Post-Training Sleep Modulates Topographical Relearning-Dependent Resting State Activity
Abstract
:1. Introduction
2. Methods
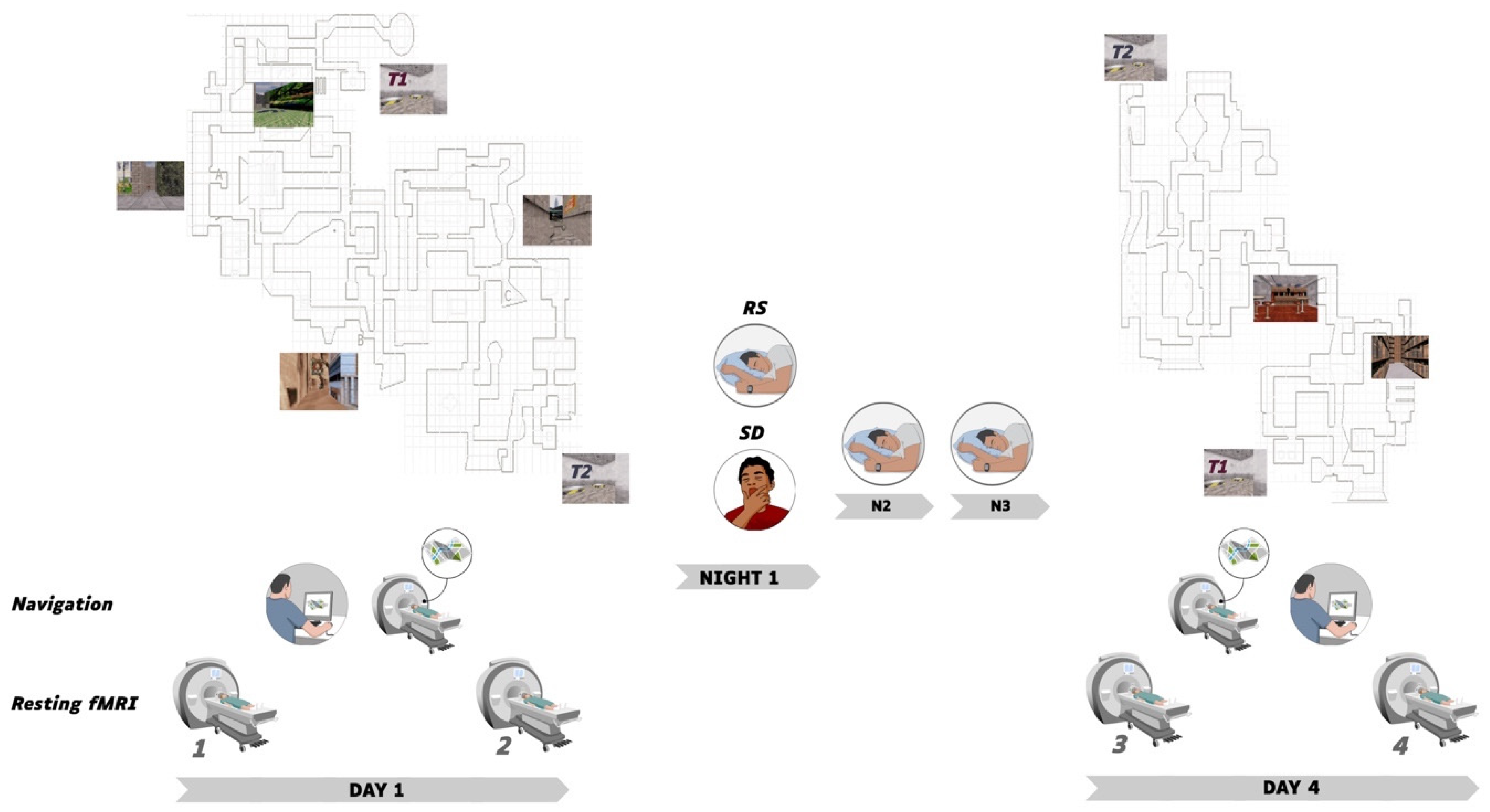
2.1. Participants and Procedure
2.2. Learning Material and Conditions
2.2.1. Behavioural Sessions
2.2.2. Task-Based fMRI
2.2.3. Resting State RS-fMRI
2.3. Brain Imaging Data Acquisition
2.4. Task-Based fMRI Data Analysis
2.5. Resting State Data Analysis
2.5.1. Preprocessing
2.5.2. ALFF Analysis
2.5.3. Seed-Based Functional Connectivity Analysis
3. Results
3.1. Behavioural Results
3.1.1. Sleep Data
3.1.2. Navigation Performance
3.2. Task-Based fMRI
3.2.1. Navigation-Related Brain Activity
3.3. Resting State ALFF
3.3.1. Spatial Learning-Related Changes in Spontaneous Activity at Rest
3.3.2. Post-Training Sleep-Dependent Learning-Related Changes in Spontaneous Activity at Rest
3.4. Resting State FC
3.4.1. Spatial Learning-Related Changes in Seed-Based FC
3.4.2. Sleep-Dependent Changes in Seed-Based Resting State FC
3.4.3. Post-Training Sleep-Dependent Relearning-Related Changes in Seed-Based Resting State FC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vorster, A.P.; Born, J. Sleep and memory in mammals, birds and invertebrates. Neurosci. Biobehav. Rev. 2015, 50, 103–119. [Google Scholar] [CrossRef] [Green Version]
- Rasch, B.; Born, J. About sleep’s role in memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef]
- Peigneux, P. Neuroimaging studies of sleep and memory in humans. Curr. Top. Behav. Neurosci. 2015, 25, 239–268. [Google Scholar] [CrossRef] [PubMed]
- Peigneux, P.; Laureys, S.; Fuchs, S.; Collette, F.; Perrin, F.; Reggers, J.; Phillips, C.; Degueldre, C.; Del Fiore, G.; Aerts, J.; et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 2004, 44, 535–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orban, P.; Rauchs, G.; Balteau, E.; Degueldre, C.; Luxen, A.; Maquet, P.; Peigneux, P. Sleep after spatial learning promotes covert reorganization of brain activity. Proc. Natl. Acad. Sci. USA 2006, 103, 7124–7129. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.D.; Tucker, M.A.; Stickgold, R.; Wamsley, E.J. Overnight Sleep Enhances Hippocampus-Dependent Aspects of Spatial Memory. Sleep 2013, 36, 1051–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, N.; Maguire, E.; O’Keefe, J. The human hippocampus and spatial and episodic memory. Neuron 2002, 35, 625. [Google Scholar] [CrossRef] [Green Version]
