Spatiotemporal Characteristics of Neural Dynamics in Theta Oscillations Related to the Inhibition of Habitual Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
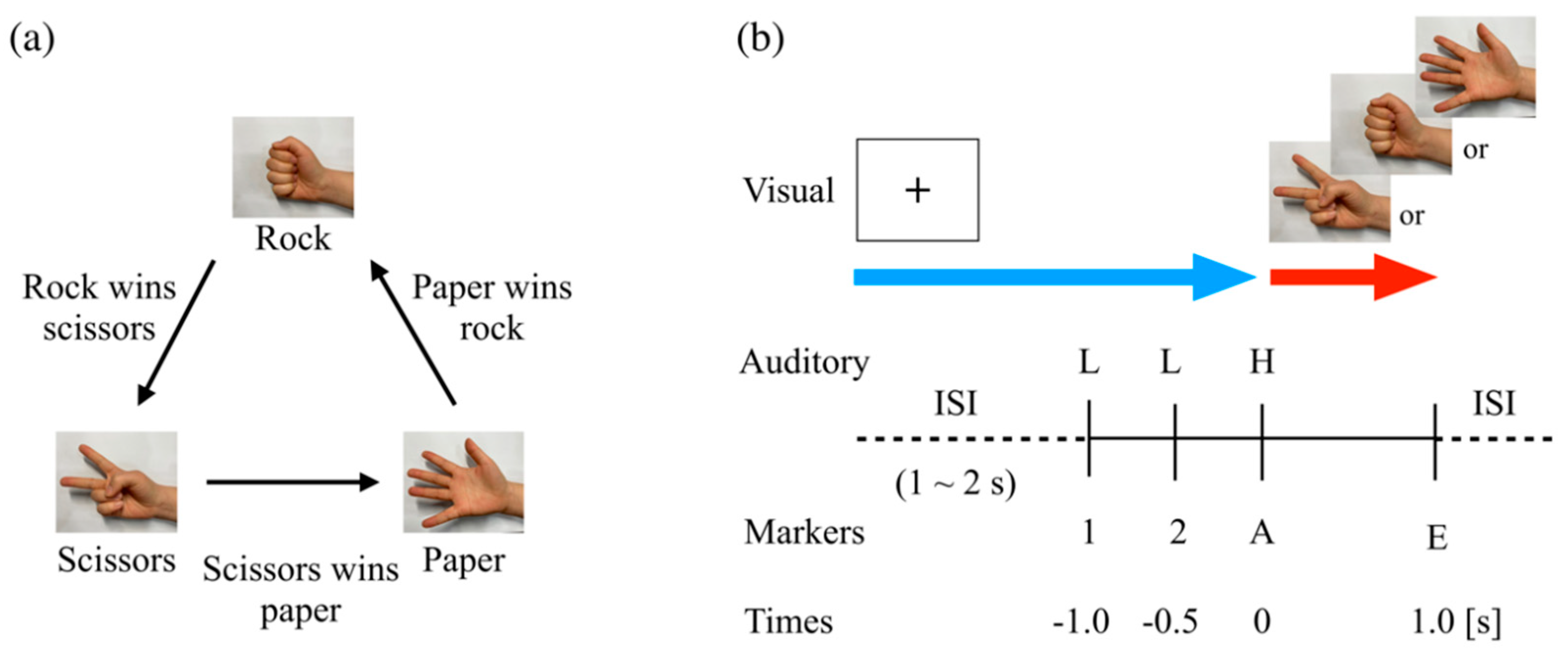
2.2. Rock–Paper–Scissors Task
2.3. EEG/EMG Recordings
2.4. Response Time and Accuracy Rate Analysis
2.5. EEG Preprocessing
2.6. EEG Spectral Analysis
2.7. EEG Phase Synchrony
2.8. Statistical Evaluation
3. Results
3.1. Response Times and Success Rates
3.2. Overall Spectral Analysis
3.3. Spectral Analysis of the Induced Theta Oscillations
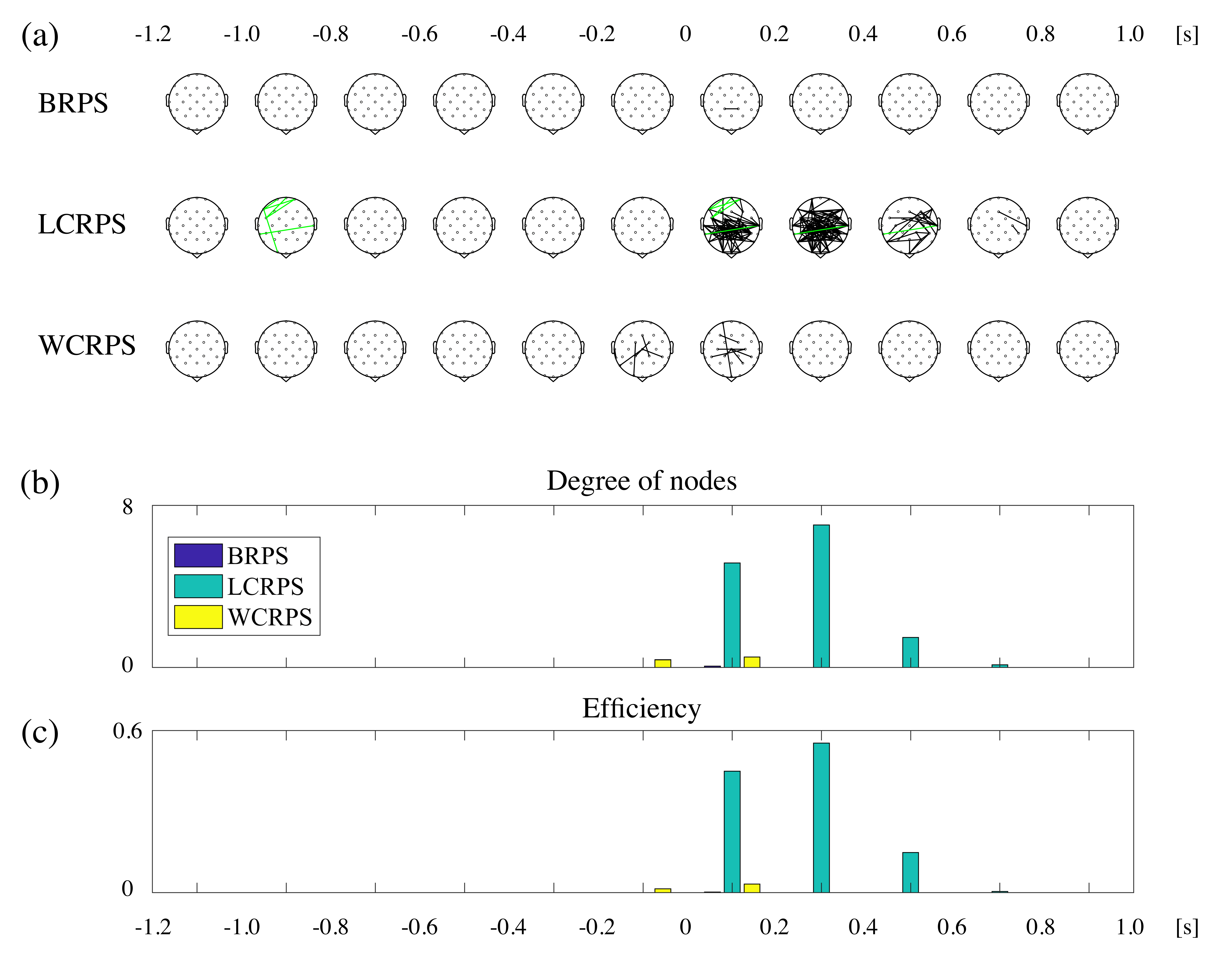
3.4. Phase Synchrony Analysis of the Theta Oscillations
4. Discussion
4.1. Temporal Variation in Frontal Theta Power Reflects a Stream of Cognitive Processes
4.2. Activation of Phase Synchronization Precedes Power Activity
4.3. The LCRPS Is More Difficult Than the WCRPS
4.4. Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dreisbach, G.; Goschke, T.; Haider, H. The role of task rules and stimulus–response mappings in the task switching paradigm. Psychol. Res. 2007, 71, 383–392. [Google Scholar] [CrossRef]
- Kiesel, A.; Steinhauser, M.; Wendt, M.; Falkenstein, M.; Jost, K.; Philipp, A.M.; Koch, I. Control and interference in task switching—A review. Psychol. Bull. 2010, 136, 849–874. [Google Scholar] [CrossRef]
- Sebanz, N.; Knoblich, G.; Prinz, W. How two share a task: Corepresenting stimulus-response mappings. J. Exp. Psychol. Hum. Percept. Perform. 2005, 31, 1234–1246. [Google Scholar] [CrossRef]
- Braver, T.S. The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn. Sci. 2012, 16, 106–113. [Google Scholar] [CrossRef]
- Yee, D.M.; Braver, T.S. Interactions of Motivation and Cognitive Control. Curr. Opin. Behav. Sci. 2018, 19, 83–90. [Google Scholar] [CrossRef]
- Bächtold, D.; Baumüller, M.; Brugger, P. Stimulus-response compatibility in representational space. Neuropsychologia 1998, 36, 731–735. [Google Scholar] [CrossRef]
- Botvinick, M.M.; Braver, T.S.; Barch, D.M.; Carter, C.S.; Cohen, J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar] [CrossRef]
- De Houwer, J. On the role of stimulus-response and stimulus-stimulus compatibility in the Stroop effect. Mem. Cogn. 2003, 31, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Hommel, B. Automatic stimulus-response translation in dual-task performance. J. Exp. Psychol. Hum. Percept. Perform. 1998, 24, 1368–1384. [Google Scholar] [CrossRef]
- Mayr, U.; Kliegl, R. Task-set switching and long-term memory retrieval. J. Exp. Psychol. Learn Mem. Cogn. 2000, 26, 1124–1140. [Google Scholar] [CrossRef]
- Rushworth, M.F.; Hadland, K.A.; Paus, T.; Sipila, P.K. Role of the human medial frontal cortex in task switching: A combined fMRI and TMS study. J. Neurophysiol. 2002, 87, 2577–2592. [Google Scholar] [CrossRef]
