2′-3′-Cyclic Nucleotide 3′-Phosphodiesterase Inhibition by Organometallic Vanadium Complexes: A Potential New Paradigm for Studying CNS Degeneration
Abstract
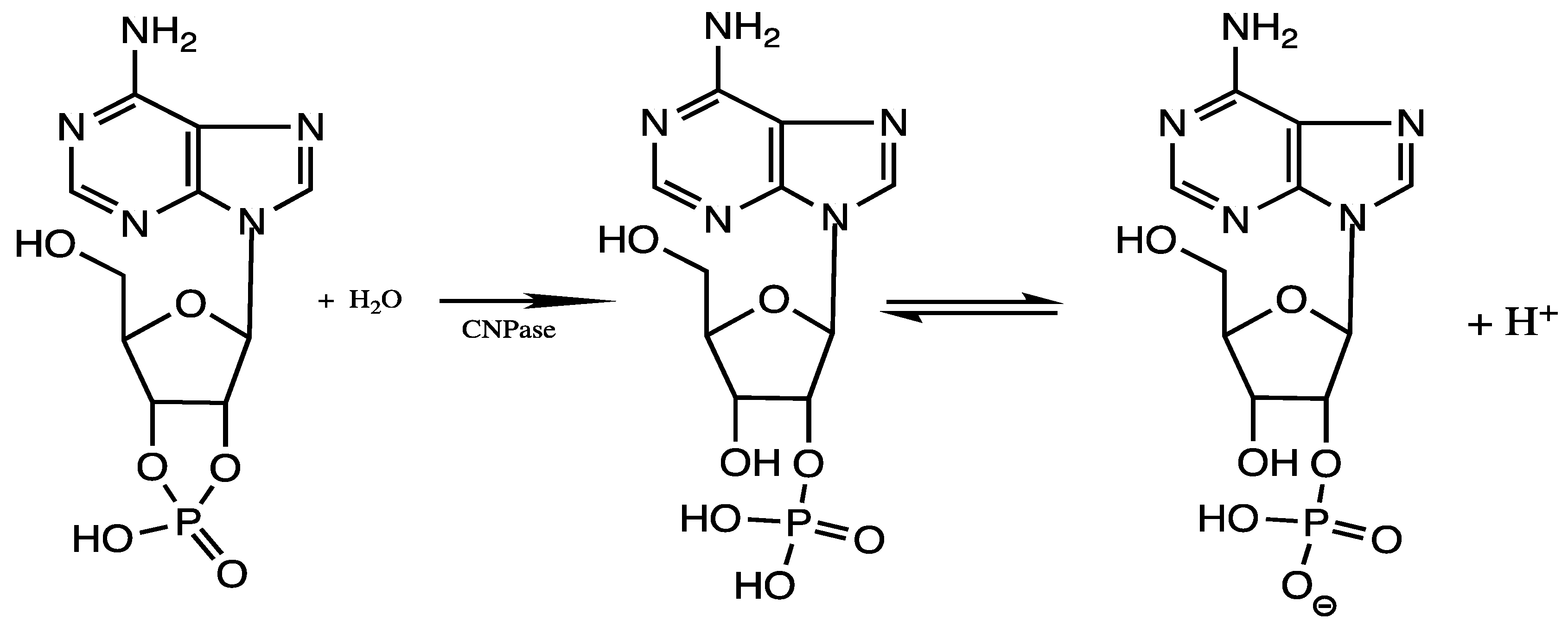
1. Introduction
2. Materials and Methods
3. Results and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raasakka, A.; Kursula, P. The myelin membrane-associated enzyme 2′,3′-cyclic nucleotide 3′-phosphodiesterase: On a highway to structure and function. Neurosci. Bull. 2014, 30, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Tsukada, Y. 2′,3′-Cyclic nucleotide 3′-phosphohydrolase in the brain of the “Jimpy” mouse, a mutant with deficient myelination. J. Neurochem. 1967, 14, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.R.; Carnegie, P.R. Advances in Neurochemistry; Agarnoff, B.W., Aprison, M.H., Eds.; Plenum Press: New York, NY, USA, 1978; Volume 3, pp. 1–41. [Google Scholar]
- Baburina, Y.L.; Krestinina, O.V.; Azarashvili, T.S. 2′,3′-cyclic nucleotide phosphodiesterase (CNPase) as a target in neurodegenerative diseases. Neurochem. J. 2013, 7, 1–15. [Google Scholar] [CrossRef]
- Sudo, R.; Kikuno, M.; Kurihara, R. 2’,3’-cyclic nucleotide 3’-phosphohydrolase in human erythrocyte membranes. Biochim. Biophys. Acta 1972, 255, 640–646. [Google Scholar] [CrossRef]
- Baburina, Y.; Azarashvivi, R.; Grachev, D.; Krestinian, O.; Galvita, A.; Stricker, R.; Reiser, G. Mitochondrial 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP) interacts with mPTP modulators and functional complexes (I–V) coupled with release of apoptotic factors. Neurochem. Int. 2015, 90, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.K.; Gillespie, D.G.; Mi, J.; Cheng, D.; Bansal, R.; Janesko-Feldman, K.; Kochanek, P.M. Role of 2’,3’-cyclic nucleotide phosphodiesterase in the renal 2’,3’-cAMP-adenosine pathway. Am. J. Physiol. Ren. Physiol. 2014, 307, F14–F24. [Google Scholar] [CrossRef] [PubMed]
- Muraro, P.A.; Kalbus, M.; Afshar, G.; McFarland, H.F.; Martin, R. T cell response to 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) in multiple sclerosis patients. J. Neuroimmunol. 2002, 130, 233–242. [Google Scholar] [CrossRef]
- Jones, R.L.; Wong, E.K.; Ibsen, K.H.; Leopold, I.H. Erythrocyte membrane 2’,3’-cyclic nucleotide 3’-phosphodiesterase activity in multiple sclerosis. Metab. PediatricSyst. Ophthalmol. 1983, 7, 25–30. [Google Scholar]
