Neuroprotective Effect of α-Mangostin in Ameliorating Propionic Acid-Induced Experimental Model of Autism in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Chemicals and Drugs
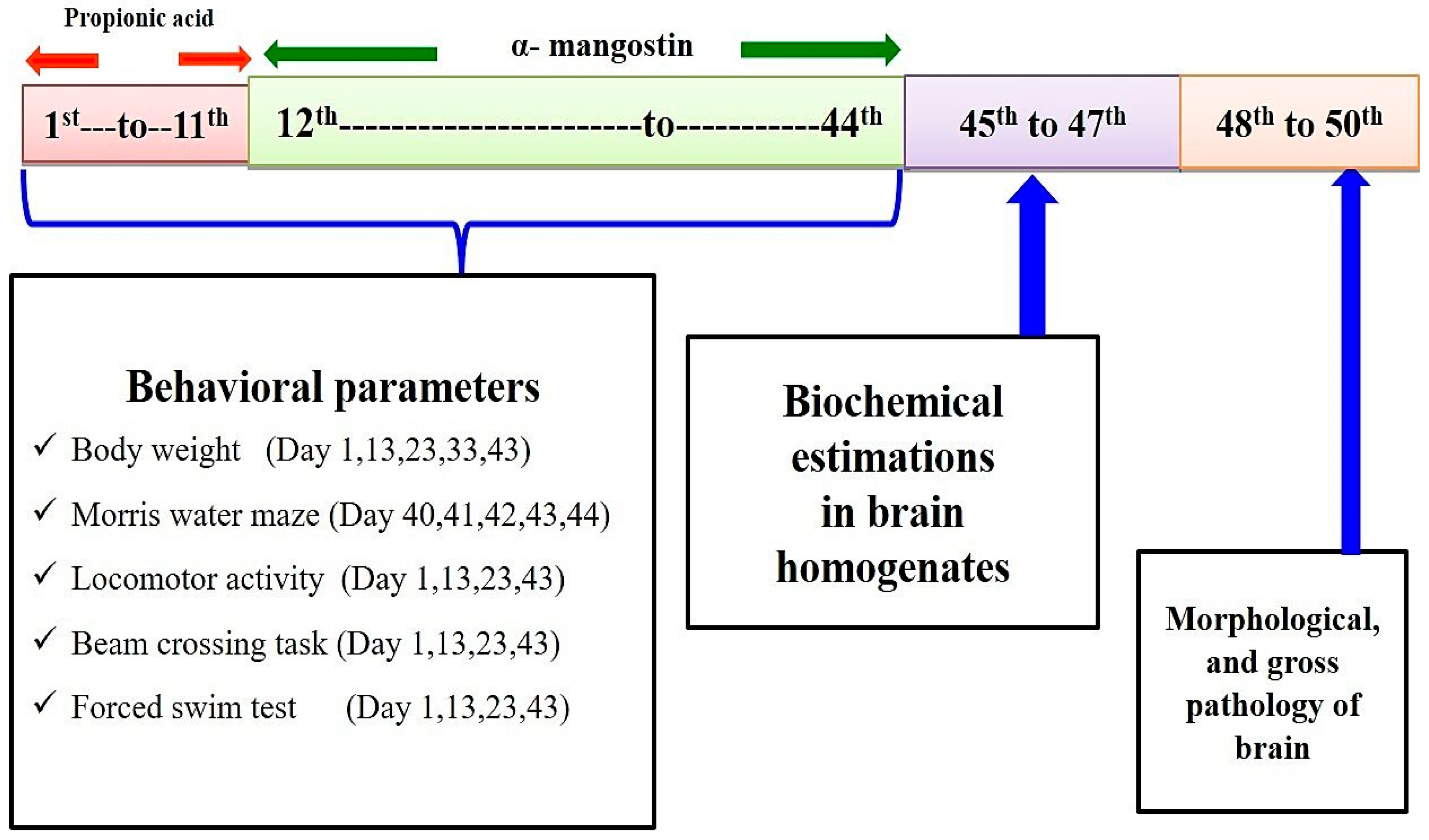
2.3. Experimental Protocol Schedule
2.4. Experimental Model of Autism
2.5. Parameters Assessed
Measurement of Body Weight
2.6. Behavioral Parameters
2.6.1. Spontaneous Locomotor Activity
2.6.2. Morris Water Maze Task
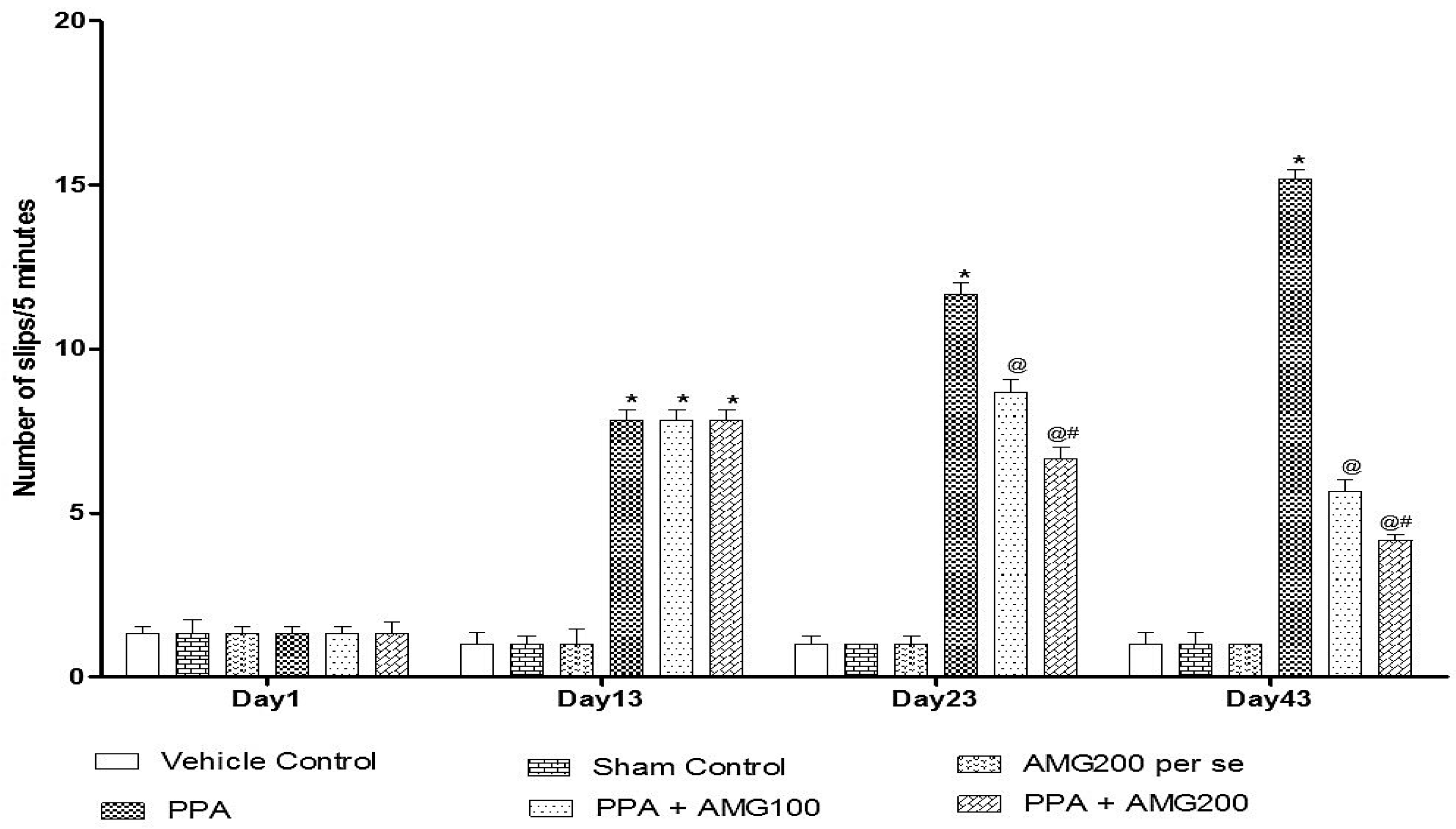
2.6.3. Beam Crossing Task
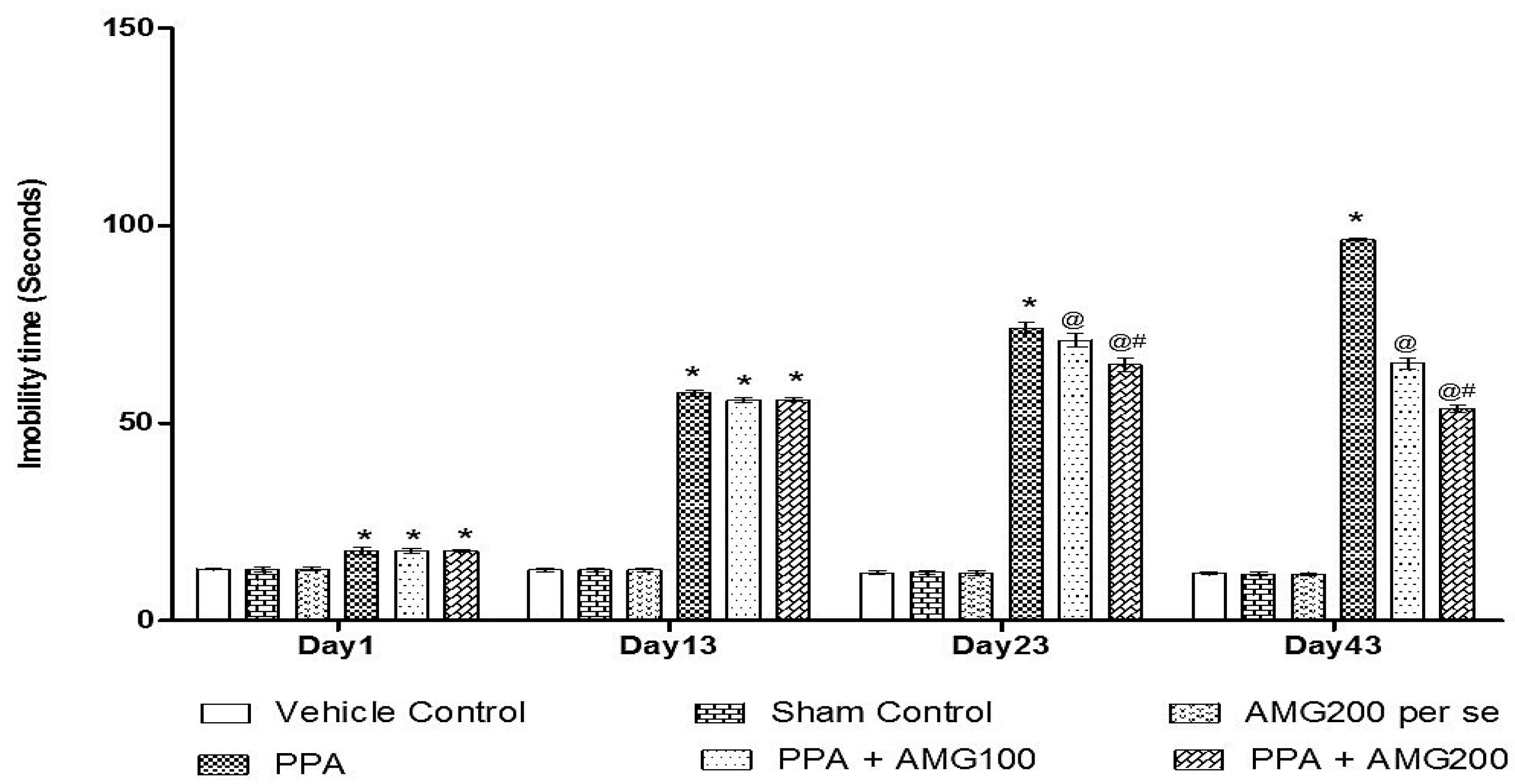
2.6.4. Force Swim Test
2.7. Measurement of Cellular and Biochemical Markers
2.7.1. Brain Homogenate Preparation
2.7.2. Measurement of ERK Levels
2.7.3. Measurement of Myelin Basic Protein (MBP)
2.8. Measurement of Apoptotic Markers
2.8.1. Caspase-3 Levels
2.8.2. Bax and Bcl-2 Levels
2.9. Neurotransmitters Evaluation
2.9.1. Serotonin Levels
2.9.2. Glutamate Levels
2.9.3. Dopamine Levels
2.9.4. Acetylcholine (Ach) Levels
2.10. Evaluation of Neuroinflammatory Biomarkers
TNF-α and IL-1β Levels
2.11. Evaluation of Oxidative Stress Parameters
2.11.1. Lactate Dehydrogenase (LDH) Levels
2.11.2. Acetylcholinesterase (AChE) Levels
2.11.3. Glutathione Levels
2.11.4. Malondialdehyde (MDA) Levels
2.11.5. Superoxide Dismutase (SOD) Levels
2.11.6. Nitrite Levels
2.12. Gross Pathological Examination and Morphology
2.13. Statistical Analysis
3. Results
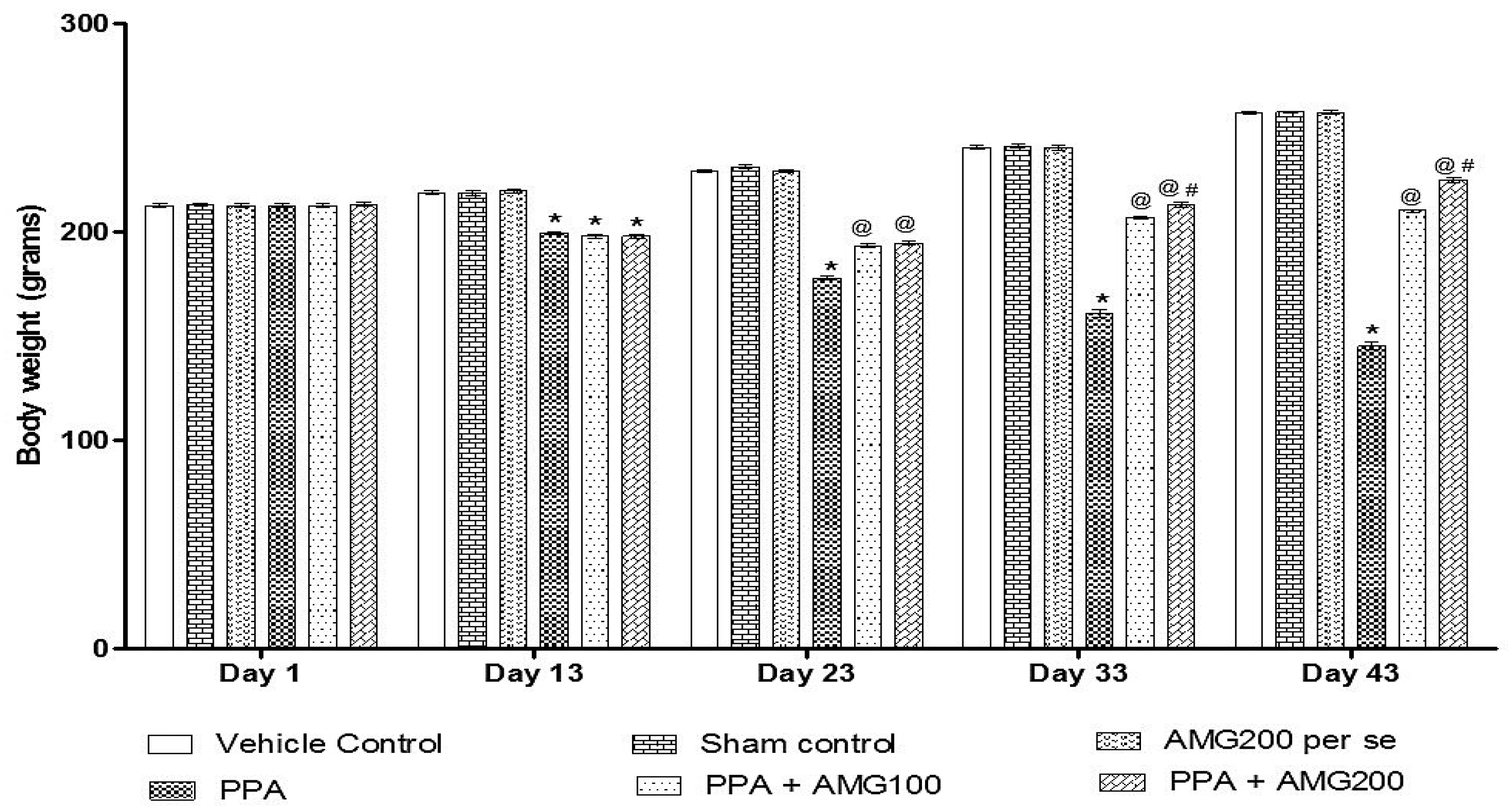
3.1. Effect of AMG on Body Weight of PPA-Treated Autistic Rats
3.2. Behavior Parameters
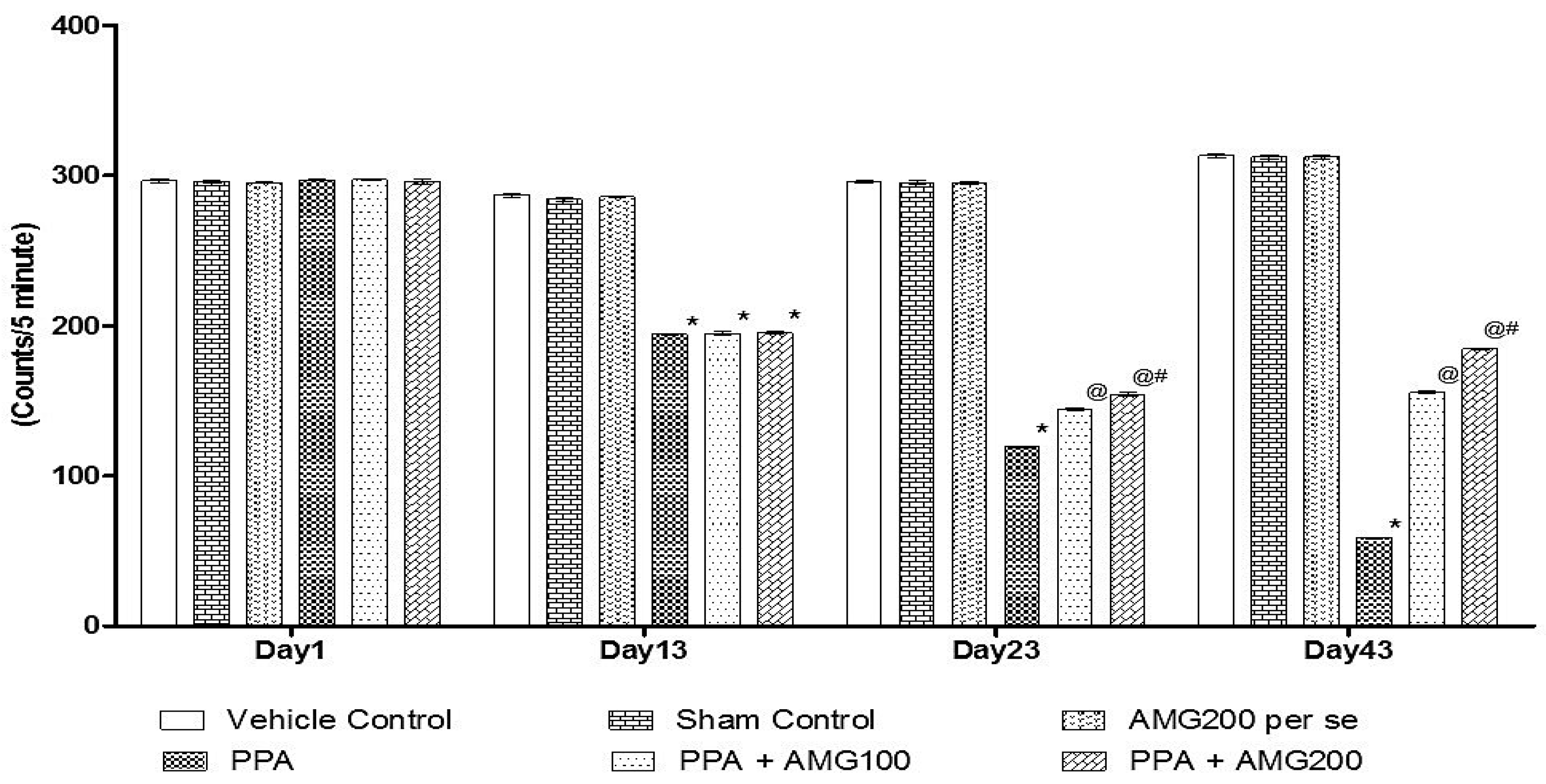
3.2.1. Effect of AMG on Locomotion Activity in PPA-Treated Autistic Rats
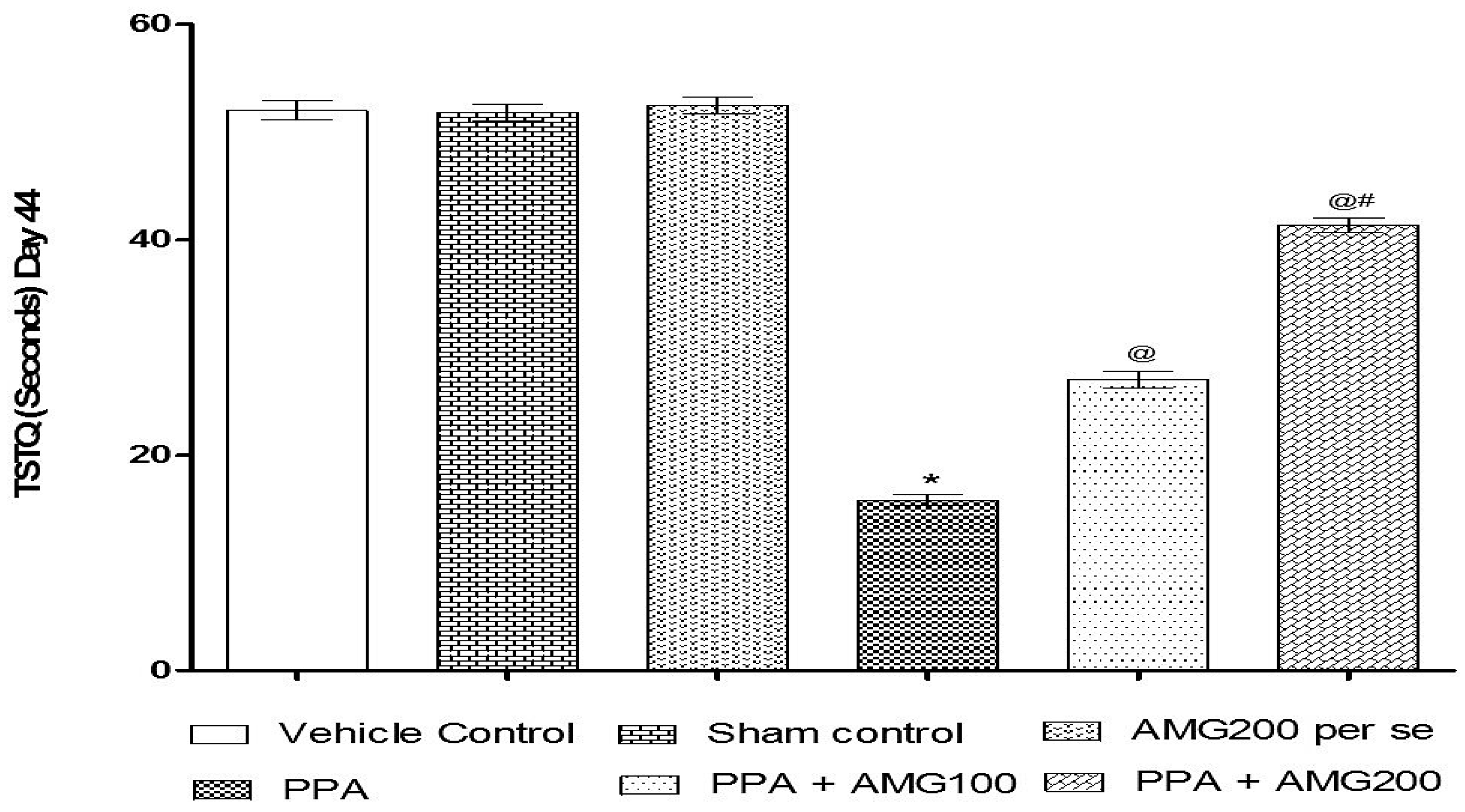
3.2.2. Effect of AMG on Spatial Memory in PPA-Treated Autistic Rats
3.2.3. Effect of AMG on Muscle Coordination in PPA-Treated Autistic Rats
3.2.4. Effect of AMG on the Immobility Phase of PPA-Treated Autistic Rats
3.3. Neurochemical Parameters
3.3.1. Effect of AMG onERK Level in PPA-Treated Autistic Rats
3.3.2. Effect of AMG on Myelin Basic Protein Levels in PPA-Treated Autistic Rats
3.4. Effect of AMG on the Apoptotic Marker of PPA-Treated Autistic Rats
Effect of AMG on Caspase-3, Bax, and Bcl-2 Levels in PPA-Treated Autistic Rats
3.5. Effect of AMG on the Measurement of Neurotransmitters in PPA-Treated Autistic Rats
3.6. Effect of AMG on the Assessment of Inflammatory Cytokines in PPA-Treated Autistic Rats
3.7. Effect of AMG on the Measurement of Oxidative Stress Markers in PPA-Treated Autistic Rats
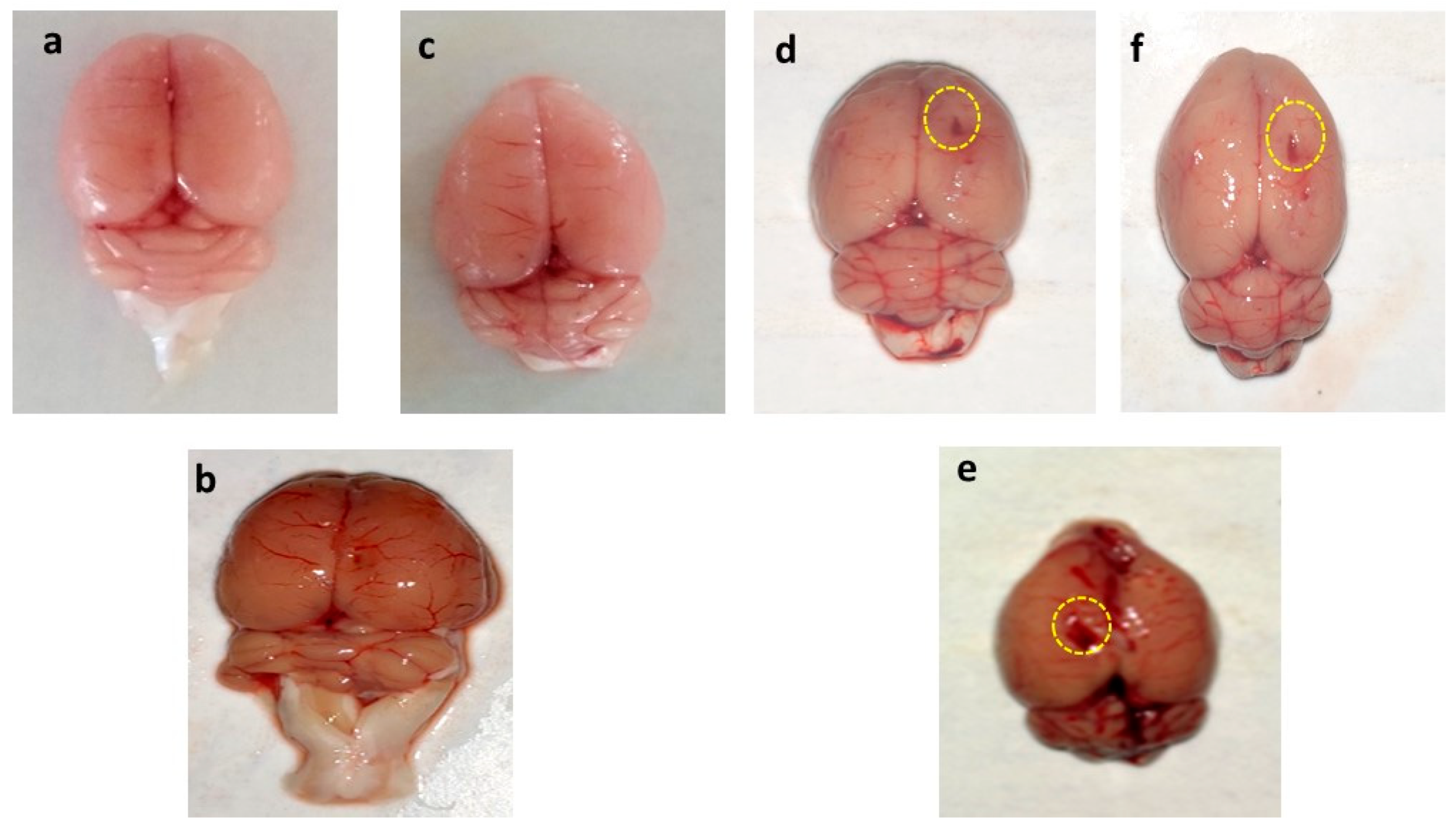
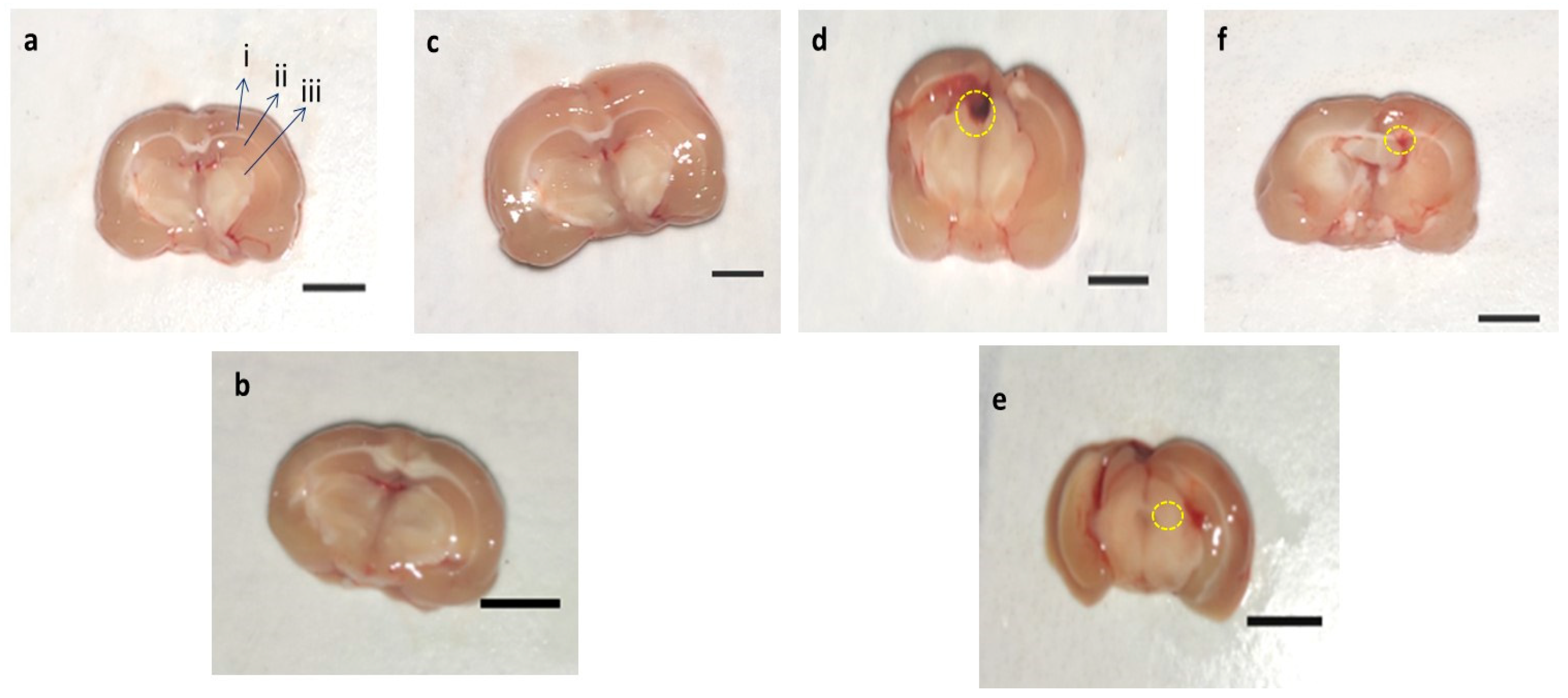
3.8. Effect of AMG on Whole Rat Brain and Brain Sections in PPA-Treated Autistic Rats
3.8.1. Assessment of the Whole Brain in PPA-Treated Autistic Rats
3.8.2. Assessment of Brain Sections in PPA-Treated Rats
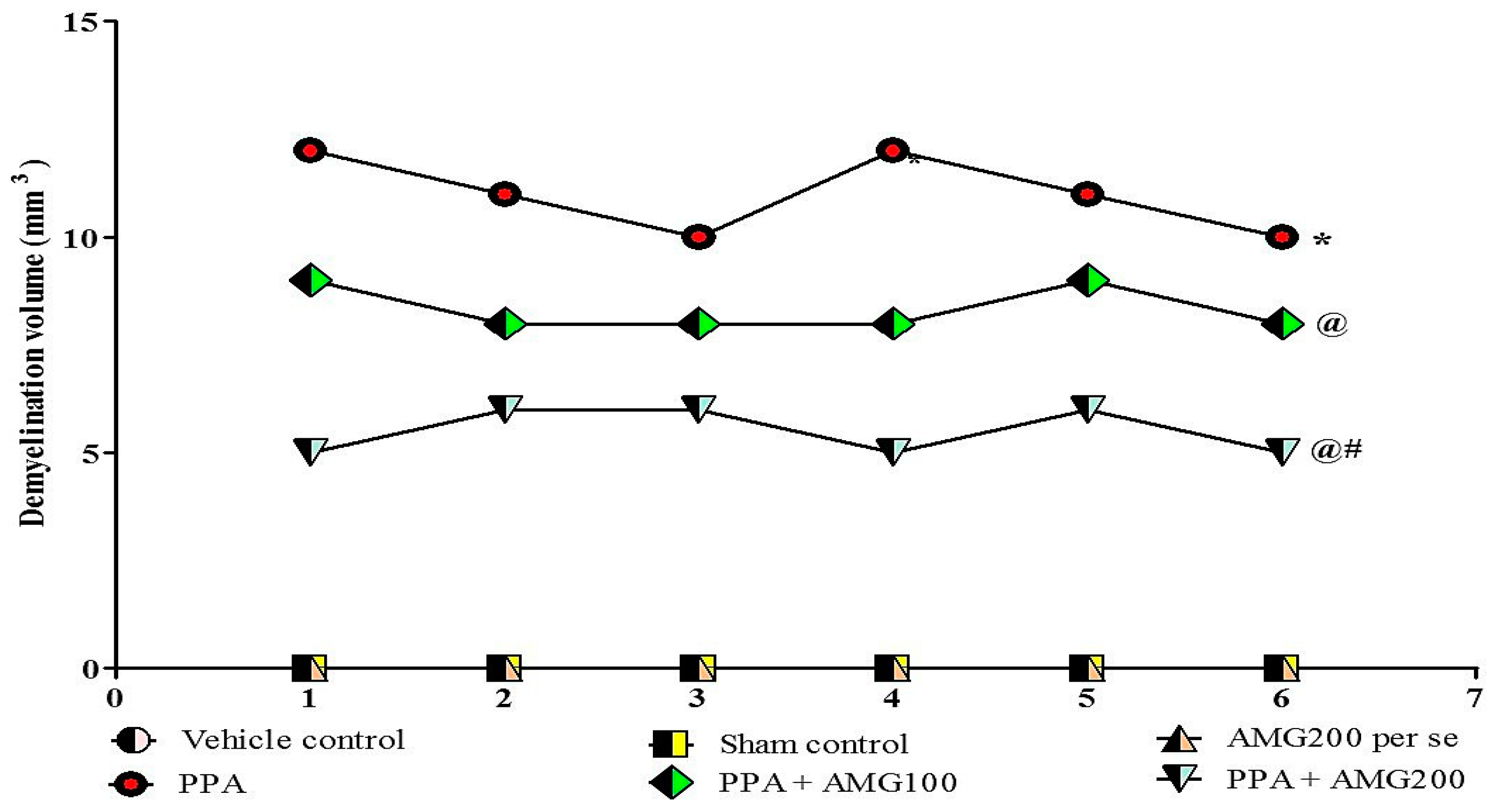
3.9. Determination of the Demyelination Volume in PPA-Treated Autistic Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Definitions
| Ach | acetylcholine |
| AChE | acetylcholinesterase |
| AD | Alzheimer’s disease |
| ALS | amyotrophic lateral sclerosis |
| AMG | alpha mangostin |
| AP | anterior/posterior |
| ASD | autism spectrum disorder |
| BCHe | butyrylcholinesterase |
| DMSO | dimethyl sulfoxide |
| DV | dorsal/ventral |
| ERK | extracellular signal-regulated kinases |
| HD | Huntington’s disease |
| HPLC | high performance liquid chromatography |
| ICV | intracerebroventricular |
| IL-β | interleukin beta |
| INCO | instruments & chemicals private limited |
| LDH | lactate dehydrogenase |
| LTD | long-term depression |
| LTP | long-term potentiation |
| MAPK | mitogen-activated protein kinase |
| MBP | myelin basic protein |
| MDA | malondialdehyde |
| ML | medial/lateral |
| MS | multiple sclerosis |
| MWM | Morris water maze |
| NO | nitric oxide |
| OPA | o-phthalaldehyde |
| p.o. | oral route |
| PD | Parkinson’s disease |
| PPA | propionic acid |
| SOD | superoxide dismutase |
| TIFF | tagged image file format |
| TNF-α | tumor necrosis factor |
| TSTQ | time spent in the target quadrant |
| β-ME | β-mercaptoethanol |
