Semi-Automatic MRI Muscle Volumetry to Diagnose and Monitor Hereditary and Acquired Polyneuropathies
Abstract
1. Introduction
2. Materials and Methods
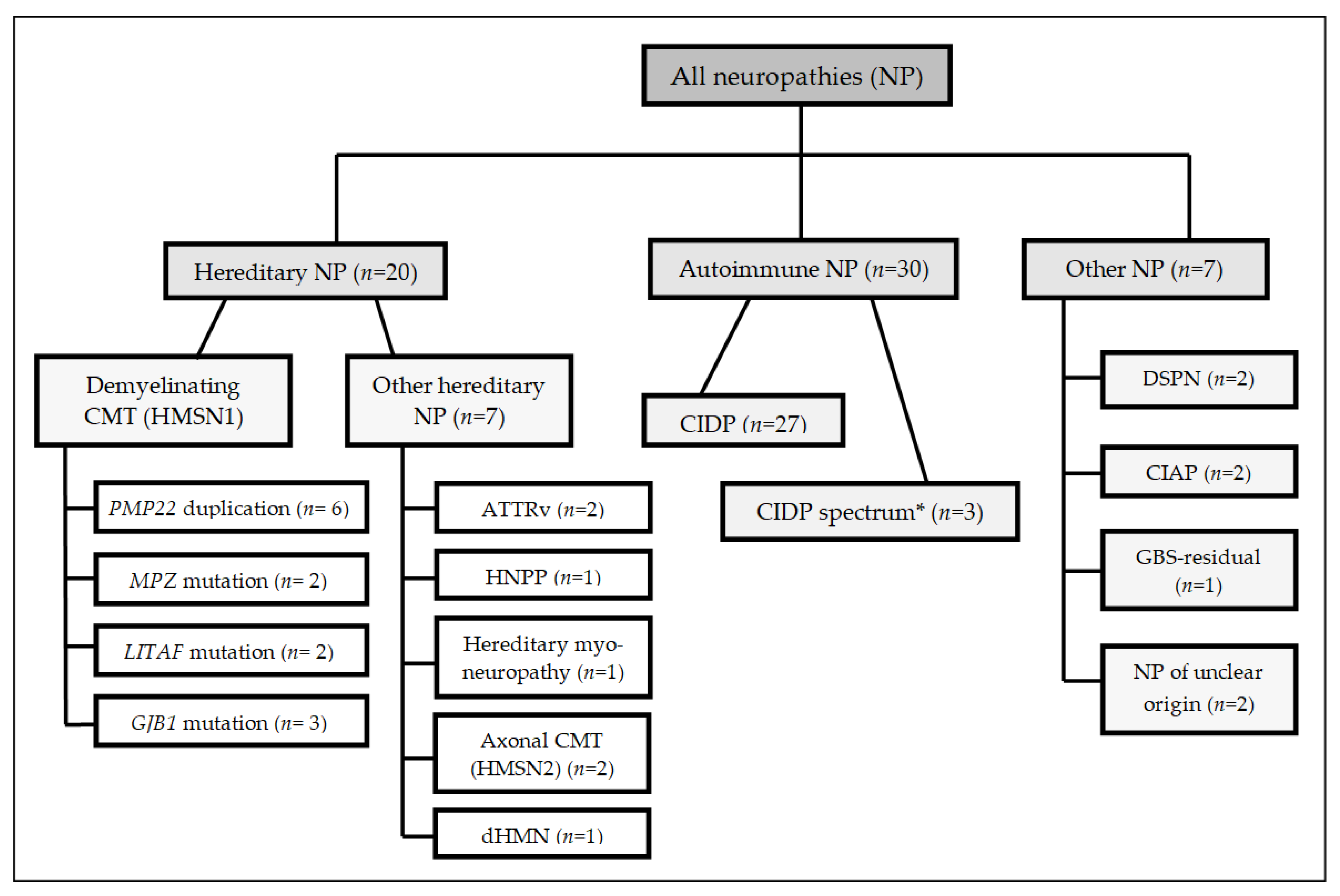
2.1. Patient Selection
2.2. Clinical Examination
2.3. Nerve Conduction Studies
2.4. MRI Studies
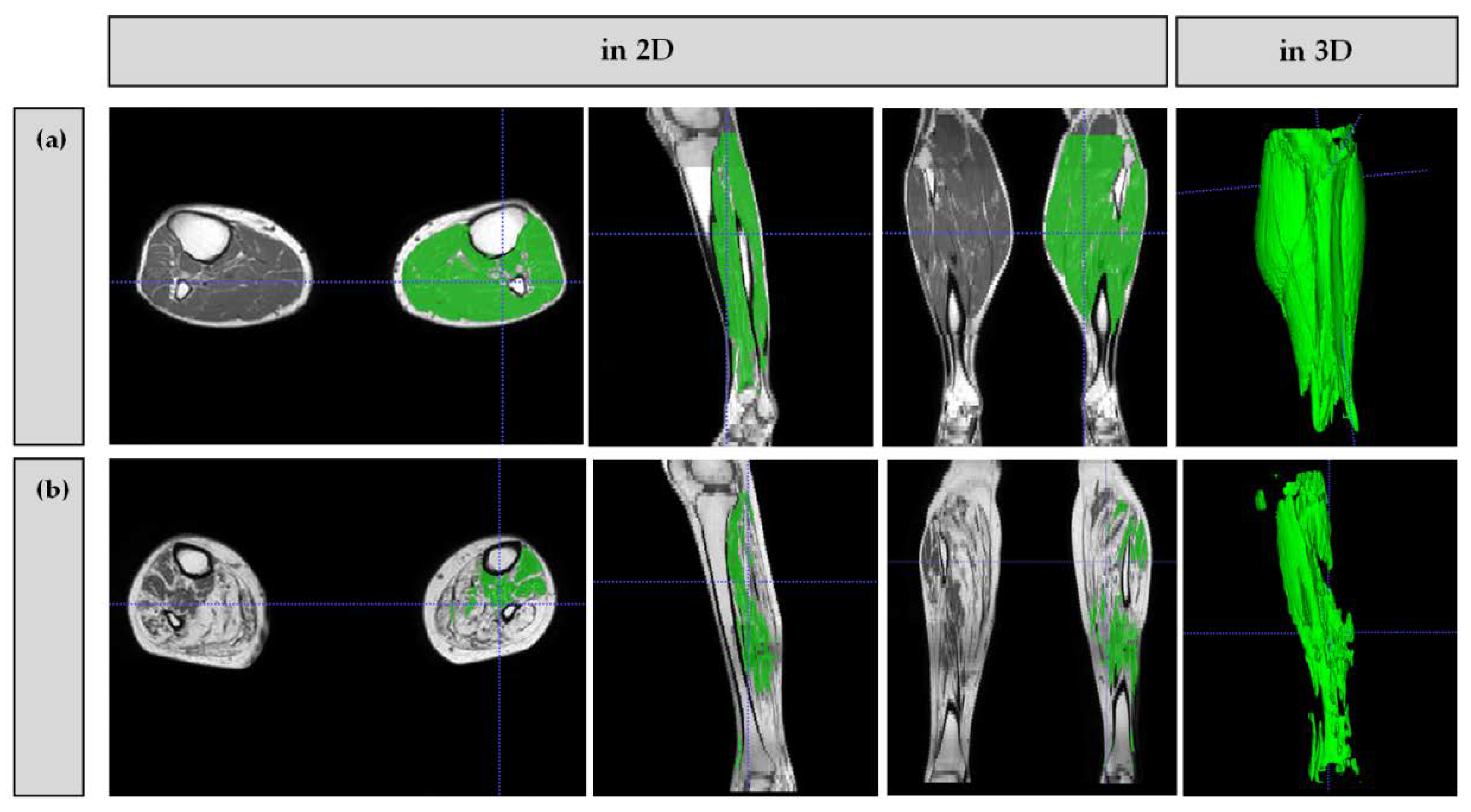
2.5. Image Analysis
2.6. Statistics
3. Results
3.1. Clinical Description of the CIDP and Demyelinating CMT Collective
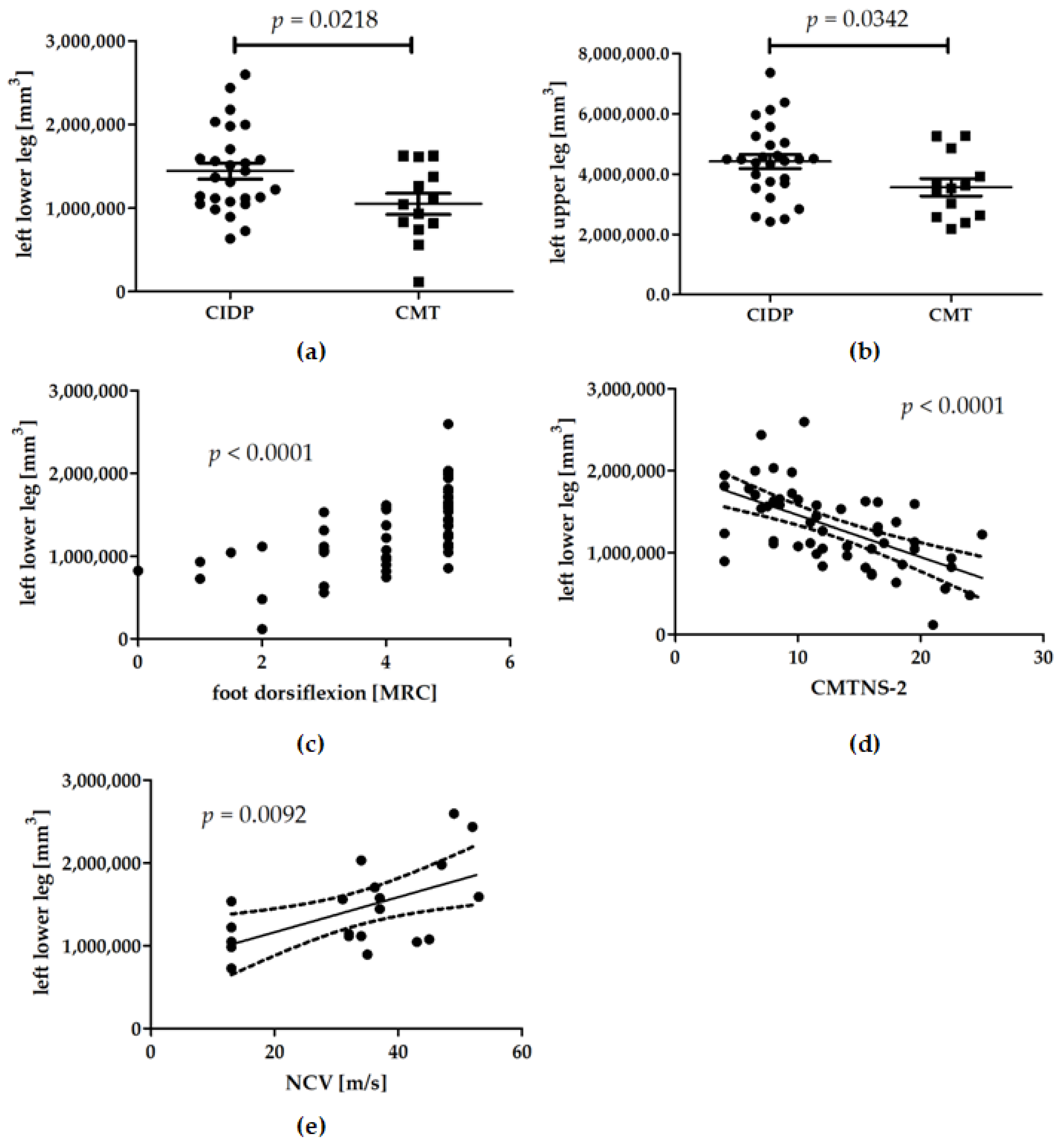
3.2. Muscle Volumes
3.3. Longitudinal Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Acquisition Matrix |
| ATTRv | Hereditary Transthyretin-Related Systemic Amyloidosis |
| CIAP | Chronic Idiopathic Axonal Polyneuropathy |
| CIDP | Chronic Inflammatory Demyelinating Disease |
| CMAP | Compound Motor Action Potentials |
| CMT | Charcot-Marie-Tooth Disease |
| CMTNS-2 | Charcot-Marie-Tooth Neuropathy Score Version 2 |
| dHMN | Distal Hereditary Motor Neuropathy |
| DICOM | Digital Imaging and Communications in Medicine |
| DML | Distal Motor Latency |
| DSPN | Diabetic Distal Symmetric Polyneuropathy |
| FA | Flip Angle |
| FOV | Field of View |
| GBS | Guillain–BarréSyndrome |
