Limitations of Flow Diverters in Posterior Communicating Artery Aneurysms
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients and Clinical and Radiological Outcomes
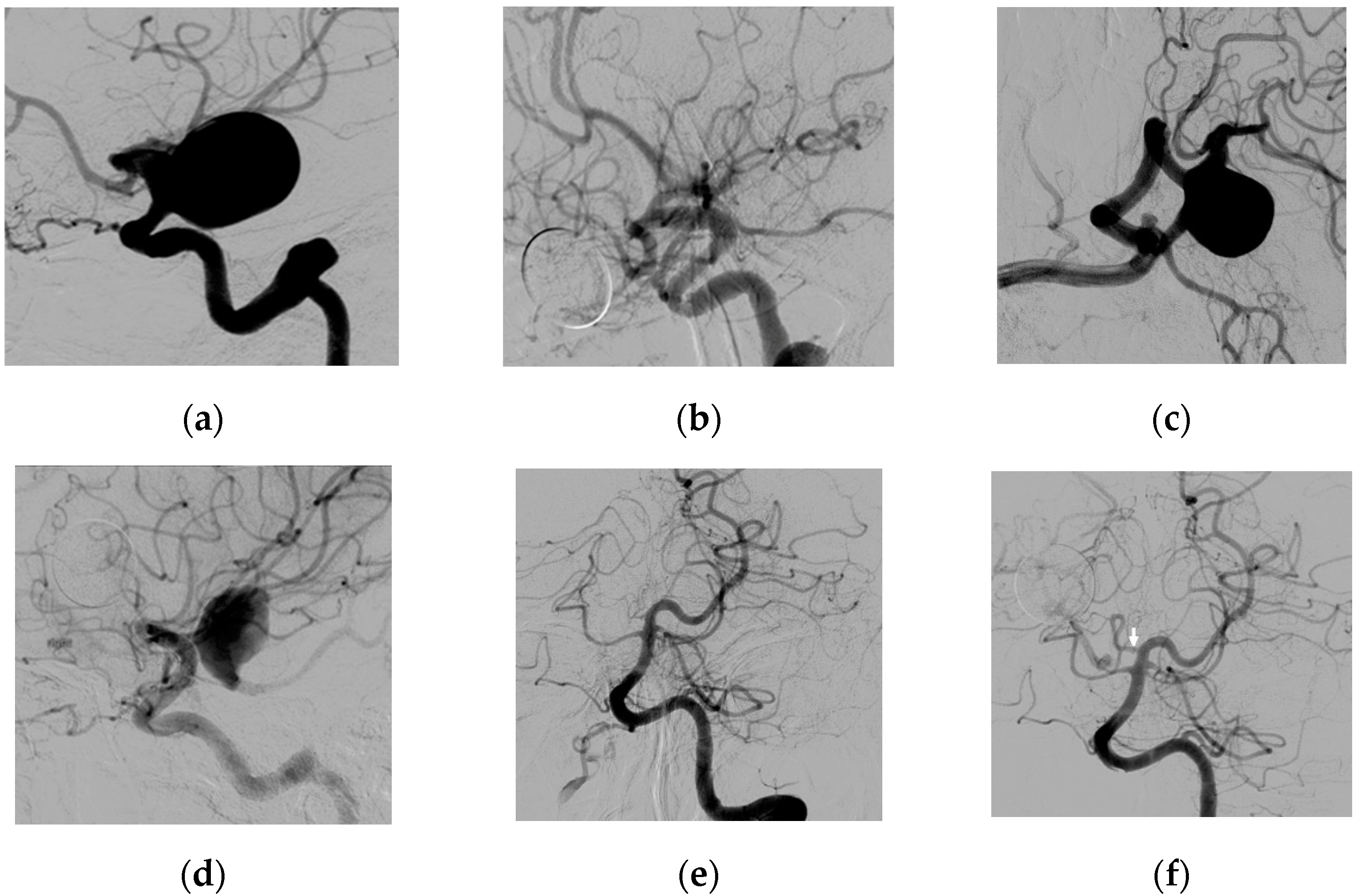
3.2. Fetal Type PcomA Patients
3.3. Analysis
3.4. Literature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brinjikji, W.; Murad, M.H.; Lanzino, G.; Cloft, H.J.; Kallmes, D.F. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke 2013, 44, 442–447. [Google Scholar] [CrossRef]
- Sadasivan, C.; Cesar, L.; Seong, J.; Rakian, A.; Hao, Q.; Tio, F.O.; Wakhloo, A.K.; Lieber, B.B. An original flow diversion device for the treatment of intracranial aneurysms: Evaluation in the rabbit elastase-induced model. Stroke 2009, 40, 952–958. [Google Scholar] [CrossRef]
- van Raamt, A.F.; Mali, W.P.; van Laar, P.J.; van der Graaf, Y. The fetal variant of the circle of Willis and its influence on the cerebral collateral circulation. Cerebrovasc. Dis. 2006, 22, 217–224. [Google Scholar] [CrossRef]
- De Vries, J.; Boogaarts, J.; Van Norden, A.; Wakhloo, A.K. New generation of Flow Diverter (surpass) for unruptured intracranial aneurysms: A prospective single-center study in 37 patients. Stroke 2013, 44, 1567–1577. [Google Scholar] [CrossRef]
- Roy, D.; Milot, G.; Raymond, J. Endovascular treatment of unruptured aneurysms. Stroke 2001, 32, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Tsang, A.C.; Fung, A.M.; Tsang, F.C.; Leung, G.K.; Lee, R.; Lui, W.M. Failure of Flow Diverter Treatment of Intracranial Aneurysms Related to the Fetal-type Posterior Communicating Artery. Neurointervention 2015, 10, 60–66. [Google Scholar] [CrossRef]
- Zanaty, M.; Chalouhi, N.; Starke, R.M.; Jabbour, P.; Ryken, K.O.; Bulsara, K.R.; Hasan, D. Failure of the Pipeline Embolization Device in Posterior Communicating Artery Aneurysms Associated with a Fetal Posterior Cerebral Artery. Case Rep. Vasc. Med. 2016, 2016, 4691275. [Google Scholar] [CrossRef][Green Version]
- Daou, B.; Valle-Giler, E.P.; Chalouhi, N.; Starke, R.M.; Tjoumakaris, S.; Hasan, D.; Rosenwasser, R.H.; Hebert, R.; Jabbour, P. Patency of the posterior communicating artery following treatment with the Pipeline Embolization Device. J. Neurosurg. 2017, 126, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.K.; Howard, B.M.; Haussen, D.C.; Osbun, J.W.; Halani, S.H.; Skukalek, S.L.; Tong, F.; Nogueira, R.G.; Dion, J.E.; Cawley, C.M.; et al. Reduced Efficacy of the Pipeline Embolization Device in the Treatment of Posterior Communicating Region Aneurysms with Fetal Posterior Cerebral Artery Configuration. Neurosurgery 2018, 82, 695–700. [Google Scholar] [CrossRef]