- Yang, L.; Kan, E.M.; Lu, J.; Wu, C.; Ling, E.-A. Expression of 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) and its roles in activated microglia in vivo and in vitro. J. Neuroinflamm. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.L.; Nguyen, V.H.; McLauchlan, C.C.; Dymon, A.; Dorsey, B.M.; Hooker, J.D.; Jones, M.A. Inhibitory effects of decavanadate on several enzymes and Leishmania tarentolae in vitro. J. Inorg. Biochem. 2012, 108, 96–104. [Google Scholar] [CrossRef] [PubMed]
- McLauchlan, E.E.; Peters, B.J.; Willsky, G.R.; Crans, D.C. Vanadium-phosphatase complexes: Phosphatase inhibitors favor the trigonal bipyramidal transition state geometries. Coord. Chem. Rev. 2015, 301–302, 163–199. [Google Scholar] [CrossRef]
- Mustapha, O.; Oke, B.; Offen, N.; Sirén, A.L.; Olopade, J. Neurobehavioral and cytotoxic effects of vanadium during oligodendrocyte maturation: A protective role for erythropoietin. Environ. Toxicol. Pharmacol. 2014, 38, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Norton, W.T.; Poduslo, S.E. Myelination in rat brain: Methods of myelin isolation. J. Neurochem. 1973, 21, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Lees, M.B.; Saundler, S.W.; Eichberg, J. Effects of detergents on 2’,3’-cyclic nucleotide-3’ phosphohydrolase. Neurobiology 1974, 4, 407–413. [Google Scholar] [PubMed]
- Jones, M.; Keenan, R.W. Specific localization of 2’,3’-cyclic nucleotide 3’ phosphohydrolase, (Ca2+, Mg2+)-ATPase, and Acetylcholinesterase in human erythrocyte membrane. Biochim. Biophys. Acta 1981, 678, 403–407. [Google Scholar] [CrossRef]
- McLauchlan, C.C.; Hooker, J.D.; Jones, M.A.; Dymon, Z.; Backhus, E.A.; Greiner, B.A.; Dorner, N.A.; Youkhana, M.A.; Manus, L.M. Inhibition of acid, alkaline, and tyrosine (PTP1B) phosphatases by novel vanadium complexes. J. Inorg. Biochem. 2010, 104, 274–281. [Google Scholar] [CrossRef] [PubMed]
- McLauchlan, C.C.; Wu, X.; Wallace, C.A.; Penn, A.S.; Tarlton, M.L.; Platt, D.C.; Rink, J.; Braich, K.; Jones, M.A. Vanadium (IV) complexes with the Kläui ligand for oxidative catalysis and enzyme inhibition. In Proceedings of the Poster Presentation, 11th International Vanadium Symposium, Montevideo, Uruguay, 5–8 November 2018. [Google Scholar]
