The Effects of 10 Hz and 20 Hz tACS in Network Integration and Segregation in Chronic Stroke: A Graph Theoretical fMRI Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
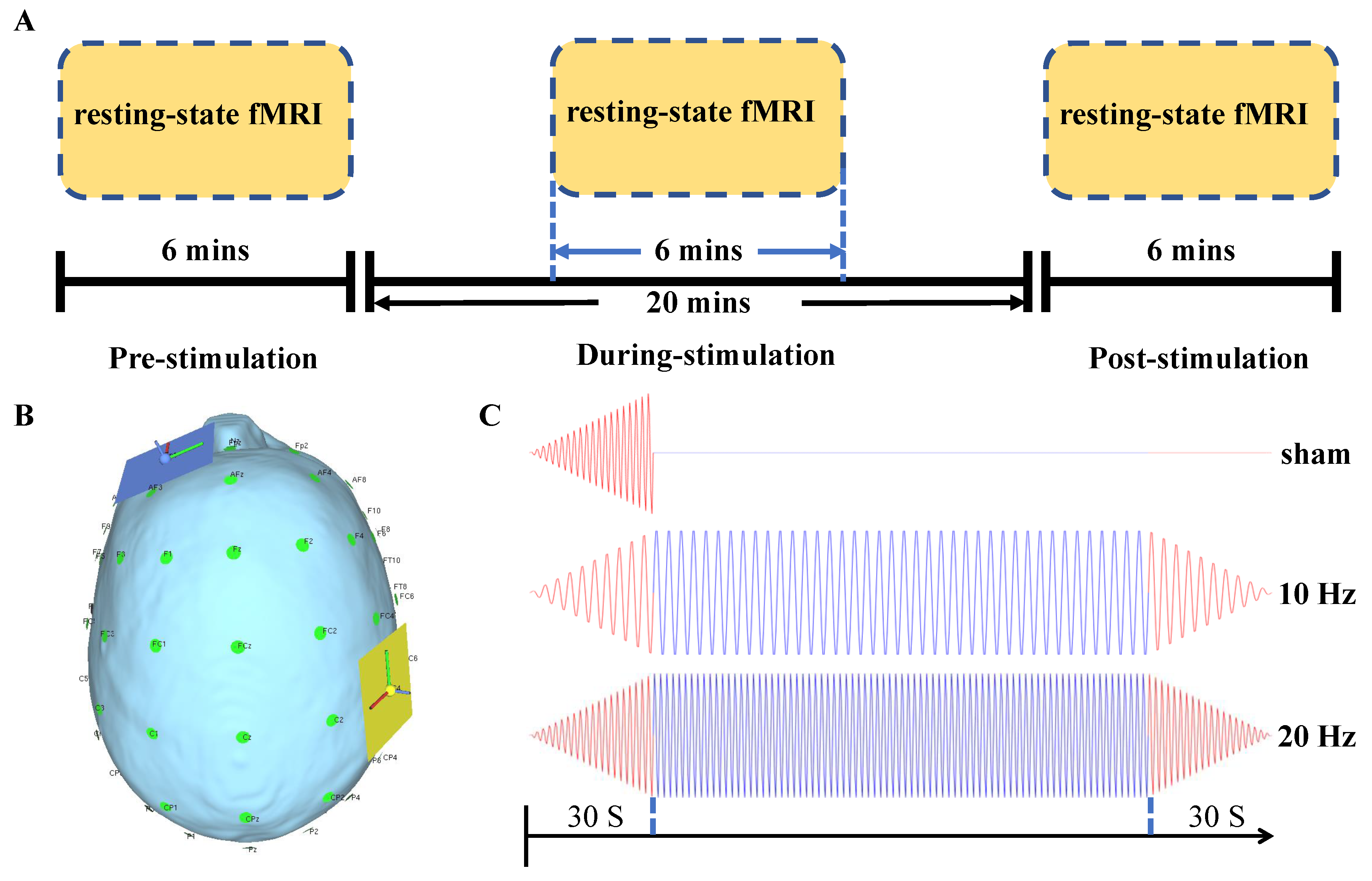
2.2. tACS Intervention
2.3. Image Acquisition and Preprocessing
2.4. Graph Theory Analysis
2.4.1. Construction of Brain Functional Networks
2.4.2. Graph Theoretical Measures
2.5. Statistical Analysis
3. Results
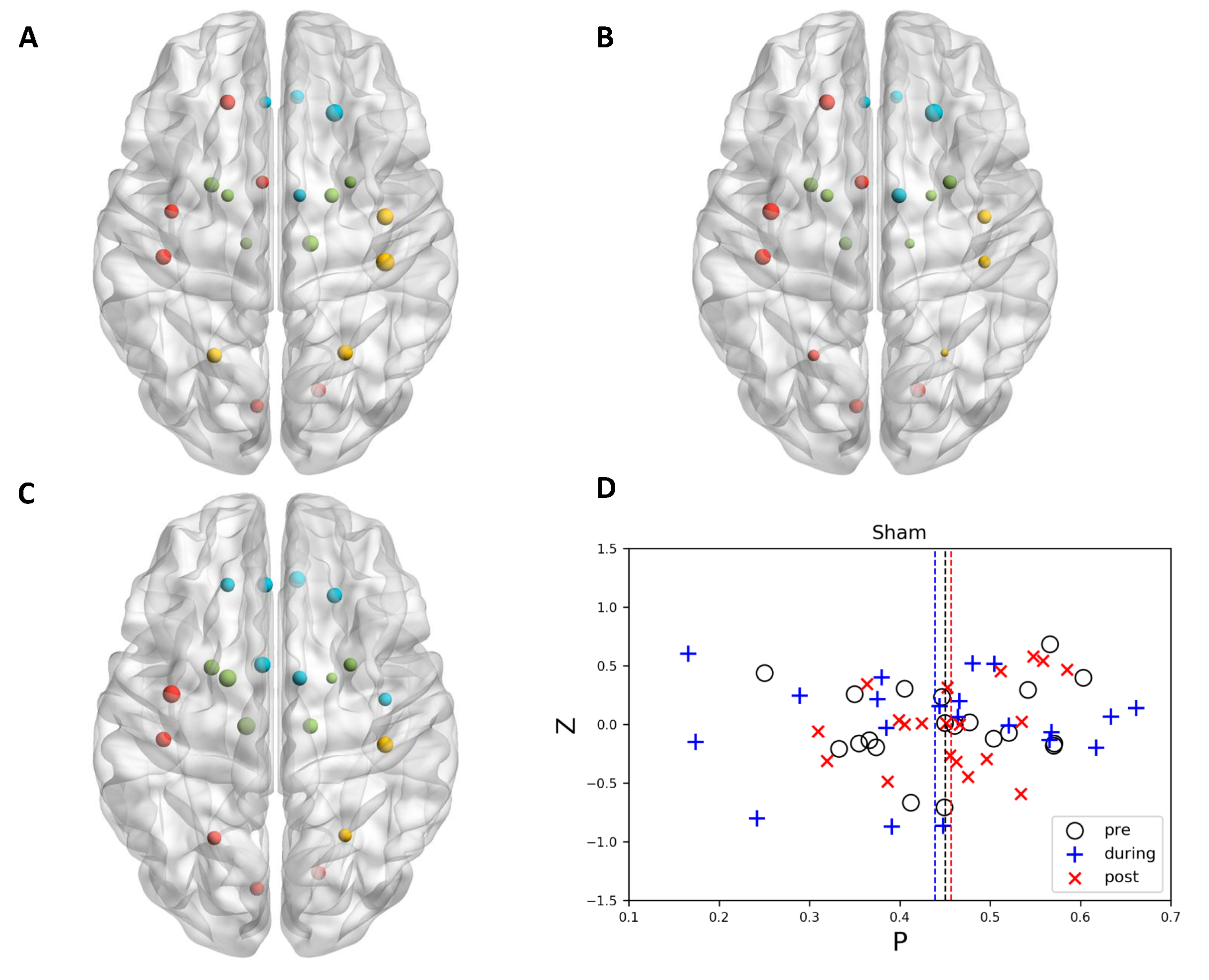
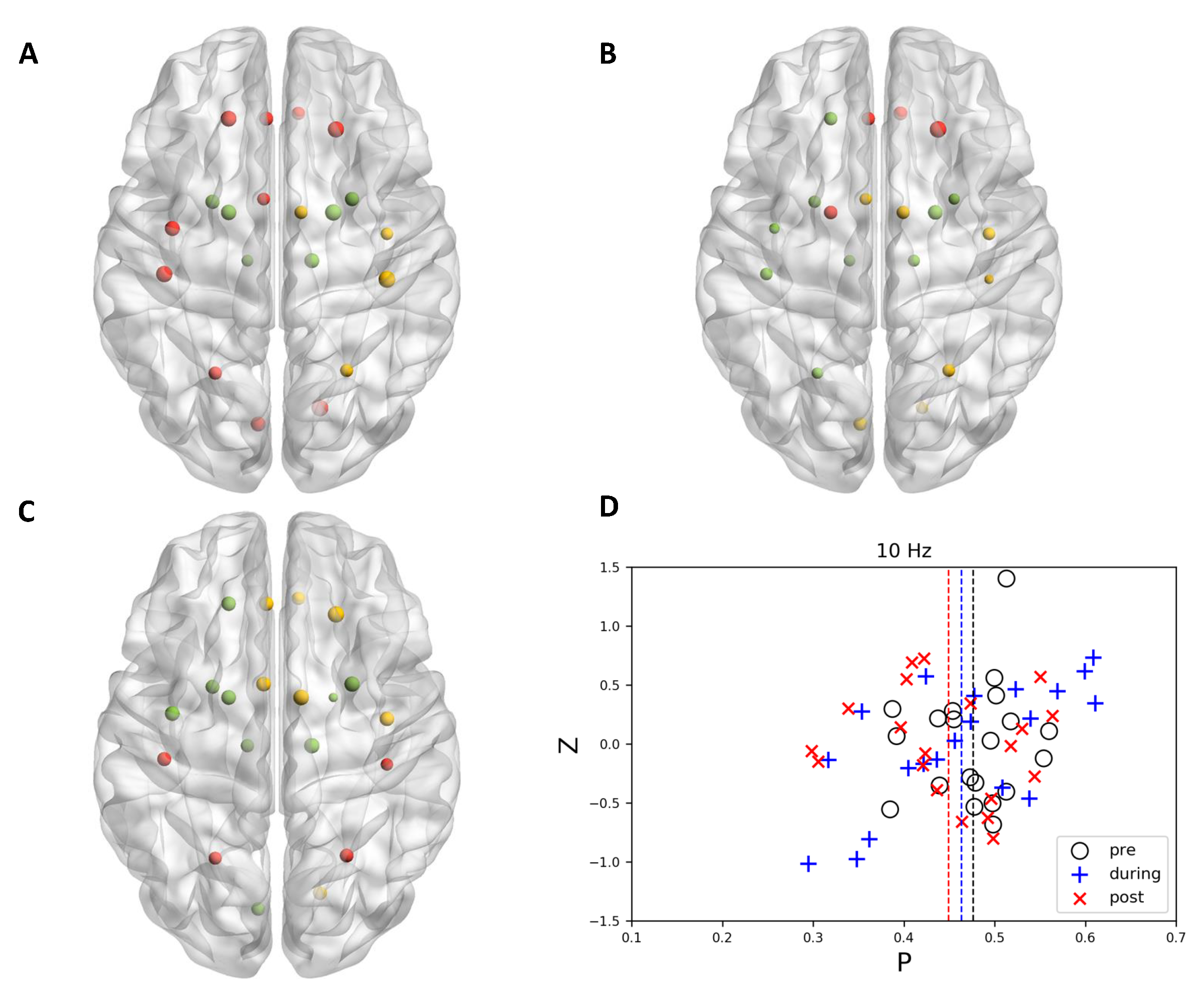
3.1. Community Structure
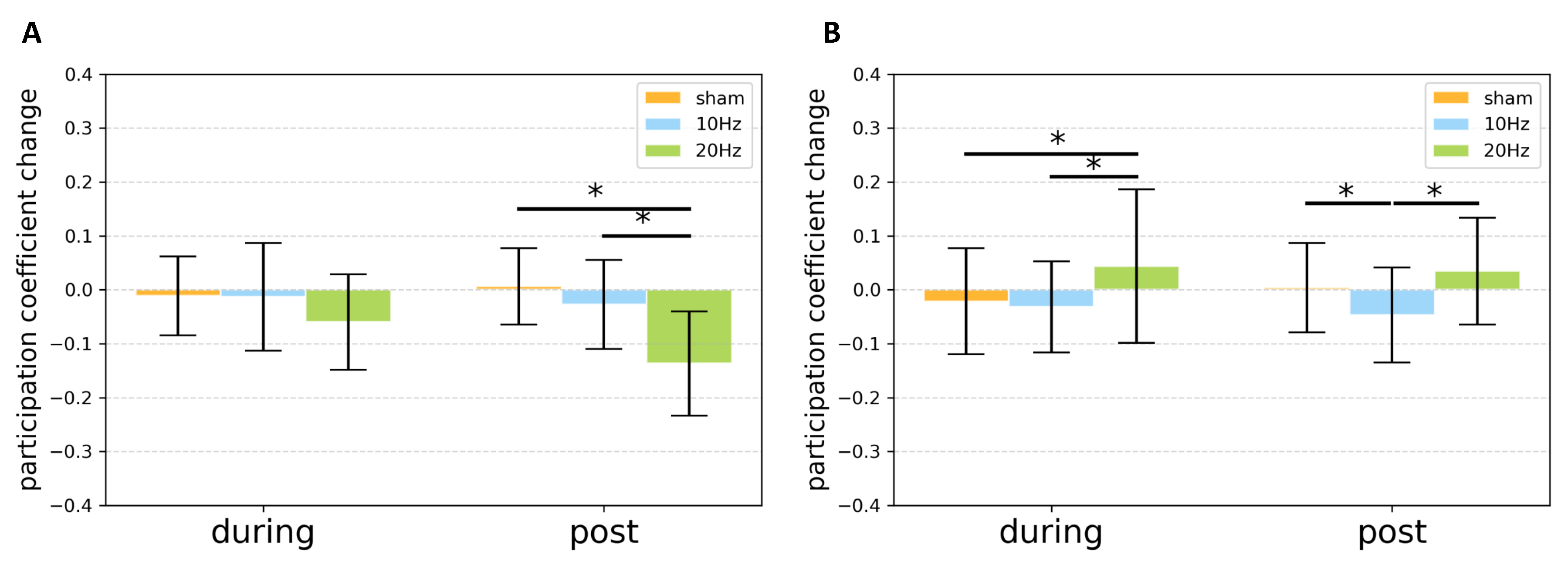
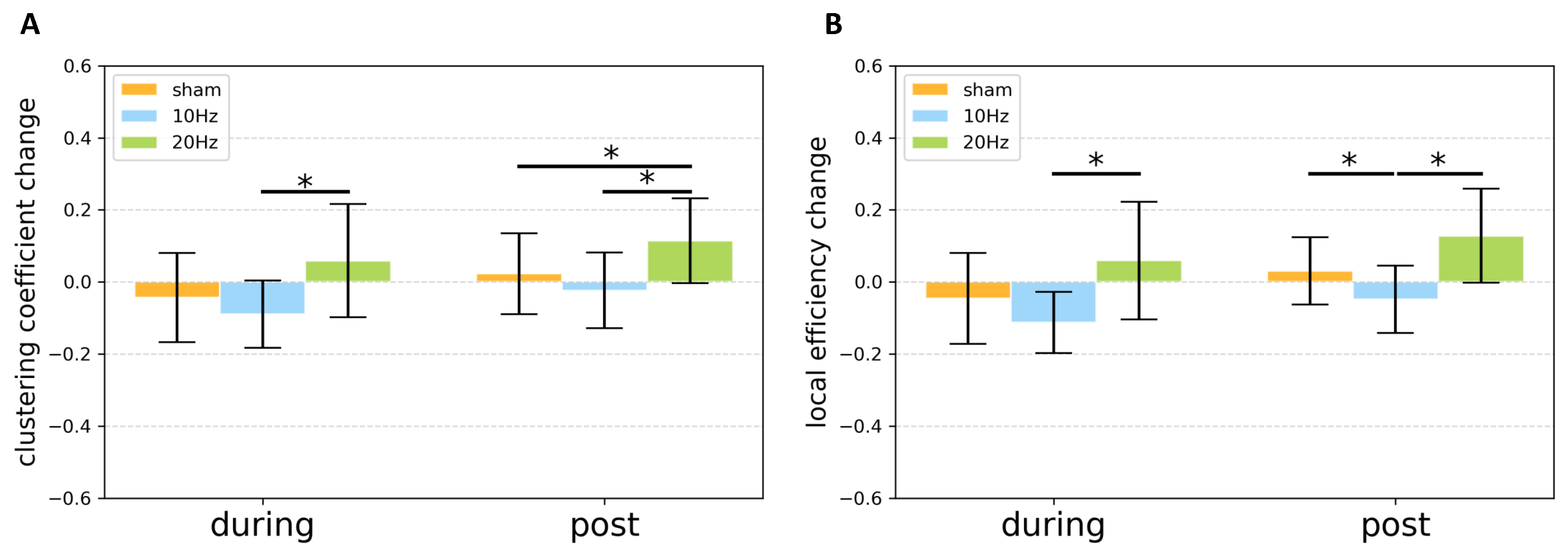
3.2. Graph Theoretically Nodal Measures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stinear, C.; Lang, C.; Zeiler, S.; Byblow, W. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020, 19. [Google Scholar] [CrossRef]
- Jäncke, L. The plastic human brain. Restor. Neurol. Neurosci. 2009, 27, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Norouzi-Gheidari, N.; Archambault, P.; Fung, J. Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: Systematic review and meta-analysis of the literature. J. Rehabil. Res. Dev. 2012, 49, 479–496. [Google Scholar] [CrossRef]
- Duret, C.; Grosmaire, A.G.; Krebs, H. Robot-Assisted Therapy in Upper Extremity Hemiparesis: Overview of an Evidence-Based Approach. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.C.; Khan, A.; Song, R.; Tong, R.K.Y. Rewiring the Lesioned Brain: Electrical Stimulation for Post-Stroke Motor Restoration. J. Stroke 2020, 22, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Purpura, D.; McMurtry, J. Intacellular activities and evoked potential changes during polarization of motor cortex. J. Neurophysiol. 1965, 28, 166–185. [Google Scholar] [CrossRef]
