Dissecting the Molecular Determinants of GABAA Receptors Current Rundown, a Hallmark of Refractory Human Epilepsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Oocyte Electrophysiology
2.2. Cell Culture and Transfection
2.3. Patch Clamp Recordings
2.4. Statistical Analysis
3. Results
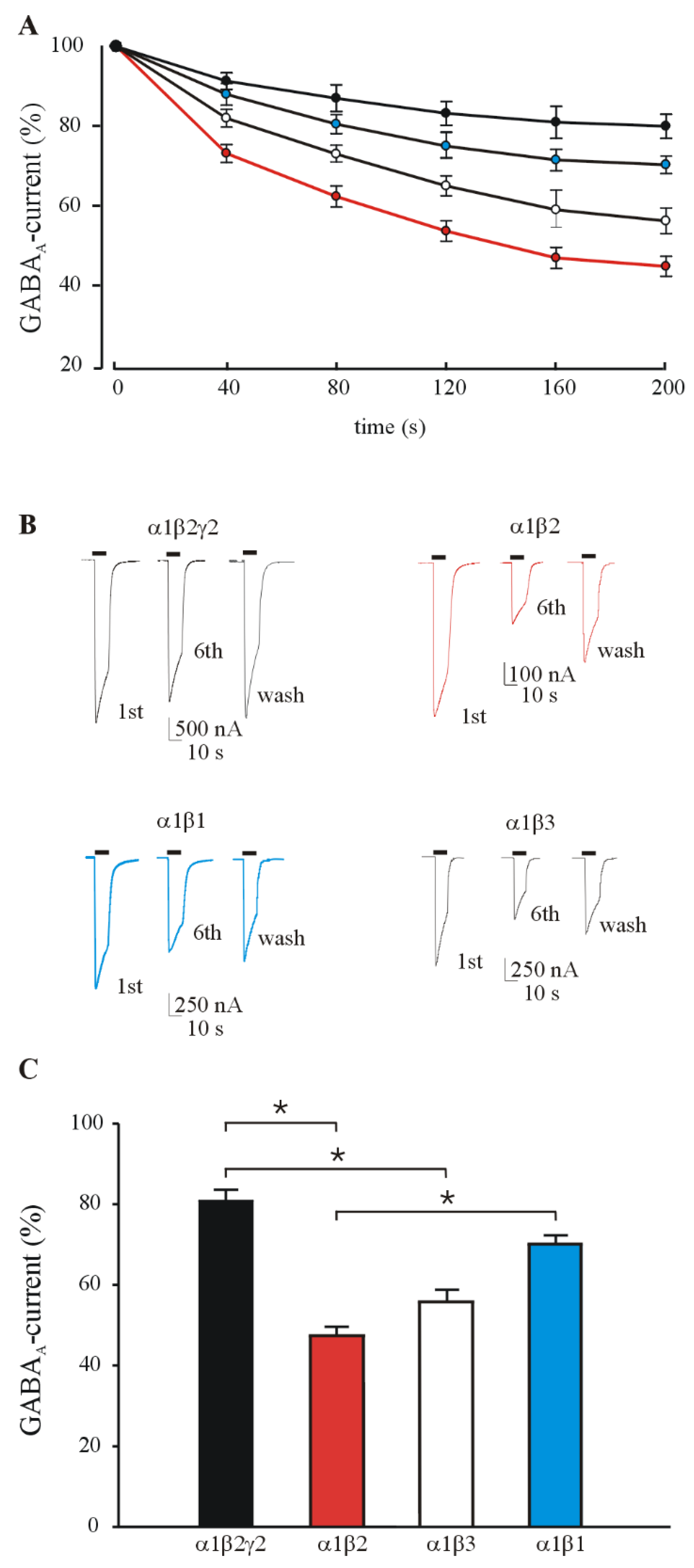
3.1. GABA Currents Evoked in Xenopus Oocytes Injected with Different Combinations of GABAAR Subunits
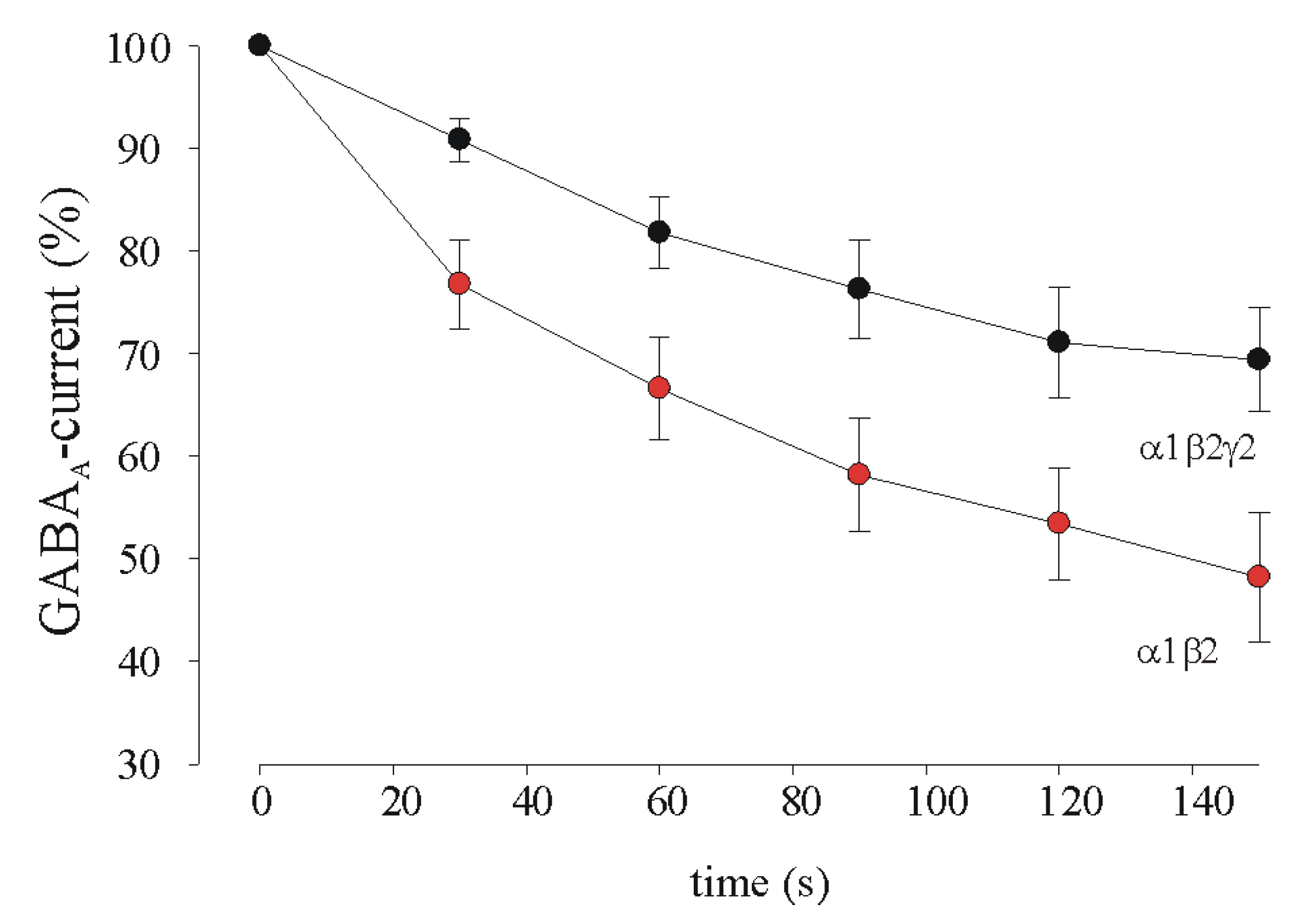
3.2. GABA Current Rundown in HEK Transfected Cells
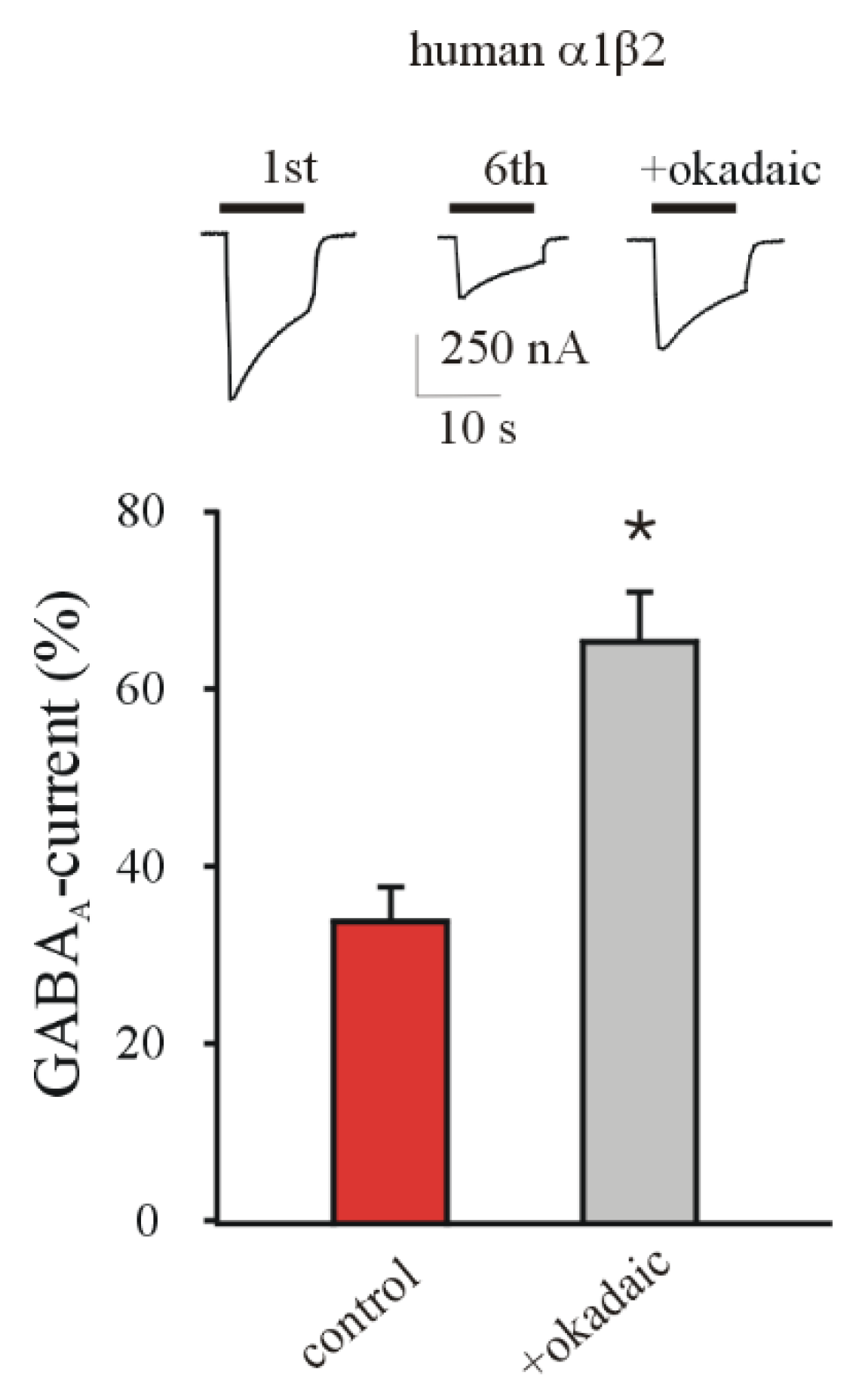
3.3. Phosphatase Inhibition Recovers GABA Current Rundown
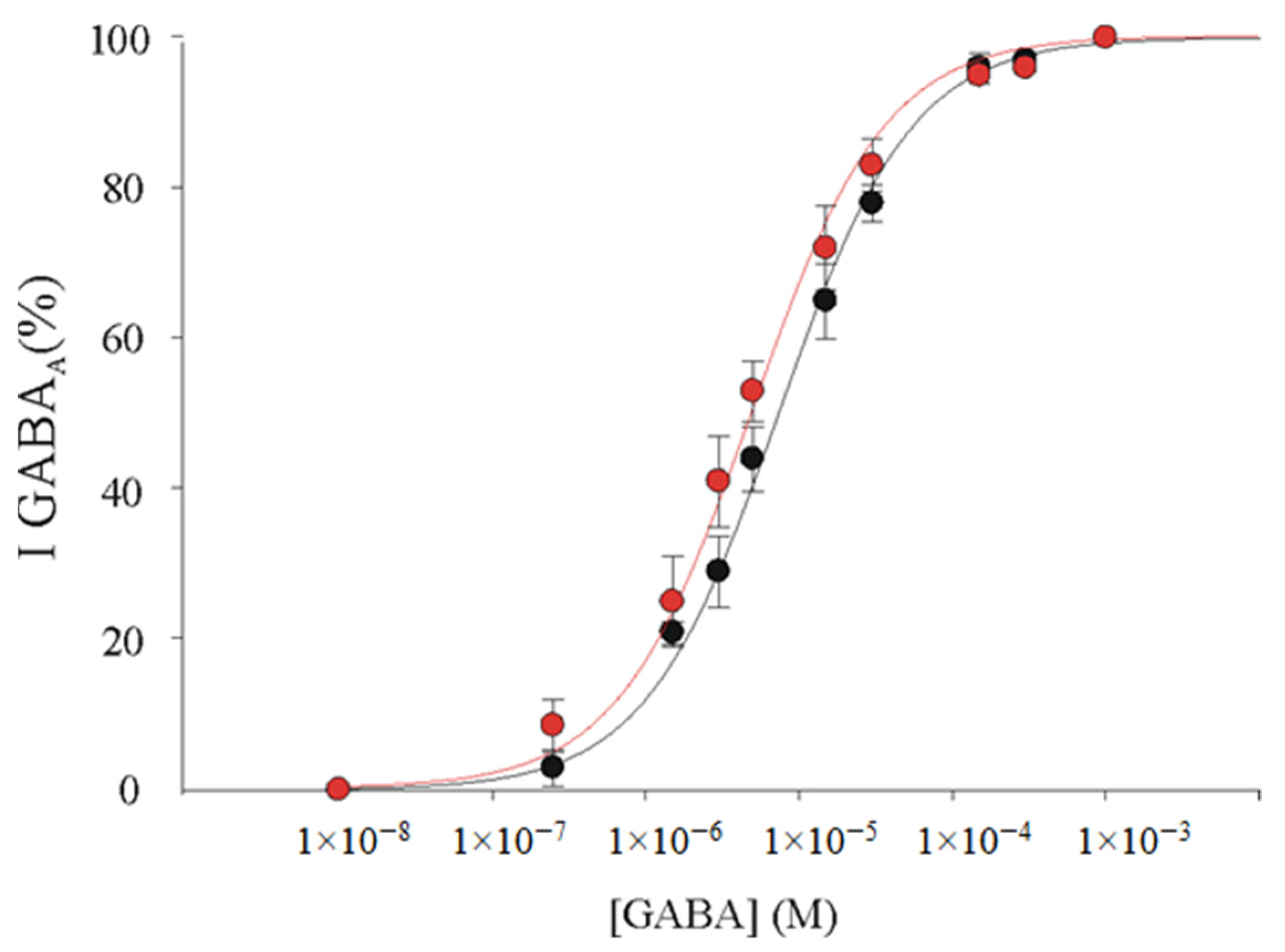
3.4. α1β2γ2 and α1β2 GABAARs Display Similar GABA Sensitivity in Xenopus Oocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sigel, E.; Steinmann, M.E. Structure, Function, and Modulation of GABAA Receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef]
- Ragozzino, D.; Palma, E.; Di Angelantonio, S.; Amici, M.; Mascia, A.; Arcella, A.; Giangaspero, F.; Cantore, G.; Di Gennaro, G.; Manfredi, M.; et al. Rundown of GABA Type A Receptors Is a Dysfunction Associated with Human Drug-Resistant Mesial Temporal Lobe Epilepsy. Proc. Natl. Acad. Sci. USA 2005, 102, 15219–15223. [Google Scholar] [CrossRef] [PubMed]
- Soukupová, M.; Binaschi, A.; Falcicchia, C.; Zucchini, S.; Roncon, P.; Palma, E.; Magri, E.; Grandi, E.; Simonato, M. Impairment of GABA Release in the Hippocampus at the Time of the First Spontaneous Seizure in the Pilocarpine Model of Temporal Lobe Epilepsy. Exp. Neurol. 