- Verbruggen, F.; Logan, G.D. Automatic and controlled response inhibition: Associative learning in the go/no-go and stop-signal paradigms. J. Exp. Psychol. Gen. 2008, 137, 649–672. [Google Scholar] [CrossRef]
- Zhang, H.; Kornblum, S. The effects of stimulus-response mapping and irrelevant stimulus-response and stimulus-stimulus overlap in four-choice Stroop tasks with single-carrier stimuli. J. Exp. Psychol. Hum. Percept. Perform. 1998, 24, 3–19. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Simon, J.R. Reactions toward the source of stimulation. J. Exp. Psychol. 1969, 81, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Donders, F.C. On the speed of mental processes. Acta Psychol. (Amst.) 1969, 30, 412–431. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Kadota, H.; Nakajima, Y.; Miyazaki, M.; Sekiguchi, H.; Kohno, Y.; Kansaku, K. Anterior prefrontal cortex activities during the inhibition of stereotyped responses in a neuropsychological rock-paper-scissors task. Neurosci. Lett. 2009, 453, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Yamaguchi, S.; Xu, J.; Kobayashi, S. Neural correlates for the suppression of habitual behavior: A functional MRI study. J. Cogn. Neurosci. 2004, 16, 944–954. [Google Scholar] [CrossRef]
- Paulus, M.P.; Feinstein, J.S.; Tapert, S.F.; Liu, T.T. Trend detection via temporal difference model predicts inferior prefrontal cortex activation during acquisition of advantageous action selection. Neuroimage 2004, 21, 733–743. [Google Scholar] [CrossRef]
- Sato, M.; Shoji, Y.; Morita, K.; Kato, Y.; Ishii, Y.; Nakano, S.; Uchimura, N. Comparison of changes in the oxygenated hemoglobin level during a ‘modified rock-paper-scissors task’ between healthy subjects and patients with schizophrenia. Psychiatry Clin. Neurosci. 2018, 72, 490–501. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Kikuchi, S.; Miwakeichi, F.; Matsumoto, K.; Nishida, M.; Ishiguro, M.; Watanabe, E.; Kato, S. Relation between Parametric Change of the Workload and Prefrontal Cortex Activity during a Modified Version of the ‘Rock, Paper, Scissors’ Task. Neuropsychobiology 2013, 68, 24–33. [Google Scholar] [CrossRef]
- Kadota, H.; Sekiguchi, H.; Takeuchi, S.; Miyazaki, M.; Kohno, Y.; Nakajima, Y. The role of the dorsolateral prefrontal cortex in the inhibition of stereotyped responses. Exp. Brain Res. 2010, 203, 593–600. [Google Scholar] [CrossRef]