- Nitsche, M.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Thut, G.; Schyns, P.; Gross, J. Entrainment of Perceptually Relevant Brain Oscillations by Non-Invasive Rhythmic Stimulation of the Human Brain. Front. Psychol. 2011, 2, 170. [Google Scholar] [CrossRef]
- Schnitzler, A.; Gross, J. Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci. 2005, 6, 285–296. [Google Scholar] [CrossRef]
- Salmelin, R.; Hari, R. Characterization of spontaneous MEG rhythms in healthy adults. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 237–248. [Google Scholar] [CrossRef]
- Feurra, M.; Blagoveshchensky, E.; Nikulin, V.; Nazarova, M.; Lebedeva, A.; Pozdeeva, D.; Yurevich, M.; Rossi, S. State-Dependent Effects of Transcranial Oscillatory Currents on the Motor System during Action Observation. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Pollok, B.; Boysen, A.C.; Krause, V. The effect of transcranial alternating current stimulation (tACS) at alpha and beta frequency on motor learning. Behav. Brain Res. 2015, 293. [Google Scholar] [CrossRef]
- Meier, A.; Krause, V.; Pollok, B. Early motor memory consolidation: Effects of 10 Hz and 20 Hz transcranial alternating current stimulation (tACS) over the left primary motor cortex (M1). Klin. Neurophysiol. 2014, 45. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Ko, M.H.; Ahn, S.; Kim, Y.H.; Song, J.; Lee, C.H.; Chang, M.; Jang, S. Primary motor cortex activation by transcranial direct current stimulation in the human brain. Neurosci. Lett. 2008, 435, 56–59. [Google Scholar] [CrossRef]