2014, 257, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, G.; Cifelli, P.; Miranda-Lourenço, C.; De Felice, E.; Limatola, C.; Sebastião, A.M.; Diógenes, M.J.; Aronica, E.; Palma, E. Rare Diseases of Neurodevelopment: Maintain the Mystery or Use a Dazzling Tool for Investigation? The Case of Rett Syndrome. Neuroscience 2020, 439, 146–152. [Google Scholar] [CrossRef]
- Ruffolo, G.; Iyer, A.; Cifelli, P.; Roseti, C.; Mühlebner, A.; van Scheppingen, J.; Scholl, T.; Hainfellner, J.A.; Feucht, M.; Krsek, P.; et al. Functional aspects of early brain development are preserved in tuberous sclerosis complex (TSC) epileptogenic lesions. Neurobiol. Dis. 2016, 95, 93–101. [Google Scholar] [CrossRef]
- Talos, D.M.; Sun, H.; Kosaras, B.; Joseph, A.; Folkerth, R.D.; Poduri, A.; Madsen, J.R.; Black, P.M.; Jensen, F.E. Altered Inhibition in Tuberous Sclerosis and Type IIb Cortical Dysplasia. Ann. Neurol. 2012, 71, 539–551. [Google Scholar] [CrossRef]
- Chuang, S.H.; Reddy, D.S. Genetic and Molecular Regulation of Extrasynaptic GABA-A Receptors in the Brain: Therapeutic Insights for Epilepsy. J. Pharmacol. Exp. Ther. 2018, 364, 180–197. [Google Scholar] [CrossRef] [PubMed]
- Mazzuferi, M.; Palma, E.; Martinello, K.; Maiolino, F.; Roseti, C.; Fucile, S.; Fabene, P.F.; Schio, F.; Pellitteri, M.; Sperk, G.; et al. Enhancement of GABA(A)-current run-down in the hippocampus occurs at the first spontaneous seizure in a model of temporal lobe epilepsy. Proc. Natl. Acad. Sci. USA 2010, 107, 3180–3185. [Google Scholar] [CrossRef]
- Ruffolo, G.; Cifelli, P.; Roseti, C.; Thom, M.; van Vliet, E.A.; Limatola, C.; Aronica, E.; Palma, E. A novel GABAergic dysfunction in human Dravet syndrome. Epilepsia 2018, 59, 2106–2117. [Google Scholar] [CrossRef]
