Disrupted Pallido-Thalamo-Cortical Functional Connectivity in Chronic Disorders of Consciousness
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
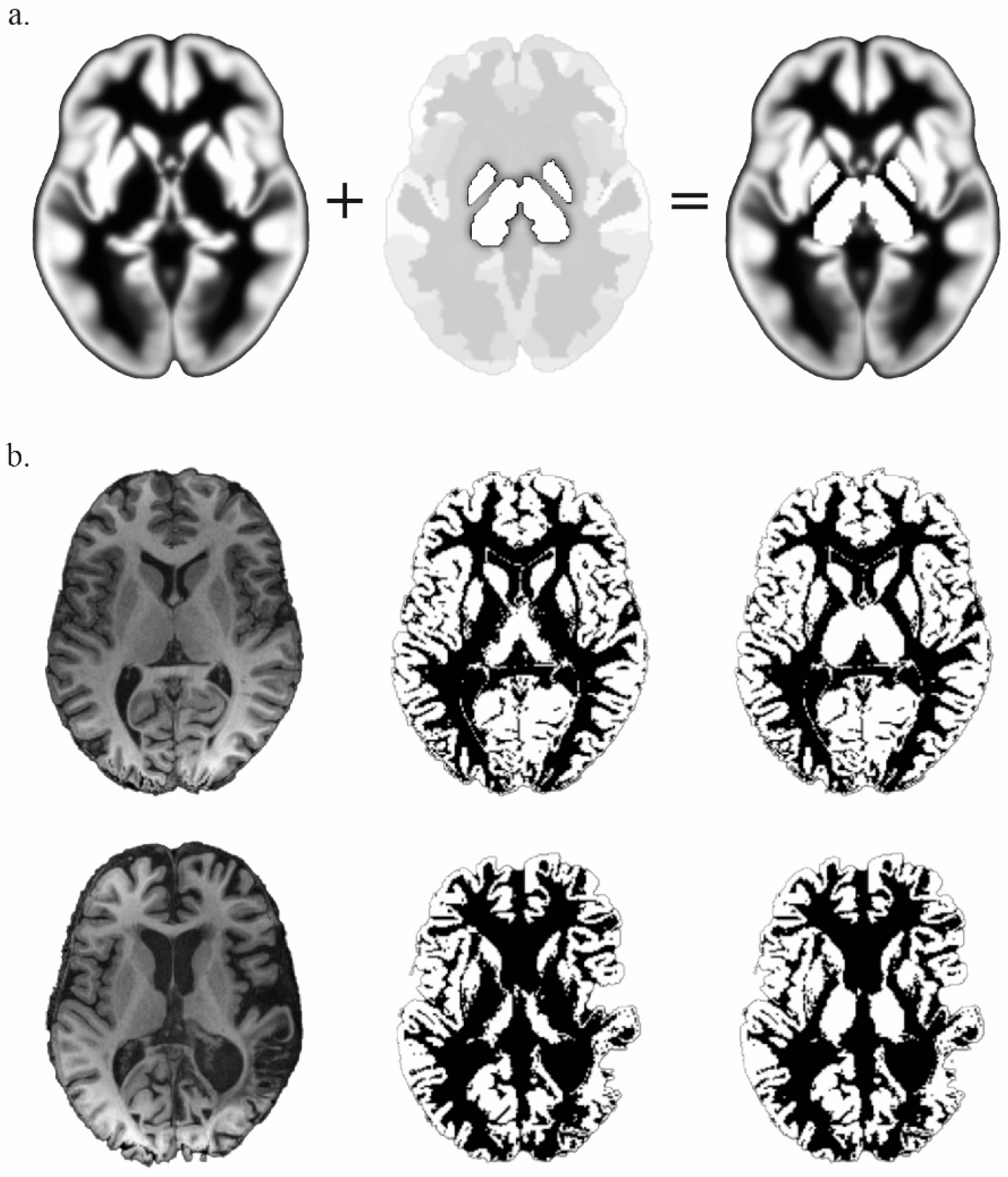
2.2. Data Acquisition and Preprocessing
2.3. Analysis of Functional Connectivity
3. Results