References
- Al-Ghamdi, M.; Al-Ayadhi, L.; El-Ansary, A. Selected biomarkers as predictive tools in testing efficacy of melatonin and coenzyme Q on propionic acid-induced neurotoxicity in rodent model of autism. BMC Neurosci. 2014, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Muratori, F.; Morales, M.A. The Role of Heavy Metal Pollution in Neurobehavioral Disorders: A Focus on Autism. Rev. J. Autism. Dev. Disord. 2014, 1, 354–372. [Google Scholar] [CrossRef]
- Miyazaki, K.; Narita, N.; Narita, M. Maternal administration of thalidomide or valproic acid causes abnormal serotonergic neurons in the offspring: Implication for pathogenesis of autism. Int. J. Dev. Neurosci. 2005, 23, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Bagasra, O.; Golkar, Z.; Garcia, M.; Rice, L.N.; Pace, D.G. Role of perfumes in pathogenesis of Autism. Med. Hypotheses 2013, 80, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wu, Y.; Wu, B.-L. Genetic architecture, epigenetic influence and environment exposure in the pathogenesis of Autism. Sci. China Life Sci. 2015, 58, 958–967. [Google Scholar] [CrossRef]
- Moessner, R.; Marshall, C.R.; Sutcliffe, J.S.; Skaug, J.; Pinto, D.; Vincent, J.; Zwaigenbaum, L.; Fernandez, B.; Roberts, W.; Szatmari, P.; et al. Contribution of SHANK3 Mutations to Autism Spectrum Disorder. Am. J. Hum. Genet. 2007, 81, 1289–1297. [Google Scholar] [CrossRef]
- Grossi, E.; Terruzzi, V. The role of intestinal dysbiosis in the pathogenesis of Autism: Minireview. Int. J. Microbiol. Adv. Immunol. 2014, 2, 41–44. [Google Scholar]
- Singh, M.; Chauhan, A.; Sahu, J.K.; Jaiswal, N.; Kumar, K.; Agarwal, A.; Kaur, J.; Singh, S. Prevalence of autism spectrum disorder in Indian children: A systematic review and meta-analysis. Neurol. India 2019, 67, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, L.S.; Samsam, A.; Naser, S.A. Propionic Acid Induces Gliosis and Neuro-inflammation through Modulation of PTEN/AKT Pathway in Autism Spectrum Disorder. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Kamen, C.L.; Zevy, D.L.; Ward, J.M.; Bishnoi, I.R.; Kavaliers, M.; Ossenkopp, K.-P. Systemic Treatment with the Enteric Bacterial Fermentation Product, Propionic Acid, Reduces Acoustic Startle Response Magnitude in Rats in a Dose-Dependent Fashion: Contribution to a Rodent Model of ASD. Neurotox. Res. 2018, 35, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Mehan, S.; Rahi, S.; Tiwari, A.; Kapoor, T.; Rajdev, K.; Sharma, R.; Khera, H.; Kosey, S.; Kukkar, U.; Dudi, R. Adenylate cyclase activator forskolin alleviates intracerebroventricular propionic acid-induced mitochondrial dysfunction of autistic rats. Neural Regen. Res. 2020, 15, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Rahi, S.; Mehan, S. Neuroprotective potential of solanesol in intracerebroventricular propionic acid induced experimental model of autism: Insights from behavioral and biochemical evidence. Toxicol. Rep. 2019, 6, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Widmann, C.; Gibson, S.; Jarpe, M.B.; Johnson, G.L. Mitogen-Activated Protein Kinase: Conservation of a Three-Kinase Module from Yeast to Human. Physiol. Rev. 1999, 79, 143–180. [Google Scholar] [CrossRef]
- Thomas, R.H.; Meeking, M.M.; Mepham, J.R.; Tichenoff, L.; Possmayer, F.; Liu, S.; MacFabe, D.F. The enteric bac-terial metabolite propionic acid alters brain and plasma phospholipid molecular species: Further development of a rodent model of autism spectrum disorders. J. Neuroinflamm. 2012, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Nan, G. The extracellular signal-regulated kinase 1/2 pathway in neurological diseases: A potential therapeutic target. Int. J. Mol. Med. 2017, 39, 1338–1346. [Google Scholar] [CrossRef]
- Bohush, A.; Niewiadomska, G.; Filipek, A. Role of mitogen activated protein kinase signaling in Parkinson’s dis-ease. Int. J. Mol. Sci. 2018, 19, 2973. [Google Scholar] [CrossRef]
- Bhowmick, S.; D’Mello, V.; Abdul-Muneer, P.M. Synergistic inhibition of ERK1/2 and JNK, not p38, phosphorylation ameliorates neuronal damages after traumatic brain injury. Mol. Neurobiol. 2019, 56, 1124–1136. [Google Scholar] [CrossRef]