- Boccia, M.; Nemmi, F.; Guariglia, C. Neuropsychology of environmental navigation in humans: Review and meta-analysis of FMRI studies in healthy participants. Neuropsychol. Rev. 2014, 24, 236–251. [Google Scholar] [CrossRef] [Green Version]
- Epstein, R.A.; Patai, E.Z.; Julian, J.B.; Spiers, H.J. The cognitive map in humans: Spatial navigation and beyond. Nat. Neurosci. 2017, 20, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Voss, P.; Fortin, M.; Corbo, V.; Pruessner, J.C.; Lepore, F. Assessment of the caudate nucleus and its relation to route learning in both congenital and late blind individuals. BMC Neurosci. 2013, 14, 113. [Google Scholar] [CrossRef] [Green Version]
- Hartley, T.; Maguire, E.; Spiers, H.J.; Burgess, N. The well-worn route and the path less traveled: Distinct neural bases of route following and wayfinding in humans. Neuron 2003, 37, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, T.K.; Gruenbaum, B.F.; Markus, E.J. Extensive training and hippocampus or striatum lesions: Effect on place and response strategies. Physiol. Behav. 2012, 105, 645–652. [Google Scholar] [CrossRef]
- Packard, M.G.; Knowlton, B.J. Learning and memory functions of the Basal Ganglia. Annu. Rev. Neurosci. 2002, 25, 563–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, M.B.; Rowland, D.C.; Moser, E.I. Place cells, grid cells, and memory. Cold Spring Harb. Perspect. Biol. 2015, 7, a021808. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.A.; Mcnaughton, B.L. Reactivation of hippocampal ensemble memories during sleep. Science 1994, 265, 676–679. [Google Scholar] [CrossRef] [Green Version]
- Nadasdy, Z.; Hirase, H.; Czurko, A.; Csicsvari, J.; Buzsaki, G. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 1999, 19, 9497–9507. [Google Scholar] [CrossRef]
- Grosmark, A.D.; Mizuseki, K.; Pastalkova, E.; Diba, K.; Buzsaki, G. REM sleep reorganizes hippocampal excitability. Neuron 2012, 75, 1001–1007. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, S.; Sirota, A.; Buzsaki, G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J. Neurosci. 2008, 28, 6731–6741. [Google Scholar] [CrossRef] [Green Version]