- Paulus, M.P.; Feinstein, J.S.; Leland, D.; Simmons, A.N. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage 2005, 25, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.M.; Cohen, J.D.; Carter, C.S. Conflict monitoring and anterior cingulate cortex: An update. Trends Cogn. Sci. 2004, 8, 539–546. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; Ullsperger, M.; Crone, E.A.; Nieuwenhuis, S. The role of the medial frontal cortex in cognitive control. Science 2004, 306, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Tschentscher, N.; Biel, A.L. Be Prepared: Tune to FM-Theta for Cognitive Control. Trends Neurosci. 2019, 42, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Nigbur, R.; Ivanova, G.; Sturmer, B. Theta power as a marker for cognitive interference. Clin. Neurophysiol. 2011, 122, 2185–2194. [Google Scholar] [CrossRef]
- Nigbur, R.; Cohen, M.X.; Ridderinkhof, K.R.; Sturmer, B. Theta dynamics reveal domain-specific control over stimulus and response conflict. J. Cogn. Neurosci. 2012, 24, 1264–1274. [Google Scholar] [CrossRef]
- Muckschel, M.; Stock, A.K.; Dippel, G.; Chmielewski, W.; Beste, C. Interacting sources of interference during sensorimotor integration processes. Neuroimage 2016, 125, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Bruns, A. Fourier-, Hilbert- and wavelet-based signal analysis: Are they really different approaches? J. Neurosci. Methods 2004, 137, 321–332. [Google Scholar] [CrossRef]
- Lachaux, J.P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef]
- Rodriguez, E.; George, N.; Lachaux, J.P.; Martinerie, J.; Renault, B.; Varela, F.J. Perception’s shadow: Long-distance synchronization of human brain activity. Nature 1999, 397, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, L.T.; Peterson, M.A.; Kaszniak, A.W.; Allen, J.J.B. EEG phase synchrony differences across visual perception conditions may depend on recording and analysis methods. Clin. Neurophysiol. 2005, 116, 172–189. [Google Scholar] [CrossRef]
- Carter, C.S.; Braver, T.S.; Barch, D.M.; Botvinick, M.M.; Noll, D.; Cohen, J.D. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 1998, 280, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Kiehl, K.A.; Smith, A.M.; Hare, R.D.; Liddle, P.F. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biol. Psychiatry 2000, 48, 210–221. [Google Scholar] [CrossRef]
- Wang, C.M.; Ulbert, I.; Schomer, D.L.; Marinkovic, K.; Halgren, E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J. Neurosci. 2005, 25, 604–613. [Google Scholar] [CrossRef]
- Fell, J.; Axmacher, N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 2011, 12, 105–118. [Google Scholar] [CrossRef]
- Guitart-Masip, M.; Barnes, G.R.; Horner, A.; Bauer, M.; Dolan, R.J.; Duzel, E. Synchronization of medial temporal lobe and prefrontal rhythms in human decision making. J. Neurosci. 2013, 33, 442–451. [Google Scholar] [CrossRef]