- Hernandez, C.C.; Macdonald, R.L. A structural look at GABAA receptor mutations linked to epilepsy syndromes. Brain Res. 2019, 1714, 234–247. [Google Scholar] [CrossRef]
- Farrant, M.; Nusser, Z. Variations on an inhibitory theme: Phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 2005, 6, 215–229. [Google Scholar] [CrossRef]
- Palma, E.; Roseti, C.; Maiolino, F.; Fucile, S.; Martinello, K.; Mazzuferi, M.; Aronica, E.; Manfredi, M.; Esposito, V.; Cantore, G.; et al. GABA(A)-current rundown of temporal lobe epilepsy is associated with repetitive activation of GABA(A) “phasic” receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 20944–20948. [Google Scholar] [CrossRef]
- Palma, E.; Ruffolo, G.; Cifelli, P.; Roseti, C.; van Vliet, E.A.; Aronica, E. Modulation of GABAA Receptors in the Treatment of Epilepsy. Curr. Pharm. Des. 2017, 23, 5563–5568. [Google Scholar] [CrossRef]
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef]
- Kwan, P.; Schachter, S.C.; Brodie, M.J. Drug-resistant epilepsy. N. Engl. J. Med. 2011, 365, 919–926. [Google Scholar] [CrossRef]
- Palma, E.; Esposito, V.; Mileo, A.M.; Di Gennaro, G.; Quarato, P.; Giangaspero, F.; Scoppetta, C.; Onorati, P.; Trettel, F.; Miledi, R.; et al. Expression of human epileptic temporal lobe neurotransmitter receptors in Xenopus oocytes: An innovative approach to study epilepsy. Proc. Natl. Acad. Sci. USA 2002, 99, 15078–15083. [Google Scholar] [CrossRef]