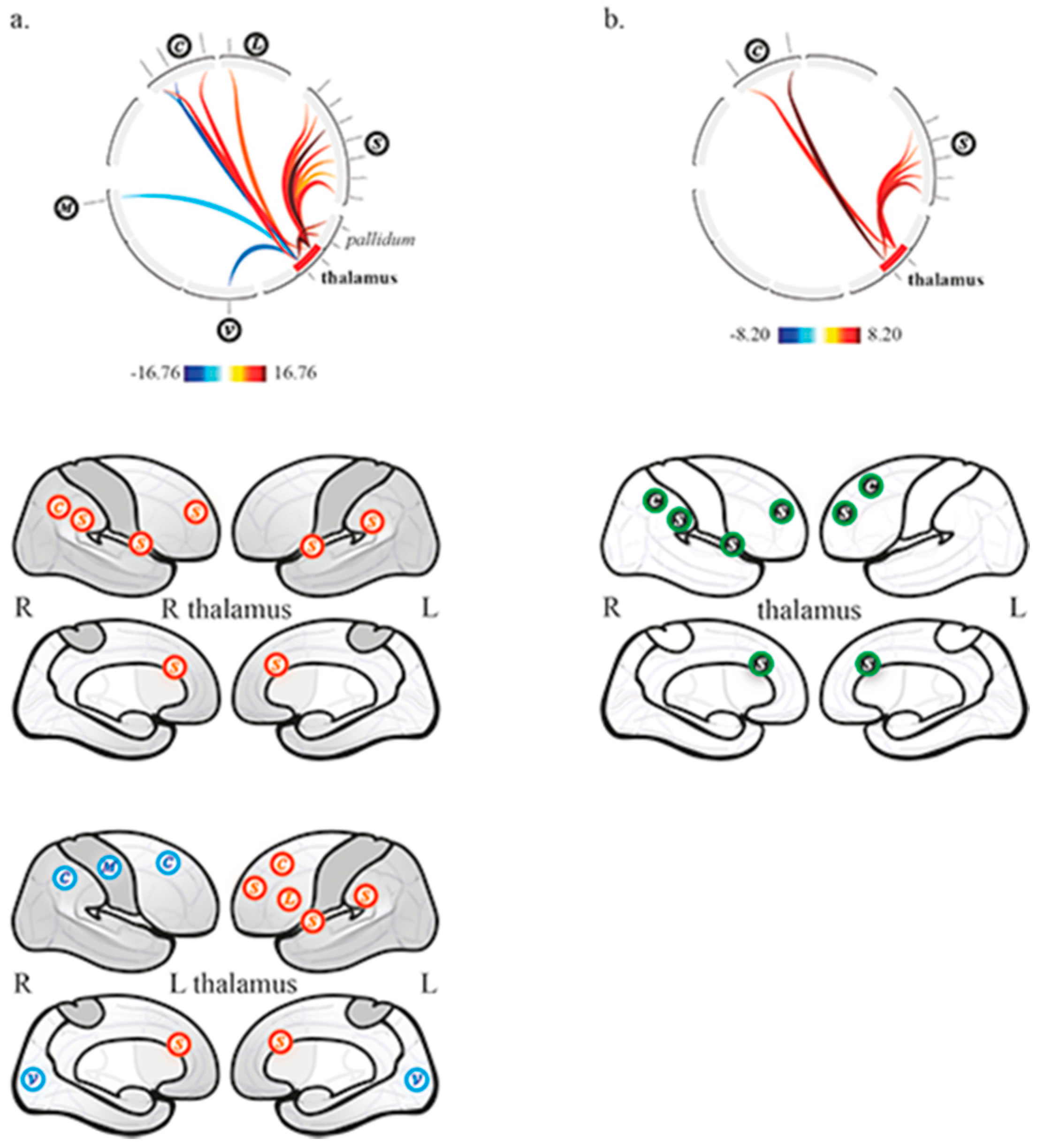
3.1. Thalamo-Cortical Functional Connectivity
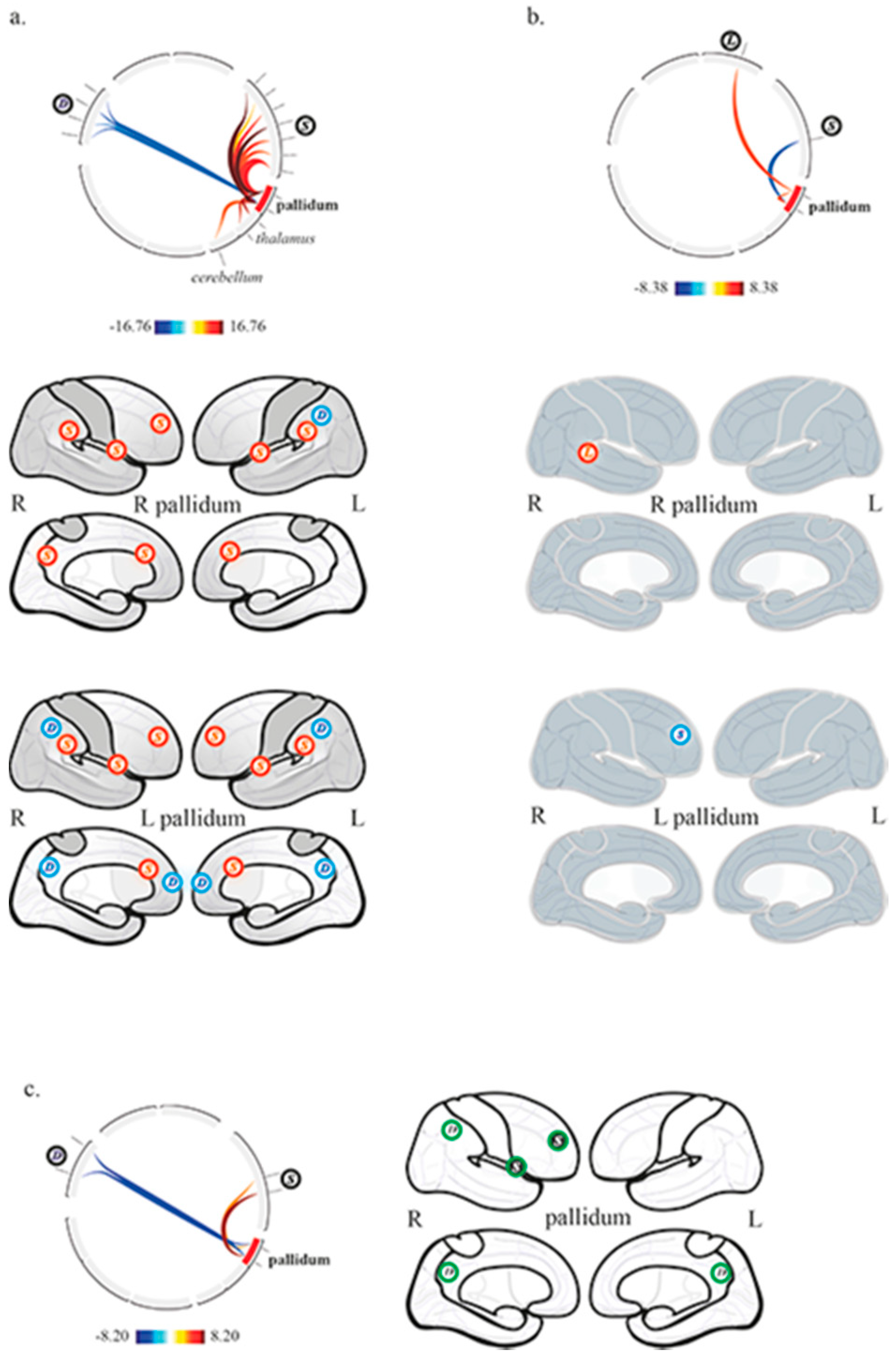
3.2. Pallido-Cortical Functional Connectivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royal College of Physicians. Prolonged Disorders of Consciousness Following Sudden Onset Brain Injury: National Clinical Guidelines; RCP: London, UK, 2020; Available online: https://www.rcplondon.ac.uk/guidelines-policy/prolonged-disorders-consciousness-following-sudden-onset-brain-injury-national-clinical-guidelines (accessed on 1 December 2020).