- Maquet, P.; Laureys, S.; Peigneux, P.; Fuchs, S.; Petiau, C.; Phillips, C.; Aerts, J.; Del Fiore, G.; Degueldre, C.; Meulemans, T.; et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat. Neurosci. 2000, 3, 831–836. [Google Scholar] [CrossRef]
- Peigneux, P.; Laureys, S.; Fuchs, S.; Destrebecqz, A.; Collette, F.; Delbeuck, X.; Phillips, C.; Aerts, J.; Del Fiore, G.; Degueldre, C.; et al. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage 2003, 20, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Huber, R.; Ghilardi, M.F.; Massimini, M.; Tononi, G. Local sleep and learning. Nature 2004, 430, 78–81. [Google Scholar] [CrossRef]
- Rasch, B.; Buchel, C.; Gais, S.; Born, J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 2007, 315, 1426–1429. [Google Scholar] [CrossRef] [Green Version]
- Rauchs, G.; Orban, P.; Schmidt, C.; Albouy, G.; Balteau, E.; Degueldre, C.; Schnackers, C.; Sterpenich, V.; Tinguely, G.; Luxen, A.; et al. Sleep modulates the neural substrates of both spatial and contextual memory consolidation. PLoS ONE 2008, 3, e2949. [Google Scholar] [CrossRef] [Green Version]
- Urbain, C.; De Tiege, X.; Op De Beeck, M.; Bourguignon, M.; Wens, V.; Verheulpen, D.; Van Bogaert, P.; Peigneux, P. Sleep in children triggers rapid reorganization of memory-related brain processes. Neuroimage 2016, 134, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Maquet, P.; Peigneux, P.; Laureys, S.; Boly, M.; Dang-Vu, T.; Desseilles, M.; Cleeremans, A. Memory processing during human sleep as assessed by functional neuroimaging. Rev. Neurol. 2003, 159, S27–S29. [Google Scholar]
- Fischer, S.; Nitschke, M.F.; Melchert, U.H.; Erdmann, C.; Born, J. Motor Memory Consolidation in Sleep Shapes More Effective Neuronal Representations. J. Neurosci. 2005, 25, 11248–11255. [Google Scholar] [CrossRef] [Green Version]
- Debas, K.; Carrier, J.; Barakat, M.; Marrelec, G.; Bellec, P.; Abdallah, H.T.; Karni, A.; Ungerleider, L.G.; Benali, H.; Doyon, J. Off-line consolidation of motor sequence learning results in greater integration within a cortico-striatal functional network. Neuroimage 2014, 99, 50–58. [Google Scholar] [CrossRef]
- Urbain, C.; Galer, S.; Van Bogaert, P.; Peigneux, P. Pathophysiology of sleep-dependent memory consolidation processes in children. Int. J. Psychophysiol. 2013, 89, 273–283. [Google Scholar] [CrossRef]