- Wallace, A.N.; Kayan, Y.; Austin, M.J.; Delgado Almandoz, J.E.; Kamran, M.; Cross, D.T.; Moran, C.J., 3rd; Osbun, J.W.; Kansagra, A.P. Pipeline embolization of posterior communicating artery aneurysms associated with a fetal origin posterior cerebral artery. Clin. Neurol. Neurosurg. 2017, 160, 83–87. [Google Scholar] [CrossRef]
- Enriquez-Marulanda, A.; Ravindran, K.; Salem, M.M.; Ascanio, L.C.; Kan, P.; Srinivasan, V.M.; Griessenauer, C.J.; Schirmer, C.M.; Jain, A.; Moore, J.M.; et al. Evaluation of Radiological Features of the Posterior Communicating Artery and Their Impact on Efficacy of Saccular Aneurysm Treatment with the Pipeline Embolization Device: A Case Series Study. World Neurosurg. 2019, 125, e998–e1007. [Google Scholar] [CrossRef]
- Rinaldo, L.; Brinjikji, W.; Cloft, H.; Lanzino, G.; Gonzalez, L.F.; Kan, P.; Castilla, L.R. Effect of Fetal Posterior Circulation on Efficacy of Flow Diversion for Treatment of Posterior Communicating Artery Aneurysms: A Multi-Institutional Study. World Neurosurg. 2019, 127, e1232–e1236. [Google Scholar] [CrossRef] [PubMed]
- Kan, P.; Duckworth, E.; Puri, A.; Velat, G.; Wakhloo, A. Treatment failure of fetal posterior communicating artery aneurysms with the pipeline embolization device. J. Neurointerv. Surg. 2016, 8, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.L.; Dabus, G.; Kan, P.; Wakhloo, A.K.; Puri, A.S. Flow-diverter stents for endovascular management of non-fetal posterior communicating artery aneurysms-analysis on aneurysm occlusion, vessel patency, and patient outcome. Interv. Neuroradiol. 2018, 24, 363–374. [Google Scholar] [CrossRef]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J., 3rd; Meissner, I.; Brown, R.D., Jr.; Piepgras, D.G.; Forbes, G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; et al. International Study of Unruptured Intracranial Aneurysms I, Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Brinjikji, W.; Lanzino, G.; Cloft, H.J.; Kallmes, D.F. Patency of the posterior communicating artery after flow diversion treatment of internal carotid artery aneurysms. Clin. Neurol. Neurosurg. 2014, 120, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Niu, Y.; Tamg, J.; Li, L.; Feng, Z.; Feng, H.; Zhu, G. Endovascular treatment of posterior communication artery aneurysms in the presence of the fetal variant of posterior cerebral artery. Interv. Neuroradiol. 2015, 21, 456–461. [Google Scholar] [CrossRef]