| HMSN | Hereditary Motor and Sensory Neuropathy |
| HNPP | Hereditary Neuropathy with Pressure Palsies |
| ITK | Insight Segmentation and Registration Toolkit |
| IVIG | Intravenous Immunoglobulin |
| MADSAM | Multifocal Acquired Demyelinating Sensory and Motor Neuropathy |
| MMN | Multifocal Motor Neuropathy |
| MRC | Medical Research Council |
| MRI | Magnet Resonance Imaging |
| NCS | Nerve Conduction Studies |
| NCV | Nerve Conduction Velocity |
| NIFTI | Neuroimaging Informatics Technology Initiative |
| NSA | Number of Signal Averages |
| ROI | Region of Interest |
| SG | Slice Gap |
| SNAP | Sensory Nerve Action Potentials |
| ST | Slice Thickness |
| STIR | Short Tau Inversion Recovery |
| T1w | T1-weighted |
| TE | Echo Time |
| TR | Repetition Time |
| TSE | Turbo Spin Echo |
| TTR | Transthyretin |
References
- Reilly, M.M.; Shy, M.E.; Muntoni, F.; Pareyson, D. 168th ENMC International Workshop: Outcome measures and clinical trials in Charcot-Marie-Tooth disease (CMT). Neuromuscul. Disord. 2010, 20, 839–846. [Google Scholar] [CrossRef]