- Peigneux, P.; Orban, P.; Balteau, E.; Degueldre, C.; Luxen, A.; Laureys, S.; Maquet, P. Offline persistence of memory-related cerebral activity during active wakefulness. PLoS Biol. 2006, 4, e100. [Google Scholar] [CrossRef]
- Kelly, C.; Castellanos, F.X. Strengthening connections: Functional connectivity and brain plasticity. Neuropsychol. Rev. 2014, 24, 63–76. [Google Scholar] [CrossRef]
- Woolley, D.G.; Mantini, D.; Coxon, J.P.; D’Hooge, R.; Swinnen, S.P.; Wenderoth, N. Virtual water maze learning in human increases functional connectivity between posterior hippocampus and dorsal caudate. Hum. Brain Mapp. 2015, 36, 1265–1277. [Google Scholar] [CrossRef] [Green Version]
- Keller, T.A.; Just, M.A. Structural and functional neuroplasticity in human learning of spatial routes. Neuroimage 2015, 125, 256–266. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Horne, J.; Ostberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Ellis, B.W.; Johns, M.W.; Lancaster, R.; Raptopoulos, P.; Angelopoulos, N.; Priest, R.G. The St. Mary’s Hospital sleep questionnaire: A study of reliability. Sleep 1981, 4, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Rauchs, G.; Orban, P.; Balteau, E.; Schmidt, C.; Degueldre, C.; Luxen, A.; Maquet, P.; Peigneux, P. Partially segregated neural networks for spatial and contextual memory in virtual navigation. Hippocampus 2007, 18, 503–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutton, C.; Bork, A.; Josephs, O.; Deichmann, R.; Ashburner, J.; Turner, R. Image distortion correction in fMRI: A quantitative evaluation. Neuroimage 2002, 16, 217–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friston, K.J.; Stephan, K.E.; Lund, T.E.; Morcom, A.; Kiebel, S. Mixed-effects and fMRI studies. Neuroimage 2005, 24, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.I.; Whiteman, A.S.; Aselcioglu, I.; Stern, C.E. Structural differences in hippocampal and prefrontal gray matter volume support flexible context-dependent navigation ability. J. Neurosci. 2014, 34, 2314–2320. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Chao-Gan, Y.; Yu-Feng, Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friston, K.J.; Williams, S.; Howard, R.; Frackowiak, R.S.; Turner, R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996, 35, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.; Hirshhorn, M.; Rosenbaum, R.S.; Winocur, G.; Moscovitch, M.; Grady, C.L. Functional