- Cifelli, P.; Palma, E.; Roseti, C.; Verlengia, G.; Simonato, M. Changes in the sensitivity of GABAA current rundown to drug treatments in a model of temporal lobe epilepsy. Front. Cell. Neurosci. 2013, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Ragozzino, D.A.; Di Angelantonio, S.; Spinelli, G.; Trettel, F.; Martinez-Torres, A.; Torchia, G.; Arcella, A.; Di Gennaro, G.; Quarato, P.P.; et al. Phosphatase inhibitors remove the run-down of gamma-aminobutyric acid type A receptors in the human epileptic brain. Proc. Natl. Acad. Sci. USA 2004, 101, 10183–10188. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, A.; Labate, A.; Cifelli, P.; Ruffolo, G.; Mumoli, L.; Aronica, E.; Palma, E. Pharmacological Modulation in Mesial Temporal Lobe Epilepsy: Current Status and Future Perspectives. Pharmacol. Res 2016, 113, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, G.; Di Bonaventura, C.; Cifelli, P.; Roseti, C.; Fattouch, J.; Morano, A.; Limatola, C.; Aronica, E.; Palma, E.; Giallonardo, A.T. A novel action of lacosamide on GABAA currents sets the ground for a synergic interaction with levetiracetam in treatment of epilepsy. Neurobiol. Dis. 2018, 115, 59–68. [Google Scholar] [CrossRef]
- Palma, E.; Ragozzino, D.; Di Angelantonio, S.; Mascia, A.; Maiolino, F.; Manfredi, M.; Cantore, G.; Esposito, V.; Di Gennaro, G.; Quarato, P.; et al. The antiepileptic drug Levetiracetam stabilizes the human epileptic GABAA receptors upon repetitive activation. Epilepsia 2007, 48, 1842–1849. [Google Scholar] [CrossRef]
- Roseti, C.; Fucile, S.; Lauro, C.; Martinello, K.; Bertollini, C.; Esposito, V.; Mascia, A.; Catalano, M.; Aronica, E.; Limatola, C.; et al. Fractalkine/CX3CL1 modulates GABAA currents in human temporal lobe epilepsy. Epilepsia 2013, 54, 1834–1844. [Google Scholar] [CrossRef]
