Transcutaneous Spinal Cord Stimulation Enhances Walking Performance and Reduces Spasticity in Individuals with Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
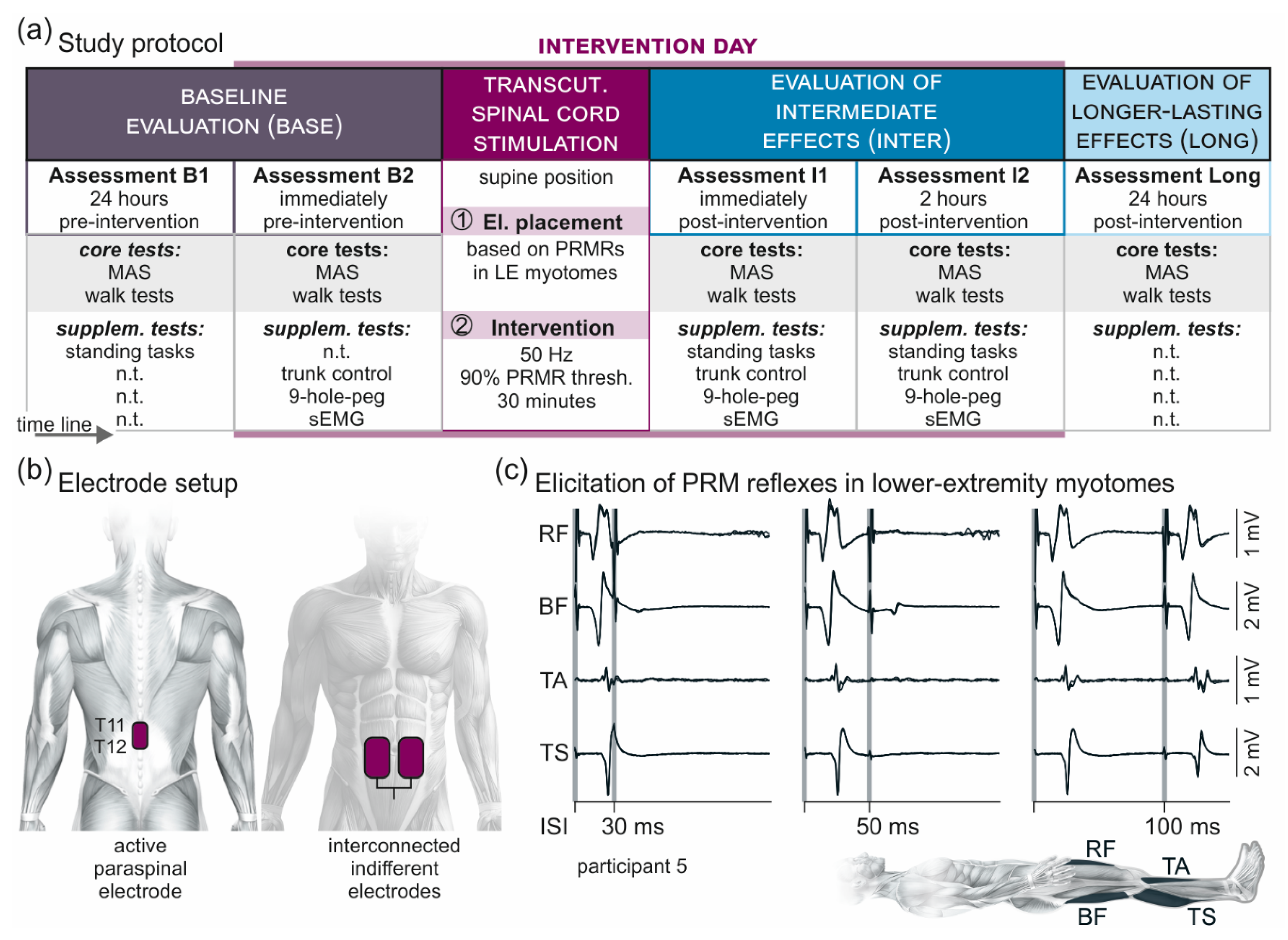
2.2. Study Protocol
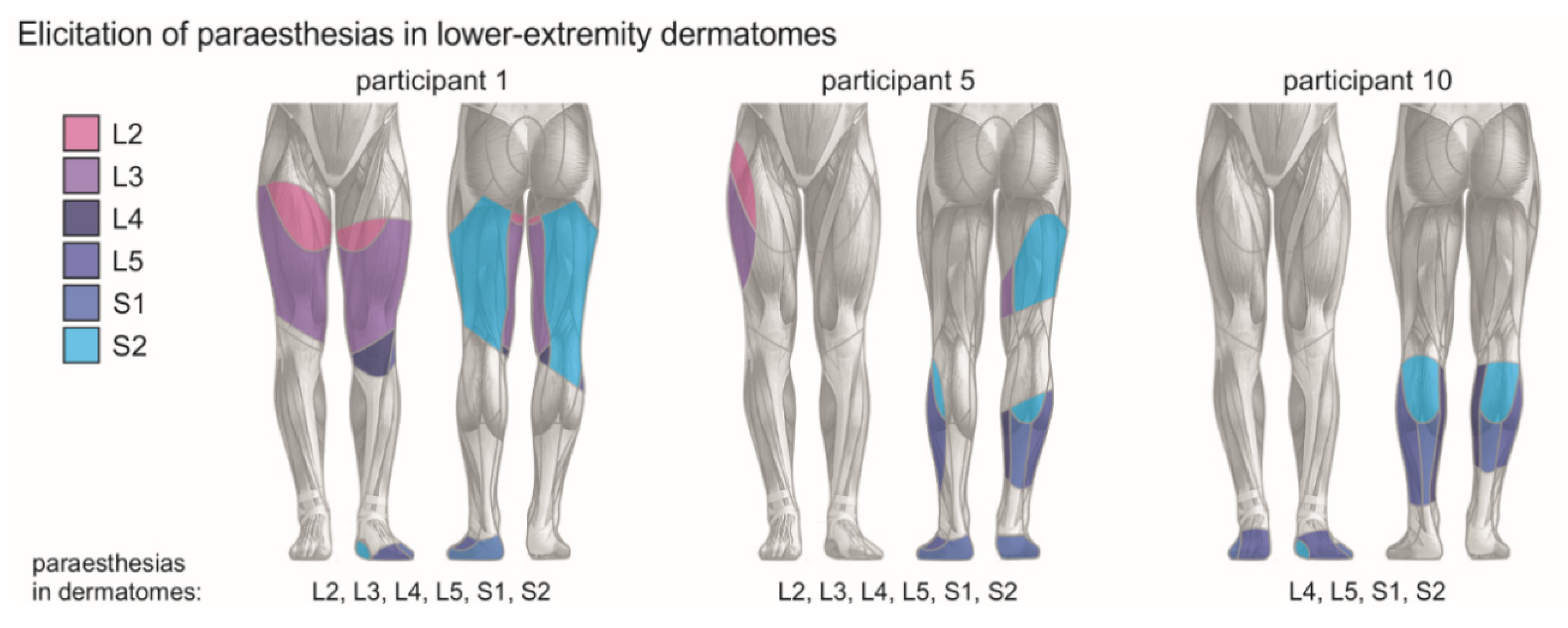
2.3. Transcutaneous Lumbar Spinal Cord Stimulation
2.4. Electromyographic Recordings
2.5. Data Analysis and Statistics
3. Results
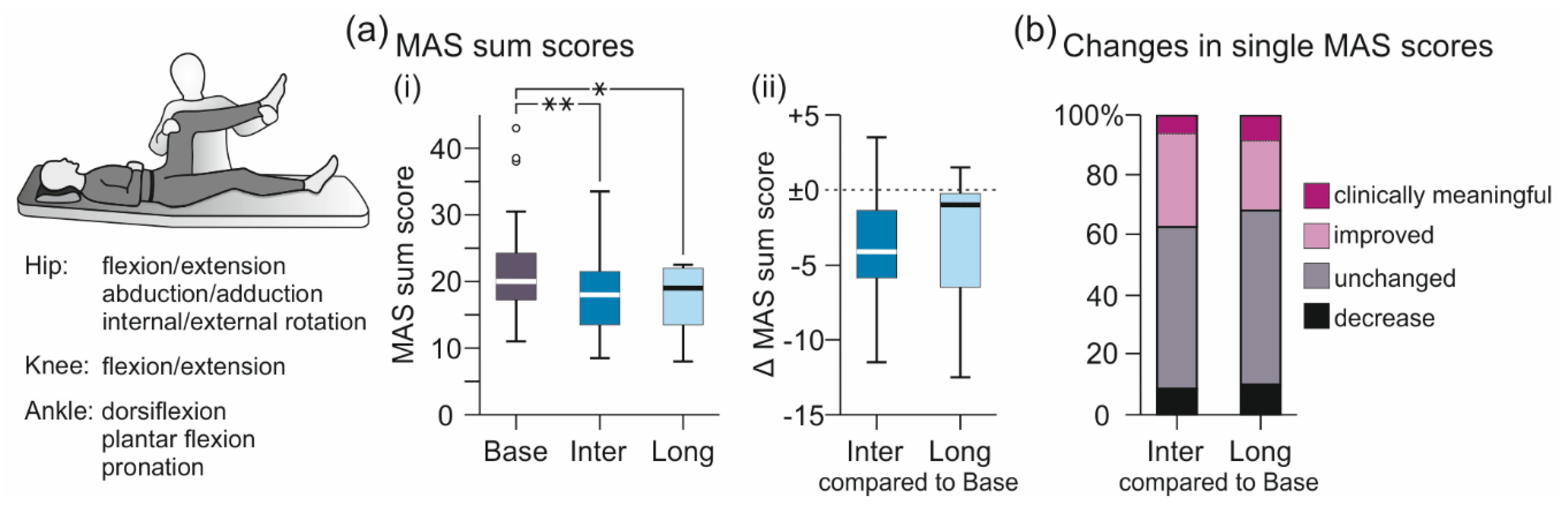
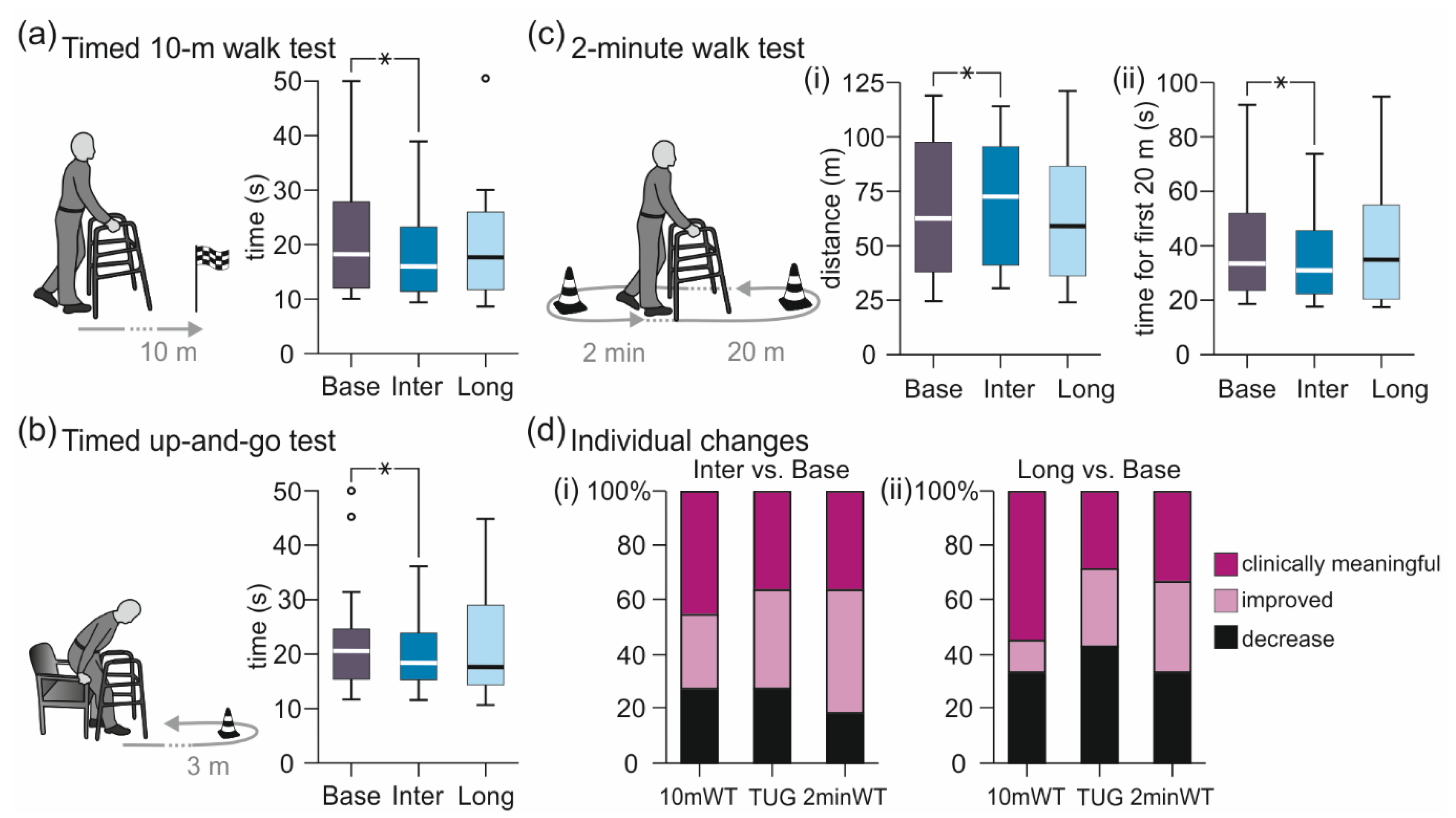
3.1. Results of Core Tests
3.1.1. MAS-Based Evaluation of Lower-Extremity Muscle Hypertonia
3.1.2. Walk Tests
3.2. Results of Supplementary Tests
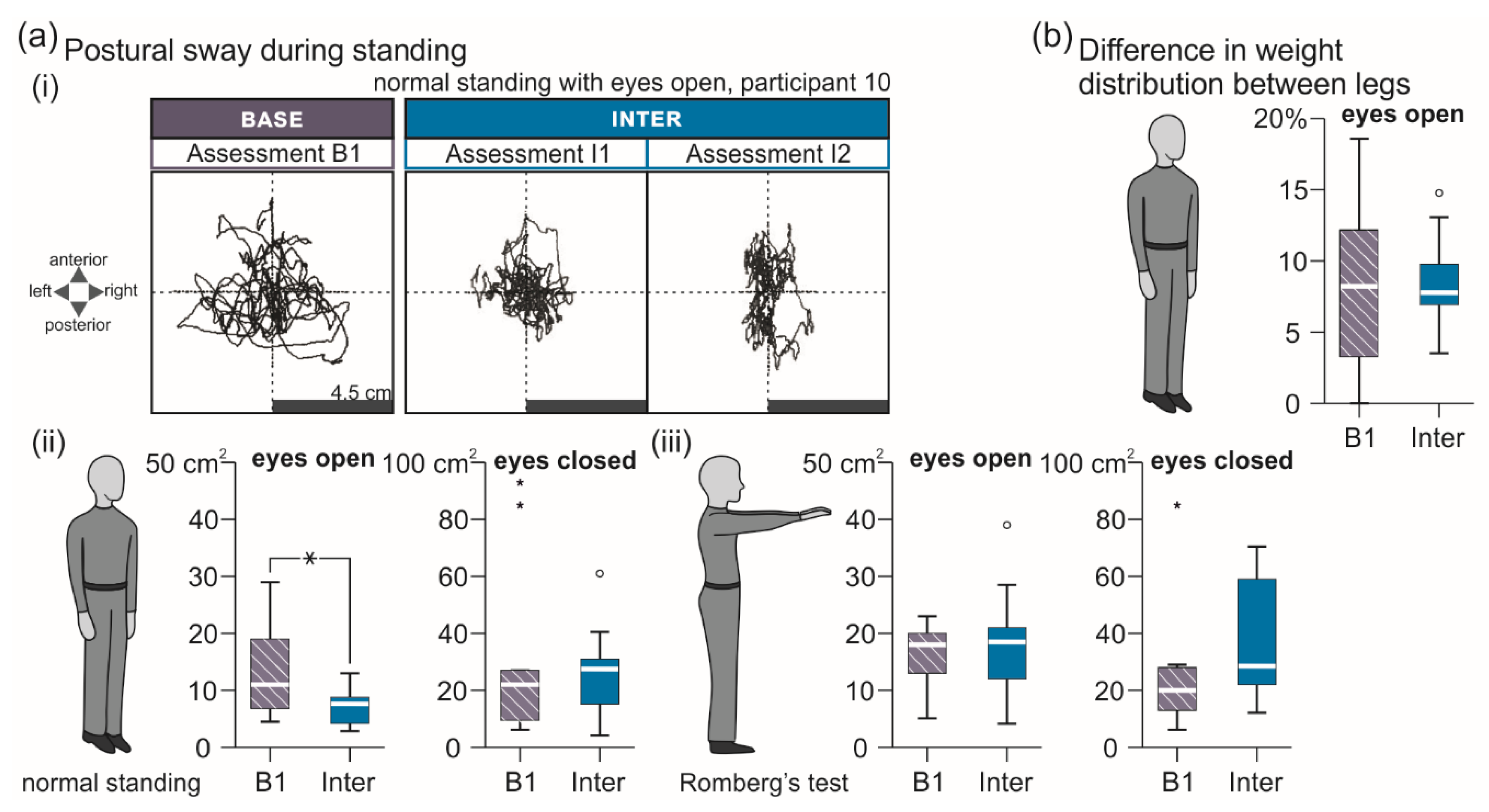
3.2.1. Standing Tasks
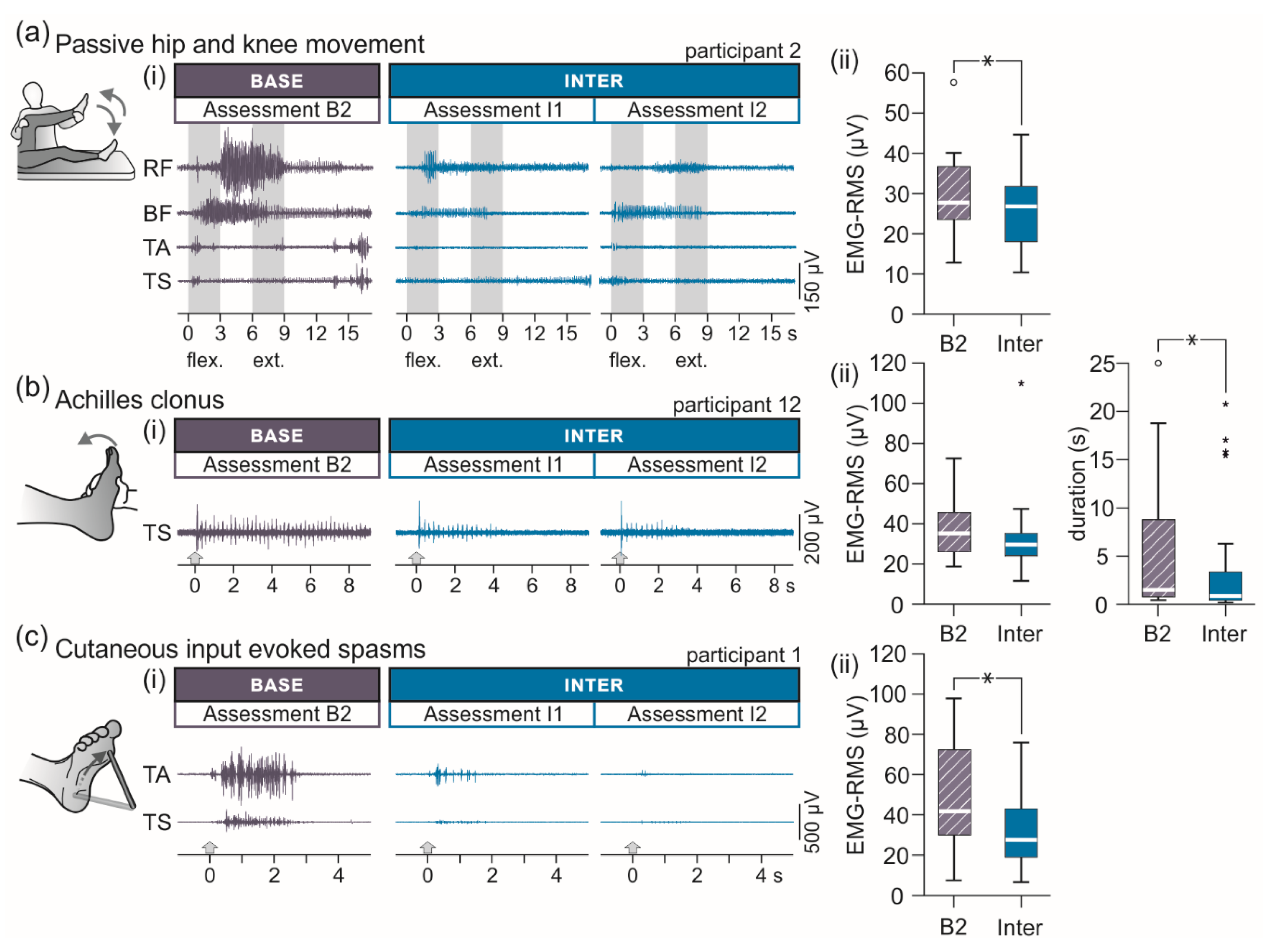
3.2.2. EMG-Based Assessment of Lower-Extremity Spasticity
3.2.3. Timed Nine-Hole-Peg Test
3.2.4. Trunk Control Test
3.2.5. Comparisons between Assessments B1 and B2 and between Assessments I1 and I2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Test | B1 | B2 | Wilcoxon Signed Rank Test |
|---|---|---|---|
| MAS sum score | 20.0 (16.0 to 24.5) | 20.8 (18.3 to 25.5) | Z = −1.230, p = 0.219, r = 0.318 |
| 10mWT (s) | 19.3 (10.5 to 30.0) | 17.2 (12.1 to 31.4) | Z = −0.533, p = 0.594, r = 0.178 |
| TUG (s) | 20.4 (14.4 to 24.7) | 20.6 (14.4 to 30.1) | Z = −1.400, p = 0.161, r = 0.495 |
| 2minWT, distance (m) | 59.0 (33.5 to 98.5) | 66.0 (37.0 to 100.0) | Z = −1.599, p = 0.110, r = 0.533 |
| 2minWT, first 20 m (s) | 33.2 (23.5 to 48.4) | 33.7 (20.2 to 58.5) | Z = −0.845, p = 0.398, r = 0.299 |
| Test | B1 | B2 | Wilcoxon Signed Rank Test |
|---|---|---|---|
| MAS sum score | 18.3 (13.3 to 23.9) | 17.5 (13.3 to 19.4) | Z = −0.910, p = 0.363, r = 0.228 |
| 10mWT (s) | 16.0 (12.7 to 23.3) | 16.0 (11.4 to 25.3) | Z = −1.778, p = 0.075, r = 0.536 |
| TUG (s) | 18.6 (15.3 to 23.9) | 18.3 (13.8 to 25.3) | Z = −0.089, p = 0.929, r = 0.027 |
| 2minWT, distance (m) | 73.0 (41.0 to 95.5) | 72.0 (40.5 to 100.0) | Z = −0.756, p = 0.449, r = 0.228 |
| 2minWT, first 20 m (s) | 31.0 (22.5 to 43.6) | 31.4 (20.5 to 49.8) | Z = −0.663, p = 0.508, r = 0.000 |
| Passive multi-joint movement, EMG-RMS (µV) | 27.1 (21.0 to 31.9) | 23.9 (16.3 to 31.1) | Z = −2.224, p = 0.026, r = 0.556 |
| Achilles clonus, EMG-RMS (µV) | 30.0 (25.5 to 36.4) | 29.7 (16.7 to 36.2) | Z = −1.190, p = 0.234, r = 0.298 |
| Achilles clonus, duration (s) | 0.9 (0.5 to 4.4) | 0.9 (0.4 to 3.7) | Z = −1.138, p = 0.255, r = 0.285 |
| Spasms, EMG-RMS (µV) | 27.3 (21.6 to 46.9) | 31.7 (17.8 to 43.8) | Z = −0.879, p = 0.379, r = 0.220 |
| Normal stance, eyes open, postural sway (cm2) | 8.1 (4.1 to 11.1) | 7.3 (4.3 to 10.4) | Z = −0.422, p = 0.673, r = 0.149 |
| Normal stance, eyes closed, postural sway (cm2) | 23.0 (7.2 to 41.0) | 22.0 (19.0 to 35.0) | Z = −0.652, p = 0.515, r = 0.217 |
| Romberg’s test, eyes open, postural sway (cm2) | 16.0 (9.5 to 24.5) | 16.0 (21.1 to 23.0) | Z = −0.059, p = 0.953, r = 0.020 |
| Romberg’s test, eyes closed, postural sway (cm2) | 33.5 (23.8 to 67.3) | 23.0 (15.2 to 54.5) | Z = −2.313, p = 0.021, r = 0.818 |
| Normal stance, eyes open, load difference (%) | 10.0 (4.5 to 16.6) | 8.0 (3.0 to 12.5) | Z = −0.845, p = 0.398, r = 0.282 |
| Timed nine-hole-peg test (s) | 30.2 (22.6 to 37.3) | 27.4 (21.6 to 37.4) | Z = −1.113, p = 0.266, r = 0.278 |
| Trunk control test | 87.0 (42.5 to 100.0) | 87.0 (42.5 to 100.0) | Z = 0.000, p = 1.000, r = 0.000 |
References
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.G.; et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.B.; Mignardot, J.-B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef]