- Rajan, A.; Siegel, S.N.; Liu, Y.; Bengson, J.; Mangun, G.R.; Ding, M. Theta Oscillations Index Frontal Decision-Making and Mediate Reciprocal Frontal-Parietal Interactions in Willed Attention. Cereb. Cortex 2019, 29, 2832–2843. [Google Scholar] [CrossRef]
- Hirvonen, J.; Monto, S.; Wang, S.H.; Palva, J.M.; Palva, S. Dynamic large-scale network synchronization from perception to action. Netw. Neurosci. 2018, 2, 442–463. [Google Scholar] [CrossRef]
- Womelsdorf, T.; Vinck, M.; Leung, L.S.; Everling, S. Selective theta-synchronization of choice-relevant information subserves goal-directed behavior. Front. Hum. Neurosci. 2010, 4, 210. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X.; Donner, T.H. Midfrontal conflict-related theta-band power reflects neural oscillations that predict behavior. J. Neurophysiol. 2013, 110, 2752–2763. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Cohen, M.X.; Allen, J.J. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J. Neurosci. 2009, 29, 98–105. [Google Scholar] [CrossRef]
- Cooper, P.S.; Karayanidis, F.; McKewen, M.; McLellan-Hall, S.; Wong, A.S.W.; Skippen, P.; Cavanagh, J.F. Frontal theta predicts specific cognitive control-induced behavioural changes beyond general reaction time slowing. Neuroimage 2019, 189, 130–140. [Google Scholar] [CrossRef]
- Michel, C.M.; Brunet, D. EEG Source Imaging: A Practical Review of the Analysis Steps. Front. Neurol. 2019, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Murray, M.M.; Lantz, G.; Gonzalez, S.; Spinelli, L.; de Peralta, R.G. EEG source imaging. Clin. Neurophysiol. 2004, 115, 2195–2222. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-H.; Kim, J.; Cho, Y.S.; Kim, S.-P. Spatiotemporal Characteristics of Neural Dynamics in Theta Oscillations Related to the Inhibition of Habitual Behavior. Brain Sci. 2021, 11, 368. https://doi.org/10.3390/brainsci11030368
Kang J-H, Kim J, Cho YS, Kim S-P. Spatiotemporal Characteristics of Neural Dynamics in Theta Oscillations Related to the Inhibition of Habitual Behavior. Brain Sciences. 2021; 11(3):368. https://doi.org/10.3390/brainsci11030368
Chicago/Turabian StyleKang, Jae-Hwan, Junsuk Kim, Yang Seok Cho, and Sung-Phil Kim. 2021. "Spatiotemporal Characteristics of Neural Dynamics in Theta Oscillations Related to the Inhibition of Habitual Behavior" Brain Sciences 11, no. 3: 368. https://doi.org/10.3390/brainsci11030368
APA StyleKang, J.-H., Kim, J., Cho, Y. S., & Kim, S.-P. (2021). Spatiotemporal Characteristics of Neural Dynamics in Theta Oscillations Related to the Inhibition of Habitual Behavior. Brain Sciences, 11(3), 368. https://doi.org/10.3390/brainsci11030368