- Cortese, A.; Wilcox, J.E.; Polke, J.M.; Poh, R.; Skorupinska, M.; Rossor, A.M.; Laura, M.; Tomaselli, P.J.; Houlden, H.; Shy, M.E.; et al. Targeted next-generation sequencing panels in the diagnosis of Charcot-Marie-Tooth disease. Neurology 2020, 94, e51–e61. [Google Scholar] [CrossRef]
- Broers, M.C.; Bunschoten, C.; Nieboer, D.; Lingsma, H.F.; Jacobs, B.C. Incidence and Prevalence of Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Systematic Review and Meta-Analysis. Neuroepidemiology 2019, 52, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marmiesse, A.; Gouveia, S.; Couce, M.L. NGS Technologies as a Turning Point in Rare Disease Research, Diagnosis and Treatment. Curr. Med. Chem. 2018, 25, 404–432. [Google Scholar] [CrossRef]
- Young, P.; Attarian, S.; Youcef, B.; Rinaudo, P.; Fitoussi, S.; Bertrand, V.; Hajj, R.; Nabirotchkin, S.; Cohen, D. Efficacy and safety of PXT3003 in patients with Charcot-Marie-Tooth type 1A (CMT1A): Results of PLEO-CMT an International Pivotal Phase 3 trial. For the PLEO CMT Investigators. In Proceedings of European Journal of Neurology; WILEY: Hoboken, NJ, USA, 2019; p. 74. [Google Scholar]