- Minassian, K.; Persy, I.; Rattay, F.; Dimitrijevic, M.R.; Hofer, C.; Kern, H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 2007, 35, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Ladenbauer, J.; Minassian, K.; Hofstoetter, U.S.; Dimitrijevic, M.R.; Rattay, F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: A computer simulation study. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Danner, S.M.; Minassian, K. Paraspinal Magnetic and Transcutaneous Electrical Stimulation. In Encyclopedia of Computational Neuroscience; Jaeger, D., Jung, R., Eds.; Springer: New York, NY, USA, 2014; pp. 1–21. [Google Scholar]
- Rattay, F.; Minassian, K.; Dimitrijevic, M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord 2000, 38, 473–489. [Google Scholar] [CrossRef]
- Capogrosso, M.; Wenger, N.; Raspopovic, S.; Musienko, P.; Beauparlant, J.; Bassi Luciani, L.; Courtine, G.; Micera, S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 19326–19340. [Google Scholar] [CrossRef] [PubMed]
- Danner, S.M.; Hofstoetter, U.S.; Ladenbauer, J.; Rattay, F.; Minassian, K. Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artif. Organs 2011, 35, 257–262. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Perret, I.; Bayart, A.; Lackner, P.; Binder, H.; Freundl, B.; Minassian, K. Spinal motor mapping by epidural stimulation of lumbosacral posterior roots in humans. iScience 2020, 24, 101930. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Krenn, M.; Danner, S.M.; Hofer, C.; Kern, H.; McKay, W.B.; Mayr, W.; Minassian, K. Augmentation of Voluntary Locomotor Activity by Transcutaneous Spinal Cord Stimulation in Motor-Incomplete Spinal Cord-Injured Individuals. Artif. Organs 2015, 39, E176–E186. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.S.; Grahn, P.J.; Strommen, J.A.; Lavrov, I.A.; Beck, L.A.; Gill, M.L.; Linde, M.B.; Brown, D.A.; Van Straaten, M.G.; Veith, D.D.; et al. Electrophysiological Guidance of Epidural Electrode Array Implantation over the Human Lumbosacral Spinal Cord to Enable Motor Function after Chronic Paralysis. J. Neurotrauma 2019, 36, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Hofstoetter, U.S.; Hubli, M.; Hassani, R.H.; Rinaldo, C.; Curt, A.; Bolliger, M. Immediate Effects of Transcutaneous Spinal Cord Stimulation on Motor Function in Chronic, Sensorimotor Incomplete Spinal Cord Injury. J. Clin. Med. 2020, 9, 3541. [Google Scholar] [CrossRef]
- Al’joboori, Y.; Massey, S.J.; Knight, S.L.; Donaldson, N.D.; Duffell, L.D. The Effects of Adding Transcutaneous Spinal Cord Stimulation (tSCS) to Sit-To-Stand Training in People with Spinal Cord Injury: A Pilot Study. J. Clin. Med. 2020, 9, 2765. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; McKay, W.B.; Tansey, K.E.; Mayr, W.; Kern, H.; Minassian, K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 2014, 37, 202–211. [Google Scholar] [CrossRef]
- Estes, S.P.; Iddings, J.A.; Field-Fote, E.C. Priming Neural Circuits to Modulate Spinal Reflex Excitability. Front. Neurol. 2017, 8, 17. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W.; Binder, H.; Minassian, K. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. J. Neurotrauma 2020, 37, 481–493. [Google Scholar] [CrossRef]
- Danner, S.M.; Krenn, M.; Hofstoetter, U.S.; Toth, A.; Mayr, W.; Minassian, K. Body Position Influences Which Neural Structures Are Recruited by Lumbar Transcutaneous Spinal Cord Stimulation. PLoS ONE 2016, 11, e0147479. [Google Scholar] [CrossRef]
- Cook, A.W.; Weinstein, S.P. Chronic dorsal column stimulation in multiple sclerosis. Preliminary report. N. Y. State J. Med. 1973, 73, 2868–2872. [Google Scholar]
- Siegfried, J.; Lazorthes, Y.; Broggi, G. Electrical spinal cord stimulation for spastic movement disorders. Appl. Neurophysiol. 1981, 44, 77–92. [Google Scholar] [CrossRef]
- Cook, A.W. Electrical stimulation in multiple sclerosis. Hosp. Pract. 1976, 11, 51–58. [Google Scholar] [CrossRef]