- Rubia, K.; Smith, A.B.; Brammer, M.J.; Toone, B.; Taylor, E. Abnormal Brain Activation During Inhibition and Error Detection in Medication-Naive Adolescents with ADHD. Am. J. Psychiatry 2005, 162, 1067–1075. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Chattopadhyay, N.; Tfelt-Hansen, J. Diverse roles of extracellular calcium-sensing receptor in the central nervous system. J. Neurosci. Res. 2010, 88, 2073–2082. [Google Scholar] [CrossRef]
- Osterweil, E.K.; Chuang, S.C.; Chubykin, A.A.; Sidorov, M.; Bianchi, R.; Wong, R.K.; Bear, M.F. Lovastatin cor-rects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron 2013, 77, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rege, K.; Medintz, I.L. Methods in Bioengineering: Nanoscale Bioengineering and Nanomedicine; Artech House: Norwood, MA, USA, 2009. [Google Scholar]
- Zhu, X.; Castellani, R.J.; Takeda, A.; Nunomura, A.; Atwood, C.S.; Perry, G.; Smith, M.A. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: The ‘two hit’ hypothesis. Mech. Ageing Dev. 2001, 123, 39–46. [Google Scholar] [CrossRef]
- Shioda, N.; Han, F.; Fukunaga, K. Chapter 26 Role of Akt and Erk Signaling in the Neurogenesis Following Brain Ischemia. Int. Rev. Neurobiol. 2009, 85, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Alam, R.; Gorska, M.M. Mitogen-activated protein kinase signalling and ERK1/2 bistability in asthma. Clin. Exp. Allergy 2011, 41, 149–159. [Google Scholar] [CrossRef]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef]
- Feld, M.; Krawczyk, M.C.; Sol Fustinana, M.; Blake, M.G.; Baratti, C.M.; Romano, A.; Boccia, M.M. Decrease of ERK/MAPK overactivation in prefrontal cortex reverses early memory deficit in a mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 40, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Wang, X.; Aoki, T.; Lo, E.H. Downregulation of Matrix Metalloproteinase-9 and Attenuation of Edema via Inhibition of ERK Mitogen Activated Protein Kinase in Traumatic Brain Injury. J. Neurotrauma 2002, 19, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Quesada, A.; Lee, B.Y.; Micevych, P.E. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev. Neurobiol. 2008, 68, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Fusco, F.R.; Anzilotti, S.; Giampà, C.; Dato, C.; Laurenti, D.; Leuti, A.; D’Amato, L.C.; Perrone, L.; Bernardi, G.; Melone, M.A. Changes in the expression of extracellular regulated kinase (ERK 1/2) in the R6/2 mouse model of Huntington’s disease after phosphodiesterase IV inhibition. Neurobiol. Dis. 2012, 46, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Brereton, C.F.; Sutton, C.E.; Lalor, S.J.; Lavelle, E.C.; Mills, K.H.G. Inhibition of ERK MAPK Suppresses IL-23- and IL-1-Driven IL-17 Production and Attenuates Autoimmune Disease. J. Immunol. 2009, 183, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Yufune, S.; Satoh, Y.; Takamatsu, I.; Ohta, H.; Kobayashi, Y.; Takaenoki, Y.; Pagès, G.; Pouysségur, J.; Endo, S.; Kazama, T. Transient Blockade of ERK Phosphorylation in the Critical Period Causes Autistic Phenotypes as an Adult in Mice. Sci. Rep. 2015, 5, 10252. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Orozco, F.; Chitchumroonchokchai, C.; Lesinski, G.B.; Suksamrarn, S.; Failla, M.L. α-Mangostin: An-ti-inflammatory activity and metabolism by human cells. J. Agric. Food Chem. 2013, 61, 3891–3900. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Federici, G.; Bertini, E.; Piemonte, F. Analysis of glutathione: Implication in redox and detoxification. Clin. Chim. Acta 2003, 333, 19–39. [Google Scholar] [CrossRef]
