24. [Google Scholar] [CrossRef]
- Page, S.J.; Fulk, G.D.; Boyne, P. Clinically Important Differences for the Upper-Extremity Fugl-Meyer Scale in People With Minimal to Moderate Impairment Due to Chronic Stroke. Phys. Ther. 2012, 92, 791–798. [Google Scholar] [CrossRef]
- Pandian, S.; Arya, K.N.; Kumar, D. Minimal clinically important difference of the lower-extremity fugl–meyer assessment in chronic-stroke. Top. Stroke Rehabil. 2016, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Veerbeek, J.M.; van Wegen, E.; van Peppen, R.; van der Wees, P.J.; Hendriks, E.; Rietberg, M.; Kwakkel, G. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PLoS ONE 2014, 9, e87987. [Google Scholar] [CrossRef]
- Van Peppen, R.P.; Kwakkel, G.; Wood-Dauphinee, S.; Hendriks, H.J.; Van der Wees, P.J.; Dekker, J. The impact of physical therapy on functional outcomes after stroke: What’s the evidence? Clin. Rehabil. 2004, 18, 833–862. [Google Scholar] [CrossRef]
- Khan, F.; Rathore, C.; Kate, M.; Joy, J.; Zachariah, G.; Vincent, P.C.; Varma, R.P.; Radhakrishnan, K. The comparative efficacy of theta burst stimulation or functional electrical stimulation when combined with physical therapy after stroke: A randomized controlled trial. Clin. Rehabil. 2019, 33, 693–703. [Google Scholar] [CrossRef]
- Pollock, A.; Baer, G.; Langhorne, P.; Pomeroy, V. Physiotherapy treatment approaches for the recovery of postural control and lower limb function following stroke: A systematic review. Clin. Rehabil. 2007, 21, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Hodges, B.R.; Cambron, J.A.; Klein, R.M.; Madigan, D.M. Prevalence of nonmusculoskeletal versus musculoskeletal cases in a chiropractic student clinic. J. Chiropr. Educ. 2013, 27, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Glucina, T.T.; Krägeloh, C.U.; Farvid, P.; Holt, K. Moving towards a contemporary chiropractic professional identity. Complement. Ther. Clin. Pr. 2020, 39, 101105. [Google Scholar] [CrossRef]
- Coulter, I.D.; Shekelle, P.G. Chiropractic in North America: A Descriptive Analysis. J. Manip. Physiol. Ther. 2005, 28, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Sorensen, L.P.; Graesborg, K.; Grunnet-Nilsson, N. Chiropractic patients in Denmark: A short description of basic characteristics. J. Manip. Physiol. Ther. 2002, 25, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Gaumer, G. Factors Associated With Patient Satisfaction With Chiropractic Care: Survey and Review of the Literature. J. Manip. Physiol. Ther. 2006, 29, 455–462. [Google Scholar] [CrossRef]
- French, S.D.; Densley, K.; Charity, M.J.; Gunn, J. Who uses Australian chiropractic services? Chiropr. Man. Ther. 2013, 21, 31. [Google Scholar] [CrossRef][Green Version]
- Cambron, J.A.; Cramer, G.D.; Winterstein, J. Patient Perceptions of Chiropractic Treatment for Primary Care Disorders. J. Manip. Physiol. Ther. 2007, 30, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.R.; Beck, R.W. Chiropractic patients presenting to the New Zealand College of Chiropractic teaching clinic: A short description of patients and patient complaints. Chiropr. J. Aust. 2005, 35, 122. [Google Scholar]