- Ali, M.; Sellers, K.; Frohlich, F. Transcranial Alternating Current Stimulation Modulates Large-Scale Cortical Network Activity by Network Resonance. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 11262–11275. [Google Scholar] [CrossRef]
- Kimberley, T.; Khandekar, G.; Borich, M. fMRI reliability in subjects with stroke. Exp. Brain Res. 2008, 186, 183–190. [Google Scholar] [CrossRef]
- Van den Heuvel, M.P.; Hulshoff Pol, H.E. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Farahani, F.; Karwowski, W.; Lighthall, N. Application of Graph Theory for Identifying Connectivity Patterns in Human Brain Networks: A Systematic Review. Front. Neurosci. 2019, 13, 585. [Google Scholar] [CrossRef] [PubMed]
- Solomons, C. A Review of Transcranial Electrical Stimulation Methods in Stroke Rehabilitation. Neurol. India 2019, 67, 417. [Google Scholar] [CrossRef] [PubMed]
- Wach, C.; Krause, V.; Moliadze, V.; Paulus, W.; Schnitzler, A.; Pollok, B. Effects of 10 Hz and 20 Hz transcranial alternating current stimulation (tACS) on motor functions and motor cortical excitability. Behav. Brain Res. 2012. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Dong, G.; Zhang, Z.; Chen, R. Effects of Transcranial Electrical Stimulation on Human Auditory Processing and Behavior—A Review. Brain Sci. 2020, 10, 531. [Google Scholar] [CrossRef]
- Thielscher, A.; Antunes, A.; Saturnino, G. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; Volume 2015, pp. 222–225. [Google Scholar] [CrossRef]
- Yan, C.G.; Zang, Y.F. DPARSF: A MatLab toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef]
- Friston, K.; Williams, S.; Howard, R.; Frackowiak, R.; Turner, R. Movement-Related effects in fMRI time-series. Magn. Reson. Med. 1996, 35, 346–355. [Google Scholar] [CrossRef]