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.; et al. Practice guideline update recommendations summary: Disorders of consciousness. Neurology 2018, 91, 450–460. [Google Scholar] [CrossRef]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; Von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.; Kelly, J.; Rosenberg, J.; Whyte, J.; Zafonte, R.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Vanhaudenhuyse, A.; Noirhomme, Q.; Tshibanda, L.J.-F.; Bruno, M.-A.; Boveroux, P.; Schnakers, C.; Soddu, A.; Perlbarg, V.; LeDoux, D.; Brichant, J.-F.; et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 2009, 133, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Hannawi, Y.; Lindquist, M.A.; Caffo, B.S.; Sair, H.I.; Stevens, R.D. Resting brain activity in disorders of consciousness: A systematic review and meta-analysis. Neurology 2015, 84, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhang, Y.; Cui, Y.; Yang, Y.; Jiang, T. Brain network studies in chronic disorders of consciousness: Advances and perspectives. Neurosci. Bull. 2018, 34, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Schiff, N.D. Recovery of consciousness after brain injury: A mesocircuit hypothesis. Trends Neurosci. 2010, 33, 1–9. [Google Scholar] [CrossRef]
- Giacino, J.T.; Fins, J.J.; Laureys, S.; Schiff, N.D. Disorders of consciousness after acquired brain injury: The state of the science. Nat. Rev. Neurol. 2014, 10, 99–114. [Google Scholar] [CrossRef]
- Weng, L.; Xie, Q.; Zhao, L.; Zhang, R.; Ma, Q.; Wang, J.; Jiang, W.; He, Y.; Chen, Y.; Li, C.; et al. Abnormal structural connectivity between the basal ganglia, thalamus, and frontal cortex in patients with disorders of consciousness. Cortex 2017, 90, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Lutkenhoff, E.S.; Ma, J.C.; Tshibanda, L.; Kamau, E.; Kirsch, M.; Pickard, J.D.; Laureys, S.; Owen, A.M.; Monti, M.M. Thalamic and extrathalamic mechanisms of consciousness after severe brain injury. Ann. Neurol. 2015, 78, 68–76. [Google Scholar] [CrossRef]
- Akeju, O.; Loggia, M.L.; Catana, C.; Pavone, K.J.; Vazquez, R.; Rhee, J.; Ramirez, V.C.; Chonde, D.B.; Izquierdo-Garcia, D.; Arabasz, G.; et al. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. eLife 2014, 3, e04499. [Google Scholar] [CrossRef]
- He, J.; Cui, Y.; Song, M.; Yang, Y.; Dang, Y.; Jiang, T.; Xu, R. Decreased functional connectivity between the mediodorsal thalamus and default mode network in patients with disorders of consciousness. Acta Neurol. Scand. 2015, 131, 145–151. [Google Scholar] [CrossRef]
- Lemaire, J.-J.; Sontheimer, A.; Pereira, B.; Coste, J.; Rosenberg, S.; Sarret, C.; Coll, G.; Gabrillargues, J.; Jean, B.; Gillart, T.; et al. Deep brain stimulation in five patients with severe disorders of consciousness. Ann. Clin. Transl. Neurol. 2018, 5, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, G. Cortical synchronized and desynchronized responses evoked by stimulation of the putamen and pallidum in cats. J. Neurol. Sci. 1968, 7, 385–391. [Google Scholar] [CrossRef]
- Lemaire, J.-J.; Sontheimer, A.; Nezzar, H.; Pontier, B.; Luauté, J.; Roche, B.; Gillart, T.; Gabrillargues, J.; Rosenberg, S.; Sarret, C.; et al. Electrical modulation of neuronal networks in brain-injured patients with disorders of consciousness: A systematic review. Ann. Fr. Anesth. Reanim. 2014, 33, 88–97. [Google Scholar] [CrossRef]
- Qiu, M.-H.; Vetrivelan, R.; Fuller, P.M.; Lu, J. Basal ganglia control of sleep-wake behavior and cortical activation. Eur. J. Neurosci. 2010, 31, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Moll, C.K.; Sharott, A.; Hamel, W.; Münchau, A.; Buhmann, C.; Hidding, U.; Zittel, S.; Westphal, M.; Müller, D.; Engel, A.K. Waking up the brain: A case study of stimulation-induced wakeful unawareness during anaesthesia. Prog. Brain Res. 2009, 177, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Haber, N.S. Corticostriatal circuitry. Dialog. Clin. Neurosci. 2016, 18, 7–21. [Google Scholar] [CrossRef]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma recovery scale-revised: Measurement characteristics and diagnostic utility 11 no commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the authors or upon any organization with which the authors are associated. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Schnakers, C.; Majerus, S.; Giacino, J.J.; Vanhaudenhuyse, A.; Bruno, M.-A.; Boly, M.; Moonen, G.; Damas, P.; Lambermont, B.; Lamy, M.; et al. A French validation study of the Coma Recovery Scale-Revised (CRS-R). Brain Inj. 2008, 22, 786–792. [Google Scholar] [CrossRef]