- Citrome, L. Quantifying Clinical Relevance. Innov. Clin. Neurosci. 2014, 11, 26–30. [Google Scholar] [PubMed]
- Pak, S.; Patten, C. Strengthening to Promote Functional Recovery Poststroke: An Evidence-Based Review. Top. Stroke Rehabil. 2008, 15, 177–199. [Google Scholar] [CrossRef]
- Sparks, C.L.; Liu, W.C.; Cleland, J.A.; Kelly, J.P.; Dyer, S.J.; Szetela, K.M.; Elliott, J.M. Functional Magnetic Resonance Imaging of Cerebral Hemodynamic Responses to Pain Following Thoracic Thrust Manipulation in Individuals With Neck Pain: A Randomized Trial. J. Manip. Physiol. Ther. 2017, 40, 625–634. [Google Scholar] [CrossRef]
- Dishman, J.D.; Greco, D.S.; Burke, J.R. Motor-Evoked Potentials Recorded from Lumbar Erector Spinae Muscles: A Study of Corticospinal Excitability Changes Associated with Spinal Manipulation. J. Manip. Physiol. Ther. 2008, 31, 258–270. [Google Scholar] [CrossRef]
- Dishman, J.; Ball, K.A.; Burke, J. First prize central motor excitability changes after spinal manipulation: A transcranial magnetic stimulation study. J. Manip. Physiol. Ther. 2002, 25, 1–9. [Google Scholar] [CrossRef]
- DeVocht, J.W.; Vining, R.; Smith, D.L.; Long, C.; Jones, T.; Goertz, C. Effect of chiropractic manipulative therapy on reaction time in special operations forces military personnel: A randomized controlled trial. Trials 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Daligadu, J.; Haavik, H.; Yielder, P.; Baarbe, J.; Murphy, B. Alterations in cortical and cerebellar motor processingt in sub-clinical neck pain patients following spinal manipulation. J. Manip. Physiol. Ther. 2013, 36, 10. [Google Scholar] [CrossRef]
- Haavik, H.; Murphy, B. Subclinical Neck Pain and the Effects of Cervical Manipulation on Elbow Joint Position Sense. J. Manip. Physiol. Ther. 2011, 34, 88–97. [Google Scholar] [CrossRef]
- Baarbé, J.K.; Yielder, P.; Haavik, H.; Holmes, M.W.R.; Murphy, B.A. Subclinical recurrent neck pain and its treatment impacts motor training-induced plasticity of the cerebellum and motor cortex. PLoS ONE 2018, 13, e0193413. [Google Scholar] [CrossRef]
- Holt, K.R.; Haavik, H.; Lee, A.C.L.; Murphy, B.; Elley, C.R. Effectiveness of Chiropractic Care to Improve Sensorimotor Function Associated With Falls Risk in Older People: A Randomized Controlled Trial. J. Manip. Physiol. Ther. 2016, 39, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Kollen, B.; Lindeman, E. Understanding the pattern of functional recovery after stroke: Facts and theories. Restor. Neurol. Neurosci. 2004, 22, 281–299. [Google Scholar] [PubMed]