- Illis, L.S.; Oygar, A.E.; Sedgwick, E.M.; Awadalla, M.A. Dorsal-column stimulation in the rehabilitation of patients with multiple sclerosis. Lancet 1976, 1, 1383–1386. [Google Scholar] [CrossRef]
- Dooley, D.M.; Sharkey, J. Electrostimulation of the nervous system for patients with demyelinating and degenerative diseases of the nervous system and vascular diseases of the extremities. Appl. Neurophysiol. 1977, 40, 208–217. [Google Scholar] [CrossRef]
- Illis, L.S.; Sedgwick, E.M.; Tallis, R.C. Spinal cord stimulation in multiple sclerosis: Clinical results. J. Neurol. Neurosurg. Psychiatry 1980, 43, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Waltz, J.M. Chronic stimulation for motor disorders. In Textbook for Stereotactic and Functional Neurosurgery; Gindelberg, P., Tasker, R., Eds.; McGraw-Hill: New York, NY, USA, 1998; pp. 1087–1099. [Google Scholar]
- Barnes, M.P.; Kent, R.M.; Semlyen, J.K.; McMullen, K.M. Spasticity in Multiple Sclerosis. Neurorehabil. Neural Repair 2003, 17, 66–70. [Google Scholar] [CrossRef]
- Rizzo, M.A.; Hadjimichael, O.C.; Preiningerova, J.; Vollmer, T.L. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult. Scler. J. 2004, 10, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Van Asch, P. Impact of Mobility Impairment in Multiple Sclerosis 2—Patients’ Perspectives. Eur. Neurol. Rev. 2011, 6, 115. [Google Scholar] [CrossRef]
- Minassian, K.; Hofstoetter, U.; Tansey, K.; Mayr, W. Neuromodulation of lower limb motor control in restorative neurology. Clin. Neurol. Neurosurg. 2012, 114, 489–497. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.M.; McKay, W.B.; Dimitrijević, M.R. Motor control after spinal cord injury: Assessment using surface EMG. Muscle Nerve 1996, 19, 966–979. [Google Scholar] [CrossRef]
- Sherwood, A.M.; Graves, D.E.; Priebe, M.M. Altered motor control and spasticity after spinal cord injury: Subjective and objective assessment. J. Rehabil. Res. Dev. 2000, 37, 41–52. [Google Scholar]
- Kirshblum, S.; Waring, W. Updates for the International Standards for Neurological Classification of Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 505–517. [Google Scholar] [CrossRef]
- Morganti, B.; Scivoletto, G.; Ditunno, P.; Ditunno, J.F.; Molinari, M. Walking index for spinal cord injury (WISCI): Criterion validation. Spinal Cord 2005, 43, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Ditunno, J.F.; Ditunno, P.L.; Scivoletto, G.; Patrick, M.; Dijkers, M.; Barbeau, H.; Burns, A.S.; Marino, R.J.; Schmidt-Read, M. The Walking Index for Spinal Cord Injury (WISCI/WISCI II): Nature, metric properties, use and misuse. Spinal Cord 2013, 51, 346–355. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Recovery cycles of posterior root-muscle reflexes evoked by transcutaneous spinal cord stimulation and of the H reflex in individuals with intact and injured spinal cord. PLoS ONE 2019, 14, e0227057. [Google Scholar] [CrossRef] [PubMed]
- Mathias, S.; Nayak, U.S.; Isaacs, B. Balance in elderly patients: The “get-up and go” test. Arch. Phys. Med. Rehabil. 1986, 67, 387–389. [Google Scholar]
- Sebastião, E.; Sandroff, B.M.; Learmonth, Y.C.; Motl, R.W. Validity of the Timed Up and Go Test as a Measure of Functional Mobility in Persons With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2016, 97, 1072–1077. [Google Scholar] [CrossRef]
- Kempen, J.; de Groot, V.; Knol, D.; Polman, C.; Lankhorst, G.; Beckerman, H. Community walking can be assessed using a 10-metre timed walk test. Mult. Scler. J. 2011, 17, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Scalzitti, D.A.; Harwood, K.J.; Maring, J.R.; Leach, S.J.; Ruckert, E.A.; Costello, E. Validation of the 2-Minute Walk Test with the 6-Minute Walk Test and Other Functional Measures in Persons with Multiple Sclerosis. Int. J. MS Care 2018, 20, 158–163. [Google Scholar] [CrossRef]