connectivity of hippocampal and prefrontal networks during episodic and spatial memory based on real-world environments. Hippocampus 2015, 25, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Reagh, Z.M.; Yassa, M.A. Repetition strengthens target recognition but impairs similar lure discrimination: Evidence for trace competition. Learn. Mem. 2014, 21, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007, 37, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Deliens, G.; Peigneux, P. One night of sleep is insufficient to achieve sleep-to-forget emotional decontextualisation processes. Cogn. Emot. 2014, 28, 698–706. [Google Scholar] [CrossRef]
- Takashima, A.; Petersson, K.M.; Rutters, F.; Tendolkar, I.; Jensen, O.; Zwarts, M.J.; McNaughton, B.L.; Fernandez, G. Declarative memory consolidation in humans: A prospective functional magnetic resonance imaging study. Proc. Natl. Acad. Sci. USA 2006, 103, 756–761. [Google Scholar] [CrossRef] [Green Version]
- Haier, R.J.; Karama, S.; Leyba, L.; Jung, R.E. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res. Notes 2009, 2, 174. [Google Scholar] [CrossRef] [Green Version]
- Rajah, M.N.; Languay, R.; Grady, C.L. Age-related changes in right middle frontal gyrus volume correlate with altered episodic retrieval activity. J. Neurosci. 2011, 31, 17941–17954. [Google Scholar] [CrossRef] [Green Version]
- Guterstam, A.; Bjornsdotter, M.; Gentile, G.; Ehrsson, H.H. Posterior cingulate cortex integrates the senses of self-location and body ownership. Curr. Biol. 2015, 25, 1416–1425. [Google Scholar] [CrossRef] [Green Version]
- Nau, M.; Navarro Schroder, T.; Bellmund, J.L.S.; Doeller, C.F. Hexadirectional coding of visual space in human entorhinal cortex. Nat. Neurosci. 2018, 21, 188–190. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, T.; Matsuda, H.; Hirakata, M.; Ugawa, Y. Navigation ability dependent neural activation in the human brain: An fMRI study. Neurosci. Res. 2006, 55, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Olafsson, V.; Plank, M.; Snider, J.; Halgren, E.; Poizner, H.; Liu, T.T. Resting-state fMRI activity predicts unsupervised learning and memory in an immersive virtual reality environment. PLoS ONE 2014, 9, e109622. [Google Scholar] [CrossRef] [Green Version]