- Molyneux, A.J.; Kerr, R.S.; Yu, L.M.; Clarke, M.; Sneade, M.; Yarnold, J.A.; Sandercock, P. International Subarachnoid Aneurysm Trial Collaborative G, International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 2005, 366, 809–817. [Google Scholar]
- Zada, G.; Breault, J.; Liu, C.Y.; Khalessi, A.A.; Larsen, D.W.; Teitelbaum, G.P.; Giannotta, S.L. Internal carotid artery aneurysms occurring at the origin of fetal variant posterior cerebral arteries: Surgical and endovascular experience. Neurosurgery 2008, 63, ONS55-61–ONS61-52. [Google Scholar] [CrossRef]
- Perrini, P.; Montemurro, N.; Caniglia, M.; Lazzarotti, G.; Benedetto, N. Wrapping of intracranial aneurysms: Single-center series and systematic review of the literature. Br. J. Neurosurg. 2015, 29, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yuan, Y.; Guo, Y.; Yu, J. Intracranial post-embolization residual or recurrent aneurysms: Current management using surgical clipping. Interv. Neuroradiol. 2016, 22, 413–419. [Google Scholar] [CrossRef] [PubMed]
| PCA Configuration | All Patients | p-Value 1 | |||
|---|---|---|---|---|---|
| Normal and Transitional Configuration | Fetal Configuration | ||||
| Number of patients/aneurysms | 17/18 | 3/3 | 19 a/21 | NA | |
| Mean age in years (SD, range) | 55.9 (9.8, 40–80) | 59.3 (12.3, 49–73) | 56.4 (9.9, 40–80) | 0.59 * | |
| Female sex (n) | 14 (77.8%) | 2 (66.7%) | 15 a (78.9%) | 1.00 | |
| Size | Small (<10 mm) | 8 (44.4%) | 0 (0%) | 8 (38.1%) | 0.26 |
| Intermediate (10–20 mm) | 7 (38.9%) | 1 (33.3%) | 8 (38.1%) | 1.00 | |
| Large (≥20 mm) | 3 (16.7%) | 2 (66.7%) | 5 (23.8%) | 0.13 | |
| Mean neck size in mm (SD, range) | 3.5 (1.2, 1.7–6.3) | 7.7 (5.9, 4.0–14.6) | 4.1 (2.6, 1.7–14.6) | 0.34 * | |
| Mean dome-to-neck ratio in mm (SD, range) | 3.4 (1.9, 1.4–9.3) | 4.3 (4.4, 1.6–9.4) | 3.5 (2.2, 1.4–9.4) | 0.76 * | |
| Number of wide-neck aneurysms (Neck ≥ 4.0 mm or dome-to-neck ratio < 2.0) | 9 (50.0%) | 3 (100%) | 12 (57.1%) | 0.23 | |
| mRS at 6 months ≤ 2 | 18 (100%) | 3 (100%) | 21 (100%) b | NA | |
| Initial closure at 6 months (%) | 14 (77.8%) | 0 (0%) | 14 (66.7%) | 0.03 | |
| Retreatment (n/n aneurysms not occluded at 6 months, (%)) | 3/4 (75%) | 2/3 (66.7%) | 5/7 (71.4%) c | 1.00 | |