- Power, J.; Barnes, K.; Snyder, A.; Schlaggar, B.; Petersen, S. Spurious but systematic conditions in functional connectivity MRI networks arise from subject motion. Neuroimage 2012, 59, 2141–2154. [Google Scholar] [CrossRef]
- Zuo, X.N.; Di Martino, A.; Kelly, C.; Shehzad, Z.; Gee, D.; Klein, D.; Castellanos, F.; Biswal, B.; Milham, M. The Oscillating Brain: Complex and Reliable. NeuroImage 2009, 49, 1432–1445. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Bear, M.; Connors, B.; Paradiso, M. Neuroscience: Exploring the Brain, 4th ed.; Jones & Bartlett Learning, LLC: Sudbury, MA, USA, 2015; pp. 1–975. [Google Scholar]
- Cambridge, U.; Cohen, P. Applied Multiple Regression/Correlation Analysis for The Behavioral Sciences. Am. J. Cardiol. 1983, 51, 187. [Google Scholar]
- Fornito, A.; Zalesky, A.; Bullmore, E. Network Scaling Effects in Graph Analytic Studies of Human Resting-State fMRI Data. Front. Syst. Neurosci. 2010, 4, 22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garrison, K.; Scheinost, D.; Finn, E.; Shen, X.; Constable, R. The (in)stability of functional brain network measures across thresholds. NeuroImage 2015, 118. [Google Scholar] [CrossRef] [PubMed]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Newman, M. Fast algorithm for detecting community structure in networks. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2004, 69, 066133. [Google Scholar] [CrossRef] [PubMed]
- Guimerà, R.; Amaral, L. Functional Cartography of Complex Metabolic Networks. Nature 2005, 23, 22–231. [Google Scholar] [CrossRef] [PubMed]