- Boccia, M.; Sulpizio, V.; Nemmi, F.; Guariglia, C.; Galati, G. Direct and indirect parieto-medial temporal pathways for spatial navigation in humans: Evidence from resting-state functional connectivity. Brain Struct. Funct. 2017, 222, 1945–1957. [Google Scholar] [CrossRef]
- Hirshhorn, M.; Grady, C.; Rosenbaum, R.; Winocur, G.; Moscovitch, M. The hippocampus is involved in mental navigation for a recently learned, but not a highly familiar environment: A longitudinal fMRI study. Hippocampus 2012, 22, 842–852. [Google Scholar] [CrossRef]
- Long, X.; Zhang, S.J. A novel somatosensory spatial navigation system outside the hippocampal formation. Cell Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Matsumoto, J.; Tran, A.H.; Ono, T.; Nishijo, H. sLORETA current source density analysis of evoked potentials for spatial updating in a virtual navigation task. Front. Behav. Neurosci. 2014, 8, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, A.J.; Powell, A.L.; Holmes, J.D.; Vann, S.D.; Aggleton, J.P. What does spatial alternation tell us about retrosplenial cortex function? Front. Behav. Neurosci. 2015, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Powell, A.L.; Nelson, A.J.D.; Hindley, E.; Davies, M.; Aggleton, J.P.; Vann, S.D. The rat retrosplenial cortex as a link for frontal functions: A lesion analysis. Behav. Brain Res. 2017, 335, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.L.; Roach, B.J.; Ford, J.M.; Turner, J.A.; van Erp, T.G.; Voyvodic, J.; Preda, A.; Belger, A.; Bustillo, J.; O’Leary, D.; et al. Relating Intrinsic Low-Frequency BOLD Cortical Oscillations to Cognition in Schizophrenia. Neuropsychopharmacology 2015, 40, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Li, R.; Zheng, Z.; Li, J. Neural Correlates of Associative Memory in the Elderly: A Resting-State Functional MRI Study. Biomed. Res. Int. 2015, 2015, 129180. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [Green Version]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef] [PubMed]
- Epstein, R.; Kanwisher, N. A cortical representation of the local visual environment. Nature 1998, 392, 598–601. [Google Scholar] [CrossRef]
- Hagewoud, R.; Whitcomb, S.; Heeringa, A.; Havekes, R.; Koolhaas, J.; Meerlo, P. A time for learning and a time for sleep: The effect of sleep deprivation on contextual fear conditioning at different times of the day. Sleep 2010, 33, 1315–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