| Full aneurysm occlusion at last follow-up (%) (Mean follow-up ± SD, range (months)) | 17 (94.4%) | 0 (0%) | 17 (81.0%) 19.1 ± 18.0 (4–69) | 0.00 | |
| Patients with complications | Total | 4 (23.5%) | 0 | 4 d/19 (21.1%) | 1.00 |
| Procedural | 1 (5.9%) | 0 (0%) | 1/19 (5.3%) | ||
| Periprocedural (≤30 days) | 3 (17.6%) | 0 (0%) | 3/19 (15.8%) | ||
| Late (>30 days) | 1 (5.9%) | 0 (0%) | 1/19 (5.3%) | ||
| Study, Year [Reference] | Number of Cases | FD Type | Aneurysm Size (Median) in mm ± SD (Range) | Aneurysms with Complete Occlusion (%) | Follow-Up Time in Months (Mean) |
|---|---|---|---|---|---|
| Tsang et al., 2015 [6] | 4 | PED | 7.6 ± 3.3 (4.4–11.0) * | 0 (0%) | NR |
| Zanaty et al., 2016 [7] | 3 | PED | 12.0 ± 2.0 (10.0–14.0) * | 0 (0%) | 5.0 |
| Daou et al., 2017 [8] | 6 | PED | NR | 4 (66.7%) | 6.0 |
| Roy et al., 2017 [9] | 9 | PED | 7.6 ± 3.8 (4.0–15.0) | 1 (11.1%) | 11.7 |
| Wallace et al., 2017 [10] | 6 | PED | 7.7 ± 4.5 (5.4–16.0) | 1 (16.7%) a | 20.0 |
| Enriquez-Marulanda et al., 2019 [11] | 4 | PED | NR | 3 (75.0%) | 11.9 |
| Rinaldo et al., 2019 [12] b | 16 | NR | 12.1 ± 8.7 (4.0–35.0) c | 7 (43.8%) | 22.8 d |
| Present series | 3 | Surpass | 26.0 ± 17.0 (10.0–44.0) | 0 (0%) | 27.6 |
| TOTAL | 51 | N.A. | N.A. | 16 (31.4%) a | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
ten Brinck, M.F.M.; Rigante, L.; Shimanskaya, V.E.; Bartels, R.H.M.A.; Meijer, F.J.A.; Wakhloo, A.K.; de Vries, J.; Boogaarts, H.D. Limitations of Flow Diverters in Posterior Communicating Artery Aneurysms. Brain Sci. 2021, 11, 349. https://doi.org/10.3390/brainsci11030349
ten Brinck MFM, Rigante L, Shimanskaya VE, Bartels RHMA, Meijer FJA, Wakhloo AK, de Vries J, Boogaarts HD. Limitations of Flow Diverters in Posterior Communicating Artery Aneurysms. Brain Sciences. 2021; 11(3):349. https://doi.org/10.3390/brainsci11030349
Chicago/Turabian Styleten Brinck, Michelle F. M., Luigi Rigante, Viktoria E. Shimanskaya, Ronald H. M. A. Bartels, Frederick J. A. Meijer, Ajay K. Wakhloo, Joost de Vries, and Hieronymus D. Boogaarts. 2021. "Limitations of Flow Diverters in Posterior Communicating Artery Aneurysms" Brain Sciences 11, no. 3: 349. https://doi.org/10.3390/brainsci11030349
APA Styleten Brinck, M. F. M., Rigante, L., Shimanskaya, V. E., Bartels, R. H. M. A., Meijer, F. J. A., Wakhloo, A. K., de Vries, J., & Boogaarts, H. D. (2021). Limitations of Flow Diverters in Posterior Communicating Artery Aneurysms. Brain Sciences, 11(3), 349. https://doi.org/10.3390/brainsci11030349