- Gijbels, D.; Eijnde, B.; Feys, P. Comparison of the 2- and 6-minute walk test in multiple sclerosis. Mult. Scler. J. 2011, 17, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Khasnis, A.; Gokula, R.M. Romberg’s test. J. Postgrad. Med. 2003, 49, 169–172. [Google Scholar] [PubMed]
- Feys, P.; Lamers, I.; Francis, G.; Benedict, R.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult. Scler. J. 2017, 23, 711–720. [Google Scholar] [CrossRef]
- Franchignoni, F.P.; Tesio, L.; Ricupero, C.; Martino, M.T. Trunk Control Test as an Early Predictor of Stroke Rehabilitation Outcome. Stroke 1997, 28, 1382–1385. [Google Scholar] [CrossRef]
- Lin, C.S.-Y.; Chan, J.H.L.; Pierrot-Deseilligny, E.; Burke, D. Excitability of human muscle afferents studied using threshold tracking of the H reflex. J. Physiol. 2002, 545, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Burke, D. Clinical uses of H reflexes of upper and lower limb muscles. Clin. Neurophysiol. Pract. 2016, 1, 9–17. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PLoS ONE 2018, 13, e0192013. [Google Scholar] [CrossRef]
- Kagamihara, Y.; Hayashi, A.; Okuma, Y.; Nagaoka, M.; Nakajima, Y.; Tanaka, R. Reassessment of H-reflex recovery curve using the double stimulation procedure. Muscle Nerve 1998, 21, 352–360. [Google Scholar] [CrossRef]
- Pinter, M.M.; Gerstenbrand, F.; Dimitrijevic, M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control Of spasticity. Spinal Cord 2000, 38, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.; Rodgers, H.; Price, C.; van Wijck, F.; Shackley, P.; Steen, N.; Barnes, M.; Ford, G.; Graham, L. BoTULS: A multicentre randomised controlled trial to evaluate the clinical effectiveness and cost-effectiveness of treating upper limb spasticity due to stroke with botulinum toxin type A. Health Technol. Assess. 2010, 14, 1–13. [Google Scholar] [CrossRef]
- Brashear, A.; Gordon, M.F.; Elovic, E.; Kassicieh, V.D.; Marciniak, C.; Do, M.; Lee, C.-H.; Jenkins, S.; Turkel, C. Intramuscular Injection of Botulinum Toxin for the Treatment of Wrist and Finger Spasticity after a Stroke. N. Engl. J. Med. 2002, 347, 395–400. [Google Scholar] [CrossRef]
- Sheikh, K.; Smith, D.S.; Meade, T.W.; Brennan, P.J.; Ide, L. Assessment of motor function in studies of chronic disability. Rheumatology 1980, 19, 83–90. [Google Scholar] [CrossRef]
- Cetisli Korkmaz, N.; Can Akman, T.; Kilavuz Oren, G.; Bir, L.S. Trunk control: The essence for upper limb functionality in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 24, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Musselman, K.E. Clinical significance testing in rehabilitation research: What, why, and how? Phys. Ther. Rev. 2007, 12, 287–296. [Google Scholar] [CrossRef]
- Hupperts, R.; Lycke, J.; Short, C.; Gasperini, C.; McNeill, M.; Medori, R.; Tofil-Kaluza, A.; Hovenden, M.; Mehta, L.R.; Elkins, J. Prolonged-release fampridine and walking and balance in MS: Randomised controlled MOBILE trial. Mult. Scler. J. 2016, 22, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Hobart, J.; Ziemssen, T.; Feys, P.; Linnebank, M.; Goodman, A.D.; Farrell, R.; Hupperts, R.; Blight, A.R.; Englishby, V.; McNeill, M.; et al. Assessment of Clinically Meaningful Improvements in Self-Reported Walking Ability in Participants with Multiple Sclerosis: Results from the Randomized, Double-Blind, Phase III ENHANCE Trial of Prolonged-Release Fampridine. CNS Drugs 2019, 33, 61–79. [Google Scholar] [CrossRef]