- Roseti, C.; Cifelli, P.; Ruffolo, G.; Barbieri, E.; Guescini, M.; Esposito, V.; Di Gennaro, G.; Limatola, C.; Giovannelli, A.; Aronica, E.; et al. Erythropoietin Increases GABAA Currents in Human Cortex from TLE Patients. Neuroscience 2019, 439, 153–162. [Google Scholar] [CrossRef]
- Morano, A.; Fanella, M.; Albini, M.; Cifelli, P.; Palma, E.; Giallonardo, A.T.; Di Bonaventura, C. Cannabinoids in the Treatment of Epilepsy: Current Status and Future Prospects. Neuropsychiatr. Dis. Treat. 2020, 16, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Morano, A.; Cifelli, P.; Nencini, P.; Antonilli, L.; Fattouch, J.; Ruffolo, G.; Roseti, C.; Aronica, E.; Limatola, C.; Di Bonaventura, C.; et al. Cannabis in epilepsy: From clinical practice to basic research focusing on the possible role of cannabidivarin. Epilepsia Open 2016, 1, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Cifelli, P.; Ruffolo, G.; De Felice, E.; Alfano, V.; van Vliet, E.A.; Aronica, E.; Palma, E. Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission? Int. J. Mol. Sci. 2020, 21, 723. [Google Scholar] [CrossRef]
- Li, G.; Yang, K.; Zheng, C.; Liu, Q.; Chang, Y.; Kerrigan, J.F.; Wu, J. Functional rundown of gamma-aminobutyric acid(A) receptors in human hypothalamic hamartomas. Ann. Neurol. 2011, 69, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Ruffolo, G.; Martinello, K.; Labate, A.; Cifelli, P.; Fucile, S.; Di Gennaro, G.; Quattrone, A.; Esposito, V.; Limatola, C.; Giangaspero, F.; et al. Modulation of GABAergic Dysfunction Due to SCN1A Mutation Linked to Hippocampal Sclerosis. Ann Clin. Transl. Neurol. 