- Hendricks, H.T.; van Limbeek, J.; Geurts, A.C.; Zwarts, M.J. Motor recovery after stroke: A systematic review of the literature. Arch. Phys. Med. Rehabil. 2002, 83, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Tsai, C.-C.; Chung, C.-Y.; Chen, C.-L.; Wu, K.P.; Chen, H.-C. Potential predictors for health-related quality of life in stroke patients undergoing inpatient rehabilitation. Health Qual. Life Outcomes 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Cinnera, A.M.; Bonnì, S.; Pellicciari, M.C.; Giorgi, F.; Caltagirone, C.; Koch, G. Health-related quality of life (HRQoL) after stroke: Positive relationship between lower extremity and balance recovery. Top. Stroke Rehabil. 2020, 27, 534–540. [Google Scholar] [CrossRef]
- Haas, M.; Bronfort, G.; Evans, R.; Schulz, C.; Vavrek, D.; Takaki, L.; Hanson, L.; Leininger, B.; Neradilek, M.B. Dose-response and efficacy of spinal manipulation for care of cervicogenic headache: A dual-center randomized controlled trial. Spine J. 2018, 18, 1741–1754. [Google Scholar] [CrossRef]
- Hays, R.D.; Spritzer, K.L.; Sherbourne, C.D.; Ryan, G.W.; Coulter, I.D. Group and Individual-level Change on Health-related Quality of Life in Chiropractic Patients with Chronic Low Back or Neck Pain. Spine 2019, 44, 647–651. [Google Scholar] [CrossRef]
- Russell, D.G.; Kimura, M.N.; Cowie, H.R.; De Groot, C.M.; McMINN, E.A.; Sherson, M.W. Changes in Quality of Life in 7 Older Adult Patients Receiving Activator Methods Chiropractic Technique. J. Chiropr. Med. 2016, 15, 59–66. [Google Scholar] [CrossRef]
- Feise, R.J. Do multiple outcome measures require p-value adjustment? BMC Med. Res. Methodol. 2002, 2, 8. [Google Scholar] [CrossRef]
- Chaibi, A.; Benth, J.Š.; Russell, M.B. Validation of Placebo in a Manual Therapy Randomized Controlled Trial. Sci. Rep. 2015, 5, 11774. [Google Scholar] [CrossRef] [PubMed]
| Variables | Chiro + PT | Sham + PT |
|---|---|---|
| Gender | ||
| Male (n) | 18 | 16 |
| Female (n) | 10 | 11 |
| Age, years (mean +/− SD) | 53.3 +/− 14.0 | 58.5 +/− 11.3 |
| Side of body affected by stroke | ||
| Left (n) | 14 | 12 |
| Right (n) | 14 | 15 |
| Time since stroke, months (mean +/− SD) | 30.0 +/− 36.6 | 27.3 +/− 31.5 |
| Subacute stage (12–24 weeks since stroke, n) | 5 | 4 |
| Chronic stage (>24 weeks since stroke, n) | 23 | 23 |
| Type of stroke | ||
| Ischemic (n) | 24 | 25 |
| Hemorrhagic (n) | 4 | 2 |
| Outcome | Time | Mean Difference ± SE [95% CI] | p-Value |
|---|---|---|---|
| Primary: | |||
| FMA | 4 weeks | 6.1 ± 2.9 [0.4, 11.9] | 0.04 * |
| 8 weeks | 4.5 ± 3.4 [−2.2, 11.2] | 0.2 | |
| Secondary: | |||
| FMA-UE | 4 weeks | 2.9 ± 2.5 [−2.0, 7.9] | 0.2 |
| 8 weeks | 3.0 ± 2.9 [−2.8, 8.8] | 0.3 | |
| FMA-LE | 4 weeks | 2.9 ± 1.2 [0.5, 5.3] | 0.02 * |
| 8 weeks | 1.9 ± 1.4 [−0.9, 4.6] | 0.2 | |
| SS-QOL | 4 weeks | −9.2 ± 10.4 [−30.0, 11.5] | 0.4 |
| 8 weeks | 16.7 ± 11.5 [−6.2, 39.6] | 0.2 | |
| TUG | 4 weeks | −1.2 ± 2.2 [−5.5, 3.2] | 0.6 |
| 8 weeks | 0.9 ± 2.7 [−4.4, 6.2] | 0.7 | |
| mRS | 4 weeks | 0.0 ± 0.3 [−0.5, 0.5] | 0.9 |
| 8 weeks | 0.1 ± 0.3 [−0.4, 0.6] | 0.7 | |
| STS | 4 weeks | −1.5 ± 4.2 [−9.6, 6.6] | 0.7 |
| 8 weeks | 1.0 ± 4.6 [−8.0, 9.9] | 0.8 |