- Saatchi, K.; Thompson, K.H.; Patrick, B.O.; Pink, M.; Yuen, V.G.; McNeill, J.H.; Orvig, C. Coordination Chemistry and Insulin-Enhancing Behavior of Vanadium Complexes with Maltol C6H6O3 Structural Isomers. Inorg. Chem. 2005, 44, 2689–2697. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Gelmini, L.; Glover, N.; Herring, F.G.; Li, H.; McNeill, J.H.; Rettig, S.J.; Setyawati, I.A.; Shuter, E.; Sun, Y.; et al. Reaction Chemistry of BMOV, Bis(maltolato)oxovanadium(IV)-A Potent Insulin Mimetic Agent. J. Am. Chem. Soc. 1995, 117, 12759–12770. [Google Scholar]

| Vanadium Complex Tested | % Activity Remaining | IC50 (µM) | Synthesis Reference | |
|---|---|---|---|---|
| No complex control | 100% | -- | -- | |
| PO4OEt | 1 | 87% | -- | 19 |
| PO4OME | 2 | 48% | -- | 19 |
| BEOV, VO (ema)2 | 3 | 73% | -- | 20 |
| BMOV, VO (ma)2 | 4 | 82% | -- | 21 |
| V (pic)3 | 5 | 9% | 20 | 18 |
| VO (pic)2 | 6 | 57% | -- | 18 |
| NH4VO2(pic)2 | 7 | 14% | 40 | 18 |
| V (imc)3 | 8 | 30% | -- | 18 |
| VO (imc)2 | 9 | 39% | -- | 18 |
| NH4VO2 (imc)2 | 10 | 52% | -- | 18 |
| V (anc)3 | 11 | 6% | 30 | 18 |
| VO (anc)2 | 12 | 65% | -- | 18 |
| NH4VO2 (anc)2 | 13 | 82% | -- | 18 |
| Na3VO4 | 14 | 77% | -- | -- |
| (NH4)6V10O28 | 15 | 36% | -- | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platt, D.C.; Rink, J.; Braich, K.; McLauchlan, C.C.; Jones, M.A. 2′-3′-Cyclic Nucleotide 3′-Phosphodiesterase Inhibition by Organometallic Vanadium Complexes: A Potential New Paradigm for Studying CNS Degeneration. Brain Sci. 2021, 11, 588. https://doi.org/10.3390/brainsci11050588
Platt DC, Rink J, Braich K, McLauchlan CC, Jones MA. 2′-3′-Cyclic Nucleotide 3′-Phosphodiesterase Inhibition by Organometallic Vanadium Complexes: A Potential New Paradigm for Studying CNS Degeneration. Brain Sciences. 2021; 11(5):588. https://doi.org/10.3390/brainsci11050588
Chicago/Turabian StylePlatt, David C., Jonathan Rink, Kamaljit Braich, Craig C. McLauchlan, and Marjorie A. Jones. 2021. "2′-3′-Cyclic Nucleotide 3′-Phosphodiesterase Inhibition by Organometallic Vanadium Complexes: A Potential New Paradigm for Studying CNS Degeneration" Brain Sciences 11, no. 5: 588. https://doi.org/10.3390/brainsci11050588
APA StylePlatt, D. C., Rink, J., Braich, K., McLauchlan, C. C., & Jones, M. A. (2021). 2′-3′-Cyclic Nucleotide 3′-Phosphodiesterase Inhibition by Organometallic Vanadium Complexes: A Potential New Paradigm for Studying CNS Degeneration. Brain Sciences, 11(5), 588. https://doi.org/10.3390/brainsci11050588