- Baert, I.; Freeman, J.; Smedal, T.; Dalgas, U.; Romberg, A.; Kalron, A.; Conyers, H.; Elorriaga, I.; Gebara, B.; Gumse, J.; et al. Responsiveness and Clinically Meaningful Improvement, According to Disability Level, of Five Walking Measures After Rehabilitation in Multiple Sclerosis. Neurorehabil. Neural Repair 2014, 28, 621–631. [Google Scholar] [CrossRef]
- Danner, S.M.; Hofstoetter, U.S.; Minassian, K. Finite Element Models of Transcutaneous Spinal Cord Stimulation. In Encyclopedia of Computational Neuroscience; Jaeger, D., Jung, R., Eds.; Springer: New York, NY, USA, 2014; pp. 1–6. [Google Scholar]
- Minassian, K.; Persy, I.; Rattay, F.; Pinter, M.M.; Kern, H.; Dimitrijevic, M.R. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum. Mov. Sci. 2007, 26, 275–295. [Google Scholar] [CrossRef]
- Hunter, J.P.; Ashby, P. Segmental effects of epidural spinal cord stimulation in humans. J. Physiol. 1994, 474, 407–419. [Google Scholar] [CrossRef]
- Holsheimer, J. Which Neuronal Elements are Activated Directly by Spinal Cord Stimulation. Neuromodulation 2002, 5, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ranck, J.B.J. Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Res. 1975, 98, 417–440. [Google Scholar] [CrossRef]
- Rattay, F. The basic mechanism for the electrical stimulation of the nervous system. Neuroscience 1999, 89, 335–346. [Google Scholar] [CrossRef]
- Struijk, J.J.; Holsheimer, J.; Boom, H.B.K. Excitation of dorsal root fibers in spinal cord stimulation: A theoretical study. IEEE Trans. Biomed. Eng. 1993, 40, 632–639. [Google Scholar] [CrossRef]
- Lloyd, D. Reflex action in relation to pattern and peripheral source of afferent stimulation. J. Neurophysiol. 1943, 6, 111–119. [Google Scholar] [CrossRef]
- Formento, E.; Minassian, K.; Wagner, F.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Rowald, A.; Bloch, J.; Micera, S.; Capogrosso, M.; Courtine, G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci. 2018, 21, 1728–1741. [Google Scholar] [CrossRef]
- Sedgwick, E.M.; Illis, L.S.; Tallis, R.C.; Thornton, A.R.; Abraham, P.; El-Negamy, E.; Docherty, T.B.; Soar, J.S.; Spencer, S.C.; Taylor, F.M. Evoked potentials and contingent negative variation during treatment of multiple sclerosis with spinal cord stimulation. J. Neurol. Neurosurg. Psychiatry 1980, 43, 15–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hofstoetter, U.S.; Danner, S.M.; Freundl, B.; Binder, H.; Mayr, W.; Rattay, F.; Minassian, K. Periodic modulation of repetitively elicited monosynaptic reflexes of the human lumbosacral spinal cord. J. Neurophysiol. 2015, 114, 400–410. [Google Scholar] [CrossRef]
- Lin, S.; Li, Y.; Lucas-Osma, A.M.; Hari, K.; Stephens, M.J.; Singla, R.; Heckman, C.J.; Zhang, Y.; Fouad, K.; Fenrich, K.K.; et al. Locomotor-related V3 interneurons initiate and coordinate muscles spasms after spinal cord injury. J. Neurophysiol. 2019, 121, 1352–1367. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R.; Gerasimenko, Y.; Pinter, M.M. Evidence for a spinal central pattern generator in humans. Ann. N. Y. Acad. Sci. 1998, 860, 360–376. [Google Scholar] [CrossRef]
- Minassian, K.; Jilge, B.; Rattay, F.; Pinter, M.M.; Binder, H.; Gerstenbrand, F.; Dimitrijevic, M.R. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: Electromyographic study of compound muscle action potentials. Spinal Cord 2004, 42, 401–416. [Google Scholar] [CrossRef]
- Minassian, K.; Hofstoetter, U.S.; Danner, S.M.; Mayr, W.; Bruce, J.A.; McKay, W.B.; Tansey, K.E. Spinal Rhythm Generation by Step-Induced Feedback and Transcutaneous Posterior Root Stimulation in Complete Spinal Cord-Injured Individuals. Neurorehabil. Neural Repair 2016, 30, 233–243. [Google Scholar] [CrossRef]
- Sivaramakrishnan, A.; Solomon, J.M.; Manikandan, N. Comparison of transcutaneous electrical nerve stimulation (TENS) and functional electrical stimulation (FES) for spasticity in spinal cord injury—A pilot randomized cross-over trial. J. Spinal Cord Med. 2018, 41, 397–406. [Google Scholar] [CrossRef]