2020, 7, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, S.; Piccioni, A.; Moriconi, C.; Trettel, F.; Cristalli, G.; Grassi, F.; Limatola, C. Adenosine A2A Receptor Induces Protein Kinase A-Dependent Functional Modulation of Human (Alpha)3(Beta)4 Nicotinic Receptor. J. Physiol. 2011, 589, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef]
- Palma, E.; Torchia, G.; Limatola, C.; Trettel, F.; Arcella, A.; Cantore, G.; Di Gennaro, G.; Manfredi, M.; Esposito, V.; Quarato, P.P.; et al. BDNF Modulates GABAA Receptors Microtransplanted from the Human Epileptic Brain to Xenopus Oocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 1667–1672. [Google Scholar] [CrossRef]
- Connolly, C.J.; Kittler, J.T.; Thomas, P.; Uren, J.M.; Brandon, N.J.; Smart, T.G.; Moss, S.J. Cell surface stability of gamma-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J. Biol. Chem. 1999, 274, 36565–36572. [Google Scholar] [CrossRef] [PubMed]
- Kittler, J.T.; Moss, S.J. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: Implications for the efficacy of synaptic inhibition. Curr. Opin. Neurobiol. 2003, 13, 341–347. [Google Scholar] [CrossRef]
- Sigel, E.; Baur, R.; Boulineau, N.; Minier, F. Impact of Subunit Positioning on GABAA Receptor Function. Biochem. Soc. Trans. 2006, 34, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.C.; Moss, S.J.; Jurd, R. GABAA Receptor Trafficking and Its Role in the Dynamic Modulation of Neuronal Inhibition. Nat. Rev. Neurosci. 2008, 9, 331–343. [Google Scholar] [CrossRef]
- Brandon, N.J.; Delmas, P.; Kittler, J.T.; McDonald, B.J.; Sieghart, W.; Brown, D.A.; Smart, T.G.; Moss, S.J. GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J. Biol. Chem. 2000, 275, 38856–38862. [Google Scholar] [CrossRef]