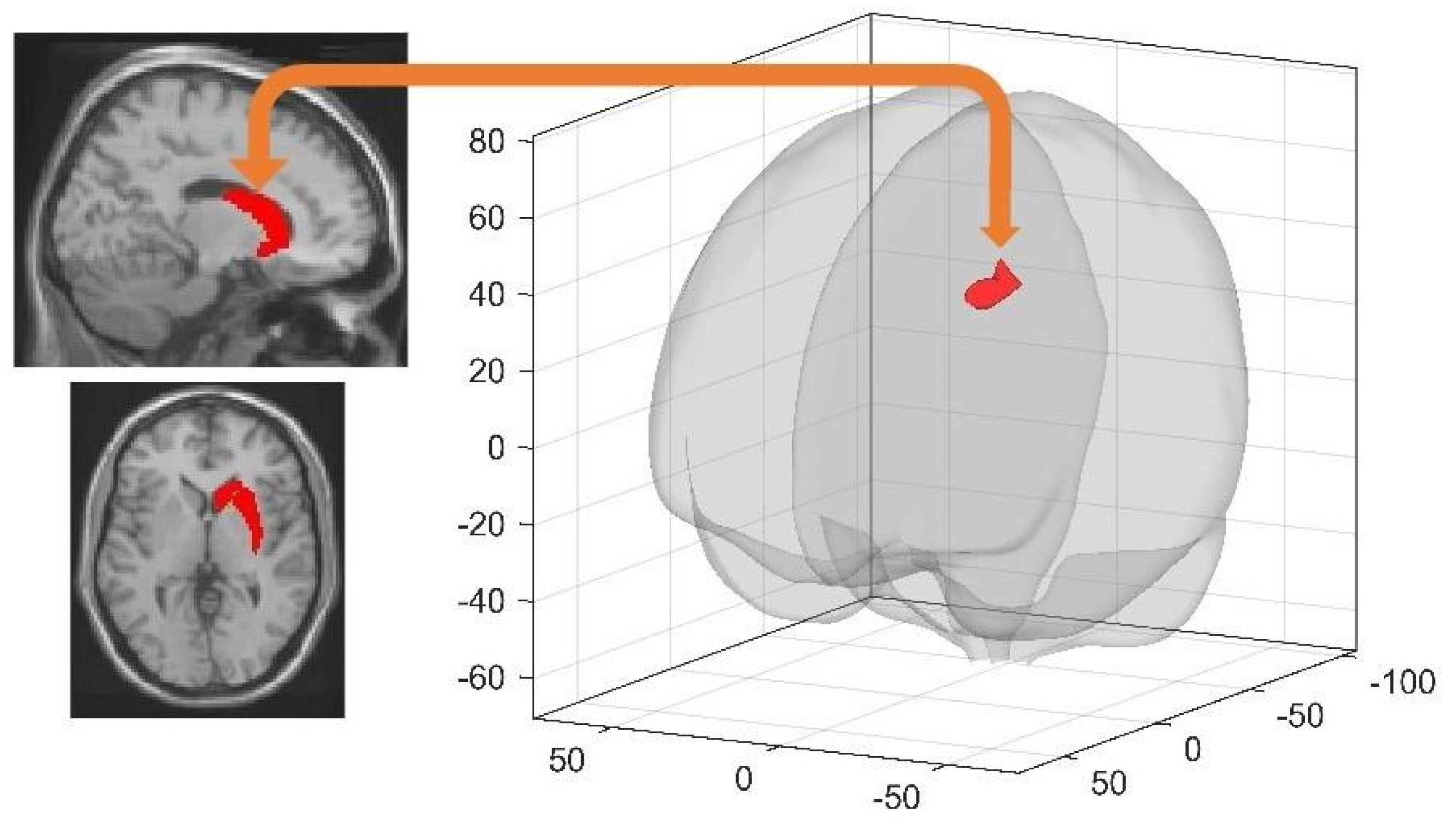
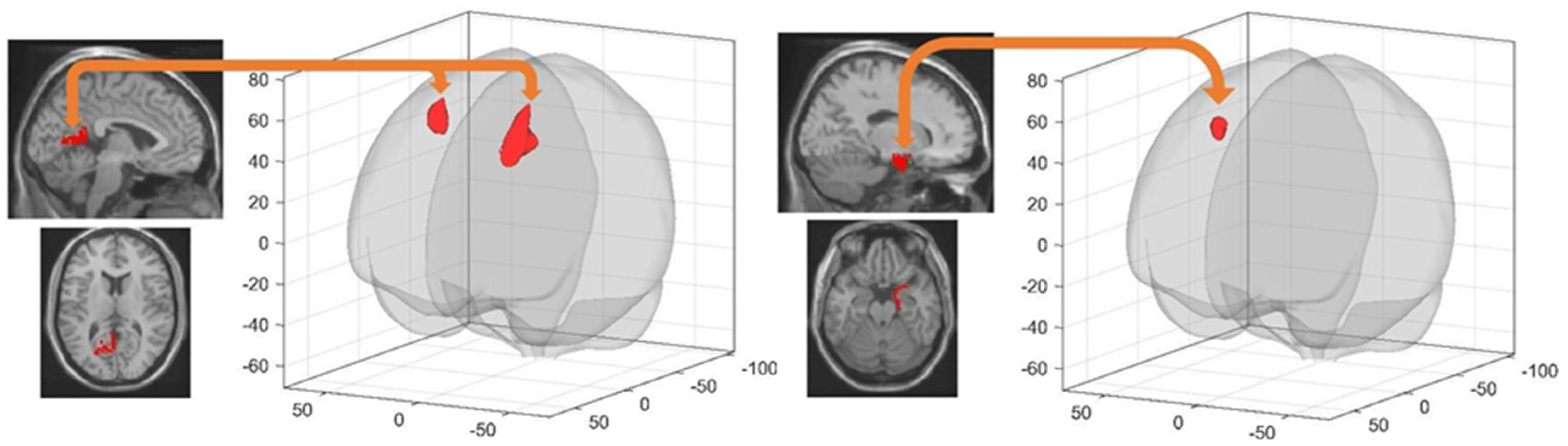
| Seed Area | Target Brain Region(s) | x y z (mm) | K | T | Cluster pFWE (Threshold < 0.0029) |
|---|---|---|---|---|---|
| Left Parahippocampal Gyrus * | Right Precuneus | 6 −60 44 | 330 | 5.09 | 0.000211 |
| Right Precuneus * | Right Middle Frontal Gyrus | 30 4 38 | 324 | 4.26 | 0.000343 |
| Cingulum_Post_L * | Right Supramarginal Gyrus | 58 −34 48 | 232 | 4.57 | 0.000377 |
| Left Hippocampus | Right Middle Frontal Gyrus | 32 4 56 | 247 | 4.95 | 0.002300 |
| Right Entorhinal Cortex | Right Inferiotemporal Gyrus | 52 −18 −24 | 351 | 5.46 | 0.000001 |
| Left Middle Temporal Gyrus | −58 −16 −24 | 401 | 5.02 | 0.000066 | |
| Right Post Cingulate Gyrus | 10 −42 28 | 605 | 4.18 | 0.000197 | |
| Left Entorhinal Cortex | Left lobule VI of cerebellar hemisphere | −30 −40 −34 | 310 | 4.88 | 0.000497 |
| Seed Area | Target Brain Region(s) | x y z (mm) | k | T | Cluster p-FWE |
|---|---|---|---|---|---|
| Left Retrosplenial Cortex | Left Superior Frontal Gyrus | −16 12 48 | 738 | 5.91 | 0.000000 |
| Right Middle Frontal Gyrus | 28 6 56 | 390 | 5.78 | 0.000047 | |
| Right Entorhinal Cortex | Right Superior Frontal Gyrus | 18 24 56 | 228 | 5.25 | 0.001829 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deantoni, M.; Villemonteix, T.; Balteau, E.; Schmidt, C.; Peigneux, P. Post-Training Sleep Modulates Topographical Relearning-Dependent Resting State Activity. Brain Sci. 2021, 11, 476. https://doi.org/10.3390/brainsci11040476
Deantoni M, Villemonteix T, Balteau E, Schmidt C, Peigneux P. Post-Training Sleep Modulates Topographical Relearning-Dependent Resting State Activity. Brain Sciences. 2021; 11(4):476. https://doi.org/10.3390/brainsci11040476
Chicago/Turabian StyleDeantoni, Michele, Thomas Villemonteix, Evelyne Balteau, Christina Schmidt, and Philippe Peigneux. 2021. "Post-Training Sleep Modulates Topographical Relearning-Dependent Resting State Activity" Brain Sciences 11, no. 4: 476. https://doi.org/10.3390/brainsci11040476
APA StyleDeantoni, M., Villemonteix, T., Balteau, E., Schmidt, C., & Peigneux, P. (2021). Post-Training Sleep Modulates Topographical Relearning-Dependent Resting State Activity. Brain Sciences, 11(4), 476. https://doi.org/10.3390/brainsci11040476