- Wannez, S.; Heine, L.; Thonnard, M.; Gosseries, O.; Laureys, S.; Coma Science Group Collaborators. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann. Neurol. 2017, 81, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Lorio, S.; Fresard, S.; Adaszewski, S.; Kherif, F.; Chowdhury, R.; Frackowiak, R.; Ashburner, J.; Helms, G.; Weiskopf, N.; Lutti, A.; et al. New tissue priors for improved automated classification of subcortical brain structures on MRI. NeuroImage 2016, 130, 157–166. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Hur, E.E.; Zaborszky, L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: A combined retrograde tracing in situ hybridization. J. Comp. Neurol. 2005, 483, 351–373. [Google Scholar] [CrossRef] [PubMed]
- Vetrivelan, R.; Qiu, M.-H.; Chang, C.; Lu, J. Role of basal ganglia in sleep–wake regulation: Neural circuitry and clinical significance. Front. Neuroanat. 2010, 4, 145. [Google Scholar] [CrossRef]
- Crone, J.S.; Lutkenhoff, E.S.; Bio, B.J.; Laureys, S.; Monti, M.M. Testing proposed neuronal models of effective connectivity within the cortico-basal ganglia-thalamo-cortical loop during loss of consciousness. Cereb. Cortex 2016, 27, 2727–2738. [Google Scholar] [CrossRef][Green Version]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Manza, P.; Zhang, S.; Hu, S.; Chao, H.H.; Leung, H.-C.; Li, C.-S.R. The effects of age on resting state functional connectivity of the basal ganglia from young to middle adulthood. NeuroImage 2015, 107, 311–322. [Google Scholar] [CrossRef][Green Version]
- Dieterich, M.; Kirsch, V.; Brandt, T. Right-sided dominance of the bilateral vestibular system in the upper brainstem and thalamus. J. Neurol. 2017, 264, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Sherman, S.M. Thalamocortical circuit motifs: A general framework. Neuron 2019, 103, 762–770. [Google Scholar] [CrossRef] [PubMed]
| Clinical Status | Gender | Age | Handedness | Nature of Initial Injury | Time Elapsed Since Initial Injury | CRS-R Score before MRI (Subscores) | |
|---|---|---|---|---|---|---|---|
| P1 | UWS | M | 32 | R | TBI | 12 y 7 m | 6 (1 1 1 1 0 2) |
| P2 | MCS | F | 63 | R | Hemorrhagic stroke | 1 y 4 m | 10 (2 3 2 1 0 2) |
| P3 | MCS | M | 23 | L | TBI | 3 y 2 m | 12 (3 2 4 1 0 2) |
| P4 | MCS | F | 22 | R | TBI | 4 y 2 m | 5 (1 1 0 1 0 2) |
| P5 | MCS | F | 47 | R | Hemorrhagic stroke | 2 y 4 m | 5 (1 1 0 1 0 2) |
| P6 | UWS | M | 42 | R | TBI | 4 y 11 m | 9 (2 1 2 2 0 2) |
| P7 | MCS | M | 26 | R | TBI with cardiopulmonary arrest | 4 y 9 m | 10 (2 3 2 1 0 2) |
| P8 | UWS | F | 36 | R | TBI | 7 y 4 m | 9 (2 1 2 2 0 2) |
| P9 | MCS | F | 50 | R | TBI | 1 y 4 m | 12 (3 3 3 1 0 2) |
| P10 | MCS | M | 61 | R | TBI | 10 m | 11 (2 2 3 2 0 2) |
| P11 | MCS | M | 20 | R | TBI with cardiopulmonary arrest | 2 y 1 m | 13 (3 3 3 2 0 2) |
| P12 | MCS | F | 34 | R | Hemorrhagic stroke | 5 y 6 m | 9 (2 2 2 1 0 2) |
| P13 | UWS | M | 45 | R | Cardiopulmonary arrest (myocardial infarction) | 6 y | 6 (1 0 2 1 0 2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sontheimer, A.; Pontier, B.; Claise, B.; Chassain, C.; Coste, J.; Lemaire, J.-J. Disrupted Pallido-Thalamo-Cortical Functional Connectivity in Chronic Disorders of Consciousness. Brain Sci. 2021, 11, 356. https://doi.org/10.3390/brainsci11030356
Sontheimer A, Pontier B, Claise B, Chassain C, Coste J, Lemaire J-J. Disrupted Pallido-Thalamo-Cortical Functional Connectivity in Chronic Disorders of Consciousness. Brain Sciences. 2021; 11(3):356. https://doi.org/10.3390/brainsci11030356
Chicago/Turabian StyleSontheimer, Anna, Bénédicte Pontier, Béatrice Claise, Carine Chassain, Jérôme Coste, and Jean-Jacques Lemaire. 2021. "Disrupted Pallido-Thalamo-Cortical Functional Connectivity in Chronic Disorders of Consciousness" Brain Sciences 11, no. 3: 356. https://doi.org/10.3390/brainsci11030356
APA StyleSontheimer, A., Pontier, B., Claise, B., Chassain, C., Coste, J., & Lemaire, J.-J. (2021). Disrupted Pallido-Thalamo-Cortical Functional Connectivity in Chronic Disorders of Consciousness. Brain Sciences, 11(3), 356. https://doi.org/10.3390/brainsci11030356