- Ping Ho Chung, B.; Kam Kwan Cheng, B. Immediate effect of transcutaneous electrical nerve stimulation on spasticity in patients with spinal cord injury. Clin. Rehabil. 2010, 24, 202–210. [Google Scholar] [CrossRef]
- Liberson, W.T.; Holmquest, H.J.; Scot, D.; Dow, M. Functional electrotherapy: Stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch. Phys. Med. Rehabil. 1961, 42, 101–105. [Google Scholar]
- Trapp, B.D.; Nave, K.-A. Multiple Sclerosis: An Immune or Neurodegenerative Disorder? Annu. Rev. Neurosci. 2008, 31, 247–269. [Google Scholar] [CrossRef]
- Pearson, O.R.; Busse, M.E.; van Deursen, R.W.M.; Wiles, C.M. Quantification of walking mobility in neurological disorders. QJM 2004, 97, 463–475. [Google Scholar] [CrossRef]
- Confavreux, C.; Vukusic, S.; Moreau, T.; Adeleine, P. Relapses and Progression of Disability in Multiple Sclerosis. N. Engl. J. Med. 2000, 343, 1430–1438. [Google Scholar] [CrossRef]
- Confavreux, C. Early clinical predictors and progression of irreversible disability in multiple sclerosis: An amnesic process. Brain 2003, 126, 770–782. [Google Scholar] [CrossRef]
- Confavreux, C.; Vukusic, S. Natural history of multiple sclerosis: A unifying concept. Brain 2006, 129, 606–616. [Google Scholar] [CrossRef]
- Motl, R.W.; Learmonth, Y.C. Neurological disability and its association with walking impairment in multiple sclerosis: Brief review. Neurodegener. Dis. Manag. 2014, 4, 491–500. [Google Scholar] [CrossRef]
- Goldman, M.D.; Motl, R.W.; Scagnelli, J.; Pula, J.H.; Sosnoff, J.J.; Cadavid, D. Clinically meaningful performance benchmarks in MS: Timed 25-Foot Walk and the real world. Neurology 2013, 81, 1856–1863. [Google Scholar] [CrossRef]
- Learmonth, Y.C.; Motl, R.W.; Sandroff, B.M.; Pula, J.H.; Cadavid, D. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.K.; Knak, K.L.; Witting, N.; Vissing, J. Two- and 6-minute walk tests assess walking capability equally in neuromuscular diseases. Neurology 2016, 86, 442–445. [Google Scholar] [CrossRef]
- Nielsen, J.; Willerslev-Olsen, M.; Lorentzen, J. Pathophysiology of Spasticity. In Neurological Rehabilitation. Spasticity and Contractures in Clinical Practice and Research; Pandyan, A., Hermens, H., Conway, B., Eds.; Imprint; CRC Press: Boca Raton, FL, USA, 2018; pp. 25–57. [Google Scholar]
- Faist, M.; Mazevet, D.; Dietz, V.; Pierrot-Deseilligny, E. A quantitative assessment of presynaptic inhibition of Ia afferents in spastics. Differences in hemiplegics and paraplegics. Brain 1994, 117, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Grey, M.J.; Klinge, K.; Crone, C.; Lorentzen, J.; Biering-Sørensen, F.; Ravnborg, M.; Nielsen, J.B. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp. Brain Res. 2008, 185, 189–197. [Google Scholar] [CrossRef]
- Schindler-Ivens, S.; Shields, R.K. Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp. Brain Res. 2000, 133, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Crone, C.; Johnsen, L.L.; Biering-Sørensen, F.; Nielsen, J.B. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 2003, 126, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010, 16, 302–307. [Google Scholar] [CrossRef]
- Hidler, J.M.; Rymer, W.Z. A simulation study of reflex instability in spasticity: Origins of clonus. IEEE Trans. Rehabil. Eng. 1999, 7, 327–340. [Google Scholar] [CrossRef]
- Dimitrijevic, M.R.; Nathan, P.W.; Sherwood, A.M. Clonus: The role of central mechanisms. J. Neurol. Neurosurg. Psychiatry 1980, 43, 321–332. [Google Scholar] [CrossRef]