| Outcome | Time | Chiro + PT Responder % [n Responders/n Total] | Sham + PT Responder % [n Responders/n Total] | p-Value |
|---|---|---|---|---|
| FMA UE, and/or LE Responder (MCID cut-off UE = 4.25, LE cut-off = 6) | 4 weeks | 100% [28/28] | 96% [26/27] | 0.3 |
| 8 weeks | 63% [12/19] | 37% [7/19] | 0.1 | |
| FMA UE, and/or LE Responder (MCID cut-off UE = 7.25, LE cut-off = 6) | 4 weeks | 96% [27/28] | 78% [21/27] | 0.04 * |
| 8 weeks | 84% [16/19] | 79% [15/19] | 0.7 |
| Outcome | Time | Baseline Mean | Mean ± SE, p-Value | |
|---|---|---|---|---|
| Chiropractic + PT | Sham + PT | |||
| Primary: | ||||
| FMA | At 4 weeks | 40.9 | 64.1 ± 2.0, <0.001 * | 58.0 ± 2.1, <0.001 * |
| At 8 weeks | 64.3 ± 2.4, <0.001 * | 59.8 ± 2.4, <0.001 * | ||
| Secondary: | ||||
| FMA-UE | At 4 weeks | 24.1 | 38.9 ± 1.7, <0.001 * | 36.0 ± 1.8, <0.001 * |
| At 8 weeks | 40.3 ± 2.1, <0.001 * | 37.3 ± 2.1, <0.001 * | ||
| FMA-LE | At 4 weeks | 16.8 | 25.0 ± 0.8, <0.001 * | 22.1 ± 0.9, <0.001 * |
| At 8 weeks | 24.2 ± 1.0, <0.001 * | 22.4 ± 1.0, <0.001 * | ||
| SS-QOL | At 4 weeks | 122.1 | 162.5 ± 7.2, <0.001 * | 171.7 ± 7.4, <0.001 * |
| At 8 weeks | 190.6 ± 8.1, <0.001 * | 174.0 ± 8.1, <0.001 * | ||
| TUG | At 4 weeks | 23.7 | 20.5 ± 1.6, 0.047 * | 21.7 ± 1.5, 0.2 |
| At 8 weeks | 21.3 ± 1.8, 0.2 | 20.4 ± 2.0, 0.1 | ||
| mRS | At 4 weeks | 2.8 | 2.4 ± 0.2, 0.01 * | 2.4 ± 0.2, 0.02 * |
| At 8 weeks | 2.2 ± 0.2, <0.001 * | 2.1 ± 0.2, <0.001 * | ||
| 5SST | Baseline | MB: 21.3 ± 2.1 | MB: 22.7 ± 2.0 | |
| At 4 weeks | 17.9 ± 2.1, 0.3 | 20.8 ± 2.0, 0.5 | ||
| At 8 weeks | 17.4 ± 2.3, 0.2 | 17.8 ± 2.6, 0.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holt, K.; Niazi, I.K.; Amjad, I.; Kumari, N.; Rashid, U.; Duehr, J.; Navid, M.S.; Shafique, M.; Haavik, H. The Effects of 4 Weeks of Chiropractic Spinal Adjustments on Motor Function in People with Stroke: A Randomized Controlled Trial. Brain Sci. 2021, 11, 676. https://doi.org/10.3390/brainsci11060676
Holt K, Niazi IK, Amjad I, Kumari N, Rashid U, Duehr J, Navid MS, Shafique M, Haavik H. The Effects of 4 Weeks of Chiropractic Spinal Adjustments on Motor Function in People with Stroke: A Randomized Controlled Trial. Brain Sciences. 2021; 11(6):676. https://doi.org/10.3390/brainsci11060676
Chicago/Turabian StyleHolt, Kelly, Imran Khan Niazi, Imran Amjad, Nitika Kumari, Usman Rashid, Jens Duehr, Muhammad Samran Navid, Muhammad Shafique, and Heidi Haavik. 2021. "The Effects of 4 Weeks of Chiropractic Spinal Adjustments on Motor Function in People with Stroke: A Randomized Controlled Trial" Brain Sciences 11, no. 6: 676. https://doi.org/10.3390/brainsci11060676
APA StyleHolt, K., Niazi, I. K., Amjad, I., Kumari, N., Rashid, U., Duehr, J., Navid, M. S., Shafique, M., & Haavik, H. (2021). The Effects of 4 Weeks of Chiropractic Spinal Adjustments on Motor Function in People with Stroke: A Randomized Controlled Trial. Brain Sciences, 11(6), 676. https://doi.org/10.3390/brainsci11060676