- Guimerà, R.; Amaral, L. Cartography of complex networks: Modules and universal roles. J. Stat. Mech. 2005, 2005, nihpa35573. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.; Strogatz, S. Collective dynamics of ’small-world’ networks. Nature 2011. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient Behavior of Small-World Networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A Network Visualization Tool for Human Brain Connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Kahana, M.; Ekstrom, A.; Fried, I. Brain Oscillations Control Timing of Single-Neuron Activity in Humans. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 3839–3844. [Google Scholar] [CrossRef]
- Fries, P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci. 2001, 9, 356–362. [Google Scholar] [CrossRef]
- Srinivasan, R.; Winter, W.; Nunez, P. Source analysis of EEG oscillations using high-resolution EEG and MEG. Prog. Brain Res. 2006, 159, 29–42. [Google Scholar] [CrossRef]
- Voytek, B.; Canolty, R.; Shestyuk, A.; Crone, N.; Parvizi, J.; Knight, R. Shifts in Gamma Phase–Amplitude Coupling Frequency from Theta to Alpha Over Posterior Cortex During Visual Tasks. Front. Hum. Neurosci. 2010, 4, 191. [Google Scholar] [CrossRef]
- Jensen, O.; Mazaheri, A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef]
- Honaga, E.; Ishii, R.; Kurimoto, R.; Ikezawa, K.; Takahashi, H.; Nakahachi, T.; Iwase, M.; Mizuta, I.; Yoshimine, T.; Takeda, M. Post-movement beta rebound abnormality as indicator of mirror neuron system dysfunction in autistic spectrum disorder: An MEG study. Neurosci. Lett. 2010, 478, 141–145. [Google Scholar] [CrossRef]
- Geerligs, L.; Renken, R.; Saliasi, E.; Maurits, N.; Lorist, M. A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cereb. Cortex 2014, 25. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Stancak, A.; Neuper, C. Post-movement beta synchronization. A correlate of an idle motor area? Electroencephalogr. Clin. Neurophysiol. 1996, 98, 281–293. [Google Scholar] [CrossRef]