- Beres-Jones, J.A.; Johnson, T.D.; Harkema, S.J. Clonus after human spinal cord injury cannot be attributed solely to recurrent muscle-tendon stretch. Exp. Brain Res. 2003, 149, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Mazzocchio, R.; Scarpini, C. Clonus in man: A rhythmic oscillation maintained by a reflex mechanism. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 56–63. [Google Scholar] [CrossRef]
- Murray, K.C.; Stephens, M.J.; Ballou, E.W.; Heckman, C.J.; Bennett, D.J. Motoneuron Excitability and Muscle Spasms Are Regulated by 5-HT 2B and 5-HT 2C Receptor Activity. J. Neurophysiol. 2011, 105, 731–748. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, J.M.; Murray, K.C.; Li, Y.; Chan, K.M.; Finlay, M.G.; Bennett, D.J.; Gorassini, M.A. Constitutively active 5-HT 2 /α 1 receptors facilitate muscle spasms after human spinal cord injury. J. Neurophysiol. 2013, 109, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.J.; Li, Y.; Harvey, P.J.; Gorassini, M. Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J. Neurophysiol. 2001, 86, 1972–1982. [Google Scholar] [CrossRef]
- Bellardita, C.; Caggiano, V.; Leiras, R.; Caldeira, V.; Fuchs, A.; Bouvier, J.; Löw, P.; Kiehn, O. Spatiotemporal correlation of spinal network dynamics underlying spasms in chronic spinalized mice. eLife 2017, 6, e23011. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E. Interneuronal relay in spinal pathways from proprioceptors. Prog. Neurobiol. 1992, 38, 335–378. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, E.; Burke, D. The Circuitry of the Human Spinal Cord: Spinal and Corticospinal Mechanisms of Movement; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Hofstoetter, U.S.; Danner, S.M.; Freundl, B.; Binder, H.; Lackner, P.; Minassian, K. Ipsi- and Contralateral Oligo- and Polysynaptic Reflexes in Humans Revealed by Low-Frequency Epidural Electrical Stimulation of the Lumbar Spinal Cord. Brain Sci. 2021, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Calancie, B. Interlimb reflexes following cervical spinal cord injury in man. Exp. Brain Res. 1991, 85, 458–469. [Google Scholar] [CrossRef]
- Butler, J.E.; Godfrey, S.; Thomas, C.K. Interlimb Reflexes Induced by Electrical Stimulation of Cutaneous Nerves after Spinal Cord Injury. PLoS ONE 2016, 11, e0153063. [Google Scholar] [CrossRef]
- McNulty, P.A.; Burke, D. Self-sustained motor activity triggered by interlimb reflexes in chronic spinal cord injury, evidence of functional ascending propriospinal pathways. PLoS ONE 2013, 8, e72725. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rancourt, A.; Di Poce, S.; Levine, A.; Hoang, J.; Ismail, F.; Boulias, C.; Phadke, C.P. Patient-Identified Factors That Influence Spasticity in People with Stroke and Multiple Sclerosis Receiving Botulinum Toxin Injection Treatments. Physiother. Can. 2015, 67, 157–166. [Google Scholar] [CrossRef]
- Oreja-Guevara, C.; González-Segura, D.; Vila, C. Spasticity in multiple sclerosis: Results of a patient survey. Int. J. Neurosci. 2013, 123, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Lucchinetti, C.; Brück, W.; Parisi, J.; Scheithauer, B.; Rodriguez, M.; Lassmann, H. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann. Neurol. 2000, 47, 707–717. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Spinal Cord Stimulation as a Neuromodulatory Intervention for Altered Motor Control Following Spinal Cord Injury. In Advanced Technologies in Rehabilitation of Gait and Balance Disorders; Sandrini, G., Homberg, V., Saltuari, L., Smania, N., Pedrocchi, A., Eds.; Springer: Berlin, Germany, 2018; pp. 501–521. [Google Scholar]
| Part. Nr. | Sex | Age (y) | Time Since Diag. (y) | EDSS Score | WISCI II Score | LEMS Total | PPSS L1 to L2 | LTSS L1 to L2 | Anti-spasticity Medication (Daily Dosage) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 52 | 24 | 3.5 | 20 | 38 | 28 | 28 | None |