- Herrera-Aco, D.R.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Sciutto-Conde, E.; Rosas-Salgado, G.; Fragoso-González, G. Alpha-mangostin: Anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem. Toxicol. 2019, 124, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, M.; Prakash, M.; Gowrishankar, S.; Rathna, J.; Pandian, S.K.; Ravi, A.V. In vitro activity of al-pha-mangostin in killing and eradicating Staphylococcus epidermidis RP62A biofilms. Appl. Microbiol. Biotechnol. 2017, 101, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Janhom, P.; Dharmasaroja, P. Neuroprotective Effects of Alpha-Mangostin on MPP + -Induced Apoptotic Cell Death in Neuroblastoma SH-SY5Y Cells. J. Toxicol. 2015, 2015, 919058. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Park, J.H.; Yoon, J.; Lee, K.Y.; Park, B. Effects of geniposide on hepatocytes undergoing epithelial-mesenchymal transition in hepatic fibrosis by targeting TGFβ/Smad and ERK-MAPK signaling pathways. Biochimie 2015, 113, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Lin, Y.; Sun, Z.; Yuan, X.; Chen, L.; Shen, B. Knowledge-guided bioinformatics model for identifying au-tism spectrum disorder diagnostic MicroRNA biomarkers. Sci. Rep. 2016, 6, 39663. [Google Scholar] [CrossRef]
- Sakagami, Y.; Iinuma, M.; Piyasena, K.; Dharmaratne, H. Antibacterial activity of α-mangostin against vancomycin resistant Enterococci (VRE) and synergism with antibiotics. Phytomedicine 2005, 12, 203–208. [Google Scholar] [CrossRef]
- Rose’Meyer, R. A review of the serotonin transporter and prenatal cortisol in the development of autism spectrum disorders. Mol. Autism. 2013, 4, 37. [Google Scholar] [CrossRef]
- Edmonson, C.; Ziats, M.N.; Rennert, O.M. Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol. Autism. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative Stress in Autism Spectrum Disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.Y.; Chong, C.W.; Murugaiyah, V. LC-QTOF-MS analysis of xanthone content in different parts of Garcinia mangostana and its influence on cholinesterase inhibition. J. Enzym. Inhib. Med. Chem. 2020, 35, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Liu, X.; Liu, J.; Cai, E.; Zhao, Y.; Li, H.; Zhang, L.; Li, P.; Gao, Y. α-Mangostin exhibits antidepressant-like effects mediated by the modification of GABAergic, serotonergic and dopaminergic systems. Nat. Prod. Res. 2018, 34, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Widowati, W.; Prahastuti, S.; Ekayanti, N.L.W.; Munshy, U.Z.; Kusuma, H.S.W.; Wibowo, S.H.B.; Amalia, A.; Widodo, W.S.; Rizal, R. Anti-Inflammation Assay of Black Soybean Extract and Its Compounds on Lipopolysaccha-ride-Induced RAW 264.7 Cell. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1374, p. 012052. [Google Scholar]
- Wang, S.-N.; Li, Q.; Jing, M.-H.; Alba, E.; Yang, X.-H.; Sabaté, R.; Han, Y.-F.; Pi, R.-B.; Lan, W.-J.; Chen, J.-K. Natural Xanthones from Garcinia mangostana with Multifunctional Activities for the Therapy of Alzheimer’s Disease. Neurochem. Res. 2016, 41, 1806–1817. [Google Scholar] [CrossRef]
- Dey, A.; De, J.N. Neuroprotective therapeutics from botanicals and phytochemicals against Huntington’s disease and related neurodegenerative disorders. J. Herb. Med. 2015, 5, 1–19. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, L.; Zhu, S.; Xu, L.; Liu, S.; Yuan, C.; Guo, Y.; Wang, X. Neuroprotection Against Parkinson’s Disease through the Activation of Akt/GSK3β Signaling Pathway by Tovophyllin A. Front. Neurosci. 2020, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Phyu, M.P.; Tangpong, J. Neuroprotective effects of xanthone derivative of Garcinia mangostana against lead-induced acetylcholinesterase dysfunction and cognitive impairment. Food Chem. Toxicol. 2014, 70, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lotter, J.S. Studies on Garcinia Mangostana Linn as a Therapeutic Intervention in an Immune-Inflammatory Model of Schizo-Phrenia. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2018. [Google Scholar]
- Oberholzer, I.; Möller, M.; Holland, B.; Dean, O.M.; Berk, M.; Harvey, B.H. Garcinia mangostana Linn displays antidepressant-like and pro-cognitive effects in a genetic animal model of depression: A bio-behavioral study in the Flinders Sensitive Line rat. Metab. Brain Dis. 2017, 33, 467–480. [Google Scholar] [CrossRef]