- Baker, S. Oscillatory interactions between sensorimotor cortex and the periphery. Curr. Opin. Neurobiol. 2008, 17, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Rosanova, M.; Casali, A.; Bellina, V.; Resta, F.; Mariotti, M.; Massimini, M. Natural Frequencies of Human Corticothalamic Circuits. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 7679–7685. [Google Scholar] [CrossRef]
- Kopell, N.; Ermentrout, B.; Whittington, M.; Traub, R. Gamma rhythms and beta rhythms have different synchronization properties. Proc. Natl. Acad. Sci. USA 2000, 97, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Haegens, S.; Barczak, A.; Musacchia, G.; Lipton, M.; Mehta, A.; Lakatos, P.; Schroeder, C. Laminar Profile and Physiology of the Rhythm in Primary Visual, Auditory, and Somatosensory Regions of Neocortex. J. Neurosci. 2015, 35, 14341–14352. [Google Scholar] [CrossRef] [PubMed]
- Halgren, M.; Ulbert, I.; Bastuji, H.; Fabó, D.; Eross, L.; Rey, M.; Devinsky, O.; Doyle, W.; Mak-McCully, R.; Halgren, E.; et al. The generation and propagation of the human alpha rhythm. Proc. Natl. Acad. Sci. USA 2019, 116, 201913092. [Google Scholar] [CrossRef] [PubMed]
- Groppe, D.; Bickel, S.; Keller, C.; Jain, S.; Hwang, S.; Harden, C.; Mehta, A. Dominant frequencies of resting human brain activity as measured by the electrocorticogram. NeuroImage 2013, 79. [Google Scholar] [CrossRef]
- Bibbig, A.; Traub, R.; Whittington, M. Long-range synchronization of γ and β oscillations and the plasticity of excitatory and inhibitory synapses: A network model. J. Neurophysiol. 2002, 88, 1634–1654. [Google Scholar] [CrossRef]