- Brandon, N.J.; Jovanovic, J.N.; Colledge, M.; Kittler, J.T.; Brandon, J.M.; Scott, J.D.; Moss, S.J. A-Kinase Anchoring Protein 79/150 Facilitates the Phosphorylation of GABA(A) Receptors by CAMP-Dependent Protein Kinase via Selective Interaction with Receptor Beta Subunits. Mol. Cell. Neurosci. 2003, 22, 87–97. [Google Scholar] [CrossRef]
- Palma, E.; Trettel, F.; Fucile, S.; Renzi, M.; Miledi, R.; Eusebi, F. Microtransplantation of Membranes from Cultured Cells to Xenopus Oocytes: A Method to Study Neurotransmitter Receptors Embedded in Native Lipids. Proc. Natl. Acad. Sci. USA 2003, 100, 2896–2900. [Google Scholar] [CrossRef] [PubMed]
- Tsunashima, K.; Schwarzer, C.; Kirchmair, E.; Sieghart, W.; Sperk, G. GABA(A) Receptor Subunits in the Rat Hippocampus III: Altered Messenger RNA Expression in Kainic Acid-Induced Epilepsy. Neuroscience 1997, 80, 1019–1032. [Google Scholar] [CrossRef]
- Brooks-Kayal, A.R.; Shumate, M.D.; Jin, H.; Lin, D.D.; Rikhter, T.Y.; Holloway, K.L.; Coulter, D.A. Human neuronal gamma-aminobutyric acid(A) receptors: Coordinated subunit mRNA expression and functional correlates in individual dentate granule cells. J. Neurosci. 1997, 19, 8312–8318. [Google Scholar] [CrossRef]
- Grabenstatter, H.L.; Russek, S.J.; Brooks-Kayal, A.R. Molecular pathways controlling inhibitory receptor expression. Epilepsia 2012, 53 (Suppl. S9), 71–78. [Google Scholar] [CrossRef]