- Lee, C.-H.; Ying, T.-H.; Chiou, H.-L.; Hsieh, S.-C.; Wen, S.-H.; Chou, R.-H.; Hsieh, Y.-H. Alhamangostin induces apoptosis through activation of reactive oxygen species and ASK1/p38 signaling pathway in cervical cancer cells. Oncotarget 2017, 8, 47425–47439. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.A.; Zheng, S.; Su, Z.; Gao, J.; Xu, H. The Pivotal Neuroinflammatory, Therapeutic and Neuroprotective Role of Alpha-Mangostin. J. Neurol. Res. 2017, 7, 67–79. [Google Scholar] [CrossRef]
- Ashton, M.M.; Dean, O.M.; Walker, A.J.; Bortolasci, C.C.; Ng, C.H.; Hopwood, M.; Harvey, B.H.; Möller, M.; McGrath, J.J.; Marx, W.; et al. The therapeutic potential of mangosteen pericarp as an adjunctive therapy for bipolar dis-order and schizophrenia. Front. Psychiatry 2019, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.T.; Lorch, J.M.; Pridgeon, J.W.; Becnel, J.J.; Clark, G.G.; Lan, Q. The Biological Activity of α-Mangostin, a Larvicidal Botanic Mosquito Sterol Carrier Protein-2 Inhibitor. J. Med. Entomol. 2010, 47, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.; Won, J.; Jin, Y.; Hong, Y.; Hur, T.Y.; Kim, J.H.; Lee, S.R.; Hong, Y. Pathophysiological and neuro-behavioral characteristics of a propionic acid-mediated autism-like rat model. PLoS ONE 2018, 13, e0192925. [Google Scholar]
- Duggal, P.; Jadaun, K.S.; Siqqiqui, E.M.; Mehan, S. Investigation of Low Dose Cabazitaxel Potential as Microtubule Stabilizer in Experimental Model of Alzheimer’s Disease: Restoring Neuronal Cytoskeleton. Curr. Alzheimer Res. 2020, 17, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Duggal, P.; Mehan, S. Neuroprotective Approach of Anti-Cancer Microtubule Stabilizers against Tauopathy Associated Dementia: Current Status of Clinical and Preclinical Findings. J. Alzheimer’s Dis. Rep. 2019, 3, 179–218. [Google Scholar] [CrossRef] [PubMed]
- Mehan, S.; Monga, V.; Rani, M.; Dudi, R.; Ghimire, K. Neuroprotective effect of solanesol against 3-nitropropionic acid-induced Huntington’s disease-like behavioral, biochemical, and cellular alterations: Restoration of coen-zyme-Q10-mediated mitochondrial dysfunction. Indian J. Pharmacol. 2018, 50, 309. [Google Scholar] [CrossRef]
- Alam, M.M.; Minj, E.; Yadav, R.K.; Mehan, S. Neuroprotective potential of adenyl cyclase/cAMP/CREB and mitochondrial CoQ10 activator in amyotrophic lateral sclerosis rats. Curr. Bioact. Compd. 2020, 16, 1–18. [Google Scholar] [CrossRef]
- Mehan, S.; Parveen, S.; Kalra, S. Adenyl cyclase activator forskolin protects against Huntington’s disease-like neurodegenerative disorders. Neural Regen. Res. 2017, 12, 290–300. [Google Scholar] [CrossRef]
- Kim, S.J.; Guerrero, N.; Wassef, G.; Xiao, J.; Mehta, H.H.; Cohen, P.; Yen, K. The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus. Oncotarget 2016, 7, 46899–46912. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Enayati, A.; Roger, H.; Binstock, T.; Redwood, L. The role of mercury in the pathogenesis of autism. Mol. Psychiatry 2002, 7, S42–S43. [Google Scholar] [CrossRef]
- Wang, X.; Mori, T.; Sumii, T.; Lo, E.H. Hemoglobin-Induced Cytotoxicity in Rat Cerebral Cortical Neurons. Stroke 2002, 33, 1882–1888. [Google Scholar] [CrossRef]
- Bai, M.; Liu, B.; Peng, M.; Jia, J.; Fang, X.; Miao, M. Effect of Sargentodoxacuneata total phenolic acids on focal cerebral ischemia reperfusion injury rats model. Saudi J. Biol. Sci. 2019, 26, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Moneim, A.E.A. The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab. Brain Dis. 2015, 30, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.A.; Arundell, M.; Parker, K.H.; Yeoman, M.S.; O’Hare, D. Simple and rapid determination of serotonin and catecholamines in biological tissue using high-performance liquid chromatography with electrochemical detection. J. Chromatogr. B 2005, 818, 269–276. [Google Scholar] [CrossRef]
- Jamwal, S.; Singh, S.; Kaur, N.; Kumar, P. Protective effect of spermidine against excitotoxic neuronal death induced by quinolinic acid in rats: Possible neurotransmitters and neuroinflammatory mechanism. Neurotox. Res. 2015, 28, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, T. Neuroprotective Methodologies in the Treatment of Multiple Sclerosis Current Status of Clinical and Pre-clinical Findings. Curr. Drug Discov. Technol. 2021, 18, 31–46. [Google Scholar] [CrossRef]