| 2 | F | 66 | 1 | 5 | 20 | 37 | 28 | 28 | None |
| 3 | M | 30 | 6 | 5 | 20 | 36 | 25 | 22 | None |
| 4 | M | 44 | 7 | 6 | 19 | 39 | 28 | 28 | 100 mg baclofen |
| 5 | F | 65 | 24 | 6 | 19 | 31 | 28 | 28 | None |
| 6 | F | 71 | 49 | 6 | 19 | 39 | 28 | 28 | None |
| 7 | F | 51 | 24 | 6.5 | 16 | 34 | 28 | 28 | 75 mg baclofen |
| 8 | F | 64 | 29 | 6.5 | 16 | 36 | 28 | 28 | None |
| 9 | F | 57 | 30 | 6.5 | 13 | 43 | 24 | 24 | 62.5 mg baclofen, 12 mg tizanidine |
| 10 | M | 39 | 18 | 6.5 | 12 | 38 | 24 | 28 | None |
| 11 | M | 61 | 9 | 6.5 | 9 | 28 | 28 | 28 | None |
| 12 | M | 48 | 3 | 7.0 | 0 | 8 | 21 | 28 | 95 mg baclofen, 300 mg pregabalin |
| 13 | F | 56 | 19 | 7.5 | 0 | 19 | 28 | 28 | 10 mg baclofen |
| 14 | M | 76 | 20 | 7.5 | 0 | 8 | 28 | 28 | None |
| 15 | F | 64 | 8 | 8 | 0 | 10 | 14 | 14 | None |
| 16 | M | 54 | 35 | 8.5 | 0 | 17 | 22 | 22 | None |
| Test | Base | Inter | Range of Changes Base vs. Inter | Long | Range of Changes Base vs. Long |
|---|---|---|---|---|---|
| MAS sum score | 20.0 (16.5 to 24.5) | 18.0 (13.3 to 21.8) | −11.5 to +3.5 | 19.0 (12.3 to 22.0) | −12.5 to +1.5 |
| 10mWT (s) | 18.2 (12.0 to 29.6) | 16.0 (11.4 to 23.8) | −14.6 to +1.3 | 17.7 (10.4 to 28.0) | −9.3 to +1.7 |
| 10mWT (m/s) | 0.55 (0.34 to 0.84) | 0.63 (0.42 to 0.88) | −0.04 to +0.20 | 0.56 (0.35 to 1.00) | −0.06 to +0.19 |
| TUG (s) | 20.6 (14.4 to 25.0) | 18.4 (14.9 to 24.2) | −12.1 to +1.5 | 17.7 (11.2 to 34.0) | −6.3 to +3.8 |
| 2minWT, distance (m) | 62.5 (37.5 to 98.8) | 72.5 (40.9 to 96.6) | −4.8 to +29.0 | 59.0 (35.6 to 100.8) | −10.0 to +16.6 |
| 2minWT, first 20 m (s) | 33.7 (21.4 to 55.6) | 31.2 (22.0 to 46.8) | −25.1 to +0.7 | 35.1 (19.6 to 60.1) | −5.7 to +6.7 |
| Standing Tasks | B1 | Inter |
| Normal stance, eyes open, sway area (cm2) | 11.0 (5.8 to 19.5) | 8.0 (4.3 to 10.4) |
| Normal stance, eyes closed, sway area (cm2) | 22.0 (9.5 to 56.0) | 22.5 (13.6 to 38.3) |
| Romberg’s test, eyes open, sway area (cm2) | 18.0 (11.5 to 20.5) | 16.0 (10.6 to 23.8) |
| Romberg’s test, eyes closed, sway area (cm2) | 20.0 (12.5 to 28.5) | 33.0 (21.0 to 59.0) |
| Diff. in weight dist. between lower extremities (%) | 8.5 (2.5 to 12.8) | 9.0 (3.5 to 13.9) |
| Surface-EMG Based Evaluation of Spasticity | B2 | Inter |
| Passive multi-joint movement, EMG-RMS (µV) | 30.5 (23.4 to 37.0) | 26.8 (17.9 to 31.9) |
| Achilles clonus, EMG-RMS (µV) | 35.3 (25.9 to 45.9) | 29.7 (23.0 to 36.2) |
| Achilles clonus, duration (s) | 1.5 (0.8 to 9.4) | 0.9 (0.4 to 3.7) |
| Muscle spasms, EMG-RMS (µV) | 41.8 (29.6 to 73.6) | 27.5 (18.8 to 43.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofstoetter, U.S.; Freundl, B.; Lackner, P.; Binder, H. Transcutaneous Spinal Cord Stimulation Enhances Walking Performance and Reduces Spasticity in Individuals with Multiple Sclerosis. Brain Sci. 2021, 11, 472. https://doi.org/10.3390/brainsci11040472
Hofstoetter US, Freundl B, Lackner P, Binder H. Transcutaneous Spinal Cord Stimulation Enhances Walking Performance and Reduces Spasticity in Individuals with Multiple Sclerosis. Brain Sciences. 2021; 11(4):472. https://doi.org/10.3390/brainsci11040472
Chicago/Turabian StyleHofstoetter, Ursula S., Brigitta Freundl, Peter Lackner, and Heinrich Binder. 2021. "Transcutaneous Spinal Cord Stimulation Enhances Walking Performance and Reduces Spasticity in Individuals with Multiple Sclerosis" Brain Sciences 11, no. 4: 472. https://doi.org/10.3390/brainsci11040472
APA StyleHofstoetter, U. S., Freundl, B., Lackner, P., & Binder, H. (2021). Transcutaneous Spinal Cord Stimulation Enhances Walking Performance and Reduces Spasticity in Individuals with Multiple Sclerosis. Brain Sciences, 11(4), 472. https://doi.org/10.3390/brainsci11040472