- Pirker, S.; Schwarzer, C.; Czech, T.; Baumgartner, C.; Pockberger, H.; Maier, H.; Hauer, B.; Sieghart, W.; Furtinger, S.; Sperk, G. Increased expression of GABA(A) receptor beta-subunits in the hippocampus of patients with temporal lobe epilepsy. J. Neuropathol. Exp. Neurol. 2003, 62, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Loup, F.; Wieser, H.G.; Yonekawa, Y.; Aguzzi, A.; Fritschy, J.M. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. J. Neurosci. 2000, 20, 5401–5419. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifelli, P.; Di Angelantonio, S.; Alfano, V.; Morano, A.; De Felice, E.; Aronica, E.; Ruffolo, G.; Palma, E. Dissecting the Molecular Determinants of GABAA Receptors Current Rundown, a Hallmark of Refractory Human Epilepsy. Brain Sci. 2021, 11, 441. https://doi.org/10.3390/brainsci11040441
Cifelli P, Di Angelantonio S, Alfano V, Morano A, De Felice E, Aronica E, Ruffolo G, Palma E. Dissecting the Molecular Determinants of GABAA Receptors Current Rundown, a Hallmark of Refractory Human Epilepsy. Brain Sciences. 2021; 11(4):441. https://doi.org/10.3390/brainsci11040441
Chicago/Turabian StyleCifelli, Pierangelo, Silvia Di Angelantonio, Veronica Alfano, Alessandra Morano, Eleonora De Felice, Eleonora Aronica, Gabriele Ruffolo, and Eleonora Palma. 2021. "Dissecting the Molecular Determinants of GABAA Receptors Current Rundown, a Hallmark of Refractory Human Epilepsy" Brain Sciences 11, no. 4: 441. https://doi.org/10.3390/brainsci11040441
APA StyleCifelli, P., Di Angelantonio, S., Alfano, V., Morano, A., De Felice, E., Aronica, E., Ruffolo, G., & Palma, E. (2021). Dissecting the Molecular Determinants of GABAA Receptors Current Rundown, a Hallmark of Refractory Human Epilepsy. Brain Sciences, 11(4), 441. https://doi.org/10.3390/brainsci11040441