- Ren, J.; Bai, Y.; Hao, L.; Dong, Y.; Pi, Z.; Jia, L. Amelioration of experimental autoimmune myasthenia gravis rats by blood purification treatment using 4-mercaptoethylpyridine-based adsorbent. J. Biomed. Mater. Res. Part A 2011, 98, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Parkhe, A.; Parekh, P.; Nalla, L.V.; Sharma, N.; Sharma, M.; Gadepalli, A.; Kate, A.; Khairnar, A. Protective effect of alpha mangostin on rotenone induced toxicity in rat model of Parkinson’s disease. Neurosci. Lett. 2020, 716, 134652. [Google Scholar] [CrossRef]
- Goudarzvand, M.; Javan, M.; Mirnajafi-Zadeh, J.; Mozafari, S.; Tiraihi, T. Vitamins E and D3 Attenuate Demyelination and Potentiate Remyelination Processes of Hippocampal Formation of Rats Following Local Injection of Ethidium Bromide. Cell. Mol. Neurobiol. 2010, 30, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Rajdev, K.; Mehan, S. Neuroprotective Methodologies of Co-Enzyme Q10 Mediated Brain Hemorrhagic Treatment: Clinical and Pre-Clinical Findings. CNS Neurol. Disord. Drug Targets (Former. Curr. Drug Targets CNS Neurol. Disord.) 2019, 18, 446–465. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Bala, R.; Khanna, D.; Mehan, S.; Kalra, S. Experimental evidence for the potential of lycopene in the management of scopolamine induced amnesia. RSC Adv. 2015, 5, 72881–72892. [Google Scholar] [CrossRef]
- Bronson, M.E.; Wages, T.D.; Beddingfield, T.; Horner, J.M.; Willis, L.L.; Scott, J.L., Jr. Morphine, MDMA, MDA, and nexus produce a conditioned place preference in newly hatched chickens. Exp. Clin. Psychopharmacol. 1996, 4, 354. [Google Scholar] [CrossRef]
- Dudi, R.; Mehan, S. Neuroprotection of brain permeable Forskolin ameliorates behavioral, biochemical and histopatho-logical alterations in rat model of intracerebral hemorrhage. Pharmaspire 2018, 10, 68–86. [Google Scholar]
- Deshmukh, R.; Sharma, V.; Mehan, S.; Sharma, N.; Bedi, K. Amelioration of intracerebroventricularstreptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine—A PDE1 inhibitor. Eur. J. Pharmacol. 2009, 620, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Rajdev, K.; Siddiqui, E.M.; Jadaun, K.S.; Mehan, S. Neuroprotective potential of solanesol in acombined model of intracerebral and intraventricular hemorrhage in rats. IBRO Rep. 2020, 8, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, C.; Liu, W.; Luo, P.; Zhang, L.; Wang, Y.; Wang, Z.; Fei, Z. A novel rat model of blast-induced traumatic brain injury simulating different damage degree: Implications for morphological, neurological, and biomarker changes. Front. Cell. Neurosci. 2015, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, V.; Liu, Z.; Allexandre, D.; Zhang, L.; Wang, X.-F.; Pioro, E.P.; Yue, G.H. Brain White Matter Shape Changes in Amyotrophic Lateral Sclerosis (ALS): A Fractal Dimension Study. PLoS ONE 2013, 8, e73614. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Vignos, M.; Dudman, J.; Chang, A.; Fisher, E.; Staugaitis, S.M.; Battapady, H.; Mork, S.; Ontaneda, D.; Jones, S.E.; et al. Cortical neuronal densities and cerebral white matter demyelination in multiple sclerosis: A retrospective study. Lancet Neurol. 2018, 17, 870–884. [Google Scholar] [CrossRef]
- Carassiti, D.; Altmann, D.R.; Petrova, N.; Pakkenberg, B.; Scaravilli, F.; Schmierer, K. Neuronal loss, demyelination and vol-ume change in the multiple sclerosis neocortex. Neuropathol. Appl. Neurobiol. 2018, 44, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Pucilowska, J.; Vithayathil, J.; Tavares, E.J.; Kelly, C.; Karlo, J.C.; Landreth, G.E. The 16p11. 2 deletion mouse model of autism exhibits altered cortical progenitor proliferation and brain cytoarchitecture linked to the ERK MAPK path-way. J. Neurosci. 