- Cohen, J.; D’Esposito, M. The Segregation and Integration of Distinct Brain Networks and Their Relationship to Cognition. J. Neurosci. 2016, 36, 12083–12094. [Google Scholar] [CrossRef]
- Feurra, M.; Pasqualetti, P.; Bianco, G.; Santarnecchi, E.; Rossi, A.; Rossi, S. State-Dependent Effects of Transcranial Oscillatory Currents on the Motor System: What You Think Matters. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 17483–17489. [Google Scholar] [CrossRef] [PubMed]
- Heise, K.F.; Kortzorg, N.; Saturnino, G.; Fujiyama, H.; Cuypers, K.; Thielscher, A.; Swinnen, S. Evaluation of a Modified High-Definition Electrode Montage for Transcranial Alternating Current Stimulation (tACS) of Pre-Central Areas. Brain Stimul. 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Naros, G.; Gharabaghi, A. Physiological and behavioral effects of β-tACS on brain self-regulation in chronic stroke. Brain Stimul. 2017, 10, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Lord, L.D.; Stevner, A.B.; Deco, G.; Kringelbach, M.L. Understanding principles of integration and segregation using whole-brain computational connectomics: Implications for neuropsychiatric disorders. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160283. [Google Scholar] [CrossRef]
- Hummel, F.; Voller, B.; Celnik, P.; Floel, A.; Giraux, P.; Gerloff, C.; Cohen, L. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci. 2006, 7, 73. [Google Scholar] [CrossRef]
- Stagg, C.; Bachtiar, V.; O’Shea, J.; Allman, C.; Bosnell, R.; Kischka, U.; Matthews, P.; Johansen-Berg, H. Cortical activation changes underlying stimulation induced behavioral gains in chronic stroke. Brain J. Neurol. 2011, 135, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Laloux, P.; Peeters, A.; Desfontaines, P.; Jamart, J.; Vandermeeren, Y. Dual-tDCS Enhances Online Motor Skill Learning and Long-Term Retention in Chronic Stroke Patients. Front. Hum. Neurosci. 2013. [Google Scholar] [CrossRef]
- Lefebvre, S.; Thonnard, J.L.; Laloux, P.; Peeters, A.; Jamart, J.; Vandermeeren, Y. Single Session of Dual-tDCS Transiently Improves Precision Grip and Dexterity of the Paretic Hand After Stroke. Neurorehabilit. Neural Repair 2013, 28, 100–110. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Yuan, K.; Chu, W.C.-w.; Tong, R.K.-y. The Effects of 10 Hz and 20 Hz tACS in Network Integration and Segregation in Chronic Stroke: A Graph Theoretical fMRI Study. Brain Sci. 2021, 11, 377. https://doi.org/10.3390/brainsci11030377
Chen C, Yuan K, Chu WC-w, Tong RK-y. The Effects of 10 Hz and 20 Hz tACS in Network Integration and Segregation in Chronic Stroke: A Graph Theoretical fMRI Study. Brain Sciences. 2021; 11(3):377. https://doi.org/10.3390/brainsci11030377
Chicago/Turabian StyleChen, Cheng, Kai Yuan, Winnie Chiu-wing Chu, and Raymond Kai-yu Tong. 2021. "The Effects of 10 Hz and 20 Hz tACS in Network Integration and Segregation in Chronic Stroke: A Graph Theoretical fMRI Study" Brain Sciences 11, no. 3: 377. https://doi.org/10.3390/brainsci11030377
APA StyleChen, C., Yuan, K., Chu, W. C.-w., & Tong, R. K.-y. (2021). The Effects of 10 Hz and 20 Hz tACS in Network Integration and Segregation in Chronic Stroke: A Graph Theoretical fMRI Study. Brain Sciences, 11(3), 377. https://doi.org/10.3390/brainsci11030377