2015, 35, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.; Braeutigam, S.; Dawes, J.M.; Krsnik, Z.; Kostovic, I.; Coutinho, E.; Dewing, J.M.; Horton, C.A.; Gomez-Nicola, D.; Menassa, D.A. Autism Spectrum Disorders: Multiple Routes to, and Multiple Consequences of, Abnormal Synaptic Function and Connectivity. Neuroscientist 2021, 27, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Kepa, A.; Medina, L.M.; Erk, S.; Srivastava, D.P.; Fernandes, A.; Toro, R.; Lévi, S.; Ruggeri, B.; Fernandes, C.; Degenhardt, F.; et al. Associations of the intellectual disability gene MYT1L with helix–loop–helix gene expression, hippo-campus volume and hippocampus activation during memory retrieval. Neuropsychopharmacology 2017, 42, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Pucilowska, J.; Vithayathil, J.; Pagani, M.; Kelly, C.; Karlo, J.C.; Robol, C.; Morella, I.; Gozzi, A.; Brambilla, R.; Landreth, G.E. Pharmacological Inhibition of ERK Signaling Rescues Pathophysiology and Behavioral Phenotype Associated with 16p11.2 Chromosomal Deletion in Mice. J. Neurosci. 2018, 38, 6640–6652. [Google Scholar] [CrossRef] [PubMed]
- Catorce, M.N.; Acero, G.; Pedraza-Chaverri, J.; Fragoso, G.; Govezensky, T.; Gevorkian, G. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in C57BL/6J mice. J. Neuroimmunol. 2016, 297, 20–27. [Google Scholar] [CrossRef]
- Yao, L.; Gu, X.; Song, Q.; Wang, X.; Huang, M.; Hu, M.; Hou, L.; Kang, T.; Chen, J.; Chen, H.; et al. Nanoformulated alpha-mangostin ameliorates Alzheimer’s disease neuropathology by elevating LDLR expression and accelerating amyloid-beta clearance. J. Control. Release 2016, 226, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Z.; Xu, J.R.; Wang, Y.X.; Hou, L.N.; Qiu, Y.; Chen, H.Z. A-mangostin, a polyphenolic xanthone derivative from mangosteen, attenuates β-amyloid oligomers-induced neurotoxicity by inhibiting amyloid aggregation. Neuropharmacology 2012, 62, 871–881. [Google Scholar] [CrossRef]
- Aliashrafi, M.; Nasehi, M.; Zarrindast, M.R.; Joghataei, M.T.; Zali, H.; Siadat, S.D. Association of microbiota-derived propionic acid and Alzheimer’s disease; bioinformatics analysis. J. Diabetes Metab. Disord. 2020, 19, 783–804. [Google Scholar] [CrossRef]
- MacFabe, D.F.; Cain, D.P.; Rodriguez-Capote, K.; Franklin, A.E.; Hoffman, J.E.; Boon, F.; Taylor, A.R.; Kavaliers, M.; Ossenkopp, K.P. Neurobiological effects of intraventricular propionic acid in rats: Possible role of short chain fatty ac-ids on the pathogenesis and characteristics of autism spectrum disorders. Behav. Brain Res. 2007, 176, 149–169. [Google Scholar] [CrossRef]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. Probiotic treatment reduces depressive-like be-haviour in rats independently of diet. Psychoneuroendocrinology 2017, 79, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Dilnashin, H.; Birla, H.; Singh, S.S.; Zahra, W.; Rathore, A.S.; Singh, B.K.; Singh, S.P. The Role of PI3K/Akt and ERK in Neurodegenerative Disorders. Neurotox. Res. 2019, 35, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Albert-Gascó, H.; Ros-Bernal, F.; Castillo-Gómez, E.; Olucha-Bordonau, F.E. MAP/ERK Signaling in Developing Cognitive and Emotional Function and Its Effect on Pathological and Neurodegenerative Processes. Int. J. Mol. Sci. 2020, 21, 4471. [Google Scholar] [CrossRef]
- Finegold, S.M. Desulfovibrio species are potentially important in regressive autism. Med. Hypotheses 2011, 77, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Vedi, M.; Rasool, M.; Sabina, E.P. Amelioration of bromobenzene hepatotoxicity by Withaniasomnifera pretreatment: Role of mitochondrial oxidative stress. Toxicol. Rep. 2014, 1, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Markus, S. Increased expression of pro-inflammatory cytokines and lack of up-regulation of anti-inflammatory cytokines in early distemper CNS lesions. J. Neuroimmunol. 2002, 125, 30–41. [Google Scholar] [CrossRef]
- Mirza, R.; Sharma, B. A selective peroxisome proliferator-activated receptor-γ agonist benefited propionic acid induced autism-like behavioral phenotypes in rats by attenuation of neuroinflammation and oxidative stress. Chem. Interact. 2019, 311, 108758. [Google Scholar] [CrossRef] [PubMed]

| Groups | ERK (ng/mL) | R-MBP (µg/mL) |
|---|---|---|
| Vehicle Control | 100.5 ± 0.85 | 100.3 ± 0.82 |
| Sham Control | 100.7 ± 0.58 | 100.7 ± 0.54 |
| AMG200 per se | 100.6 ± 0.61 | 100.7 ± 0.59 |
| PPA | 250.7 ± 0.51 * | 152.9 ± 0.55 * |
| PPA + AMG100 | 210.4 ± 0.53 @ | 145.5 ± 0.60 @ |
| PPA + AMG200 | 190 ± 0.59 @# | 130.7 ± 0.59 @# |
| Groups | Caspase-3 (ng/mL) | Bax (ng/mg Protein) | Bcl-2 (ng/mg Protein) |
|---|---|---|---|
| Vehicle Control | 100.32 ± 0.52 | 4.80 ± 0.25 | 28.19 ± 0.25 |
| Sham Control | 100.85 ± 0.54 | 4.80 ± 0.25 | 28.21 ± 0.26 |
| AMG200 per se | 100.50 ± 0.52 | 4.80 ± 0.25 | 28.20 ± 0.26 |
| PPA | 150.93 ± 0.91 * | 10.73 ± 0.25 * | 18.67 ± 0.56 * |
| PPA + AMG100 | 140.92 ± 0.56 @ | 8.70 ± 0.23 @ | 21.16 ± 0.58 @ |
| PPA + AMG200 | 130.48 ± 0.55 @# | 6.92 ± 0.25 @# | 23.67 ± 0.24 @# |
| Neurotransmitters Levels | ||||
|---|---|---|---|---|
| Groups | 5-HT (ng/mg Protein) | Glutamate (ng/mg Protein) | Dopamine (ng/mg Protein) | Ach (ng/mg Protein) |
| Vehicle Control | 36.63 ± 0.55 | 103.66 ± 0.57 | 85.48 ± 0.34 | 9.48 ± 0.26 |
| Sham Control | 37.57 ± 0.63 | 103.52 ± 0.38 | 87.54 ± 0.54 | 9.49 ± 0.36 |
| AMG200 per se | 36.72 ± 0.59 | 101.52 ± 0.47 | 85.35 ± 0.60 | 9.49 ± 0.26 |
| PPA | 12.81 ± 0.52 * | 278.90 ± 0.58 * | 29.36 ± 0.55 * | 0.80 ± 0.01 * |
| PPA + AMG100 | 15.51 ± 0.60 @ | 201.82 ± 0.60 @ | 44.48 ± 0.52 @ | 5.71 ± 0.61 @ |
| PPA + AMG200 | 18.29 ± 0.59 @# | 172.34 ± 0.59 @# | 48.85 ± 0.56 @# | 7.18 ± 0.37 @# |
| Groups | Neuroinflammatory Markers | |
|---|---|---|
| TNF-α (pg/mg Protein) | IL-1β (pg/mg Protein) | |
| Vehicle Control | 28.83 ± 0.84 | 12.48 ± 0.54 |
| Sham Control | 28.93 ± 0.85 | 12.56 ± 0.79 |
| AMG200 per se | 28.41 ± 0.57 | 12.62 ± 0.68 |
| PPA | 66.93 ± 0.99 * | 25.90 ± 0.37 * |
| PPA + AMG100 | 60.29 ± 0.58 @ | 20.93 ± 0.37 @ |
| PPA + AMG200 | 48.37 ± 0.50 @# | 16.82 ± 0.33 @# |
| Oxidative Stress Markers | ||||||
|---|---|---|---|---|---|---|
| Groups | AchE (µM/mg Protein) | LDH (Unit/mg Protein) | SOD (µM/mg Protein) | MDA (nM/mg Protein) | Nitrite (µM/mg Protein) | GSH (µM/mg Protein) |
| Vehicle Control | 16.30 ± 0.72 | 103.41 ± 0.88 | 453.25 ± 0.56 | 28.69 ± 0.58 | 4.14 ± 0.43 | 31.45 ± 0.35 |
| Sham Control | 16.92 ± 0.72 | 104.50 ± 0.97 | 453.54 ± 0.59 | 28.91 ± 0.58 | 4.14 ± 0.36 | 30.65 ± 0.40 |
| AMG200 per se | 16.31 ± 0.79 | 103.89 ± 0.70 | 453.53 ± 0.34 | 28.92 ± 0.37 | 4.15 ± 0.35 | 31.01 ± 0.40 |
| PPA | 48.89 ± 0.85 * | 377.45 ± 0.79 * | 310.53 ± 0.61 * | 66.86 ± 0.55 * | 9.92 ± 0.25 * | 6.60 ± 0.37 * |
| PPA + AMG100 | 35.38 ± 0.62 @ | 282.72 ± 0.89 @ | 323.38 ± 0.54 @ | 60.71 ± 0.69 @ | 7.42 ± 0.36 @ | 11.67 ± 0.36 @ |
| PPA + AMG200 | 28.32 ± 0.59 @# | 257.21 ± 0.56 @# | 340.25 ± 0.57 @# | 53.25 ± 0.58 @# | 5.87 ± 0.24 @# | 18.84 ± 0.57 @# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.; Khera, R.; Rahi, S.; Mehan, S.; Makeen, H.A.; Khormi, Y.H.; Rehman, M.U.; Khan, A. Neuroprotective Effect of α-Mangostin in Ameliorating Propionic Acid-Induced Experimental Model of Autism in Wistar Rats. Brain Sci. 2021, 11, 288. https://doi.org/10.3390/brainsci11030288
Tiwari A, Khera R, Rahi S, Mehan S, Makeen HA, Khormi YH, Rehman MU, Khan A. Neuroprotective Effect of α-Mangostin in Ameliorating Propionic Acid-Induced Experimental Model of Autism in Wistar Rats. Brain Sciences. 2021; 11(3):288. https://doi.org/10.3390/brainsci11030288
Chicago/Turabian StyleTiwari, Aarti, Rishabh Khera, Saloni Rahi, Sidharth Mehan, Hafiz Antar Makeen, Yahya H. Khormi, Muneeb U Rehman, and Andleeb Khan. 2021. "Neuroprotective Effect of α-Mangostin in Ameliorating Propionic Acid-Induced Experimental Model of Autism in Wistar Rats" Brain Sciences 11, no. 3: 288. https://doi.org/10.3390/brainsci11030288
APA StyleTiwari, A., Khera, R., Rahi, S., Mehan, S., Makeen, H. A., Khormi, Y. H., Rehman, M. U., & Khan, A. (2021). Neuroprotective Effect of α-Mangostin in Ameliorating Propionic Acid-Induced Experimental Model of Autism in Wistar Rats. Brain Sciences, 11(3), 288. https://doi.org/10.3390/brainsci11030288