- Dohrn, M.F.; Ihne, S.; Hegenbart, U.; Medina, J.; Züchner, S.L.; Coelho, T.; Hahn, K. Targeting transthyretin—Mechanism-based treatment approaches and future perspectives in hereditary amyloidosis. J. Neurochem. 2020. [Google Scholar] [CrossRef]
- Allen, J.A.; Gelinas, D.F.; Freimer, M.; Runken, M.C.; Wolfe, G.I. Immunoglobulin administration for the treatment of CIDP: IVIG or SCIG? J. Neurol. Sci. 2019, 408, 116497. [Google Scholar] [CrossRef] [PubMed]
- Nobile-Orazio, E.; Cocito, D.; Jann, S.; Uncini, A.; Beghi, E.; Messina, P.; Antonini, G.; Fazio, R.; Gallia, F.; Schenone, A.; et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: A randomised controlled trial. Lancet Neurol. 2012, 11, 493–502. [Google Scholar] [CrossRef]
- Rajabally, Y.A. Long-term immunoglobulin therapy for chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve 2015, 51, 657–661. [Google Scholar] [CrossRef]
- Van Schaik, I.N.; Bril, V.; van Geloven, N.; Hartung, H.P.; Lewis, R.A.; Sobue, G.; Lawo, J.P.; Praus, M.; Mielke, O.; Durn, B.L.; et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2018, 17, 35–46. [Google Scholar] [CrossRef]
- Morrow, J.M.; Sinclair, C.D.; Fischmann, A.; Machado, P.M.; Reilly, M.M.; Yousry, T.A.; Thornton, J.S.; Hanna, M.G. MRI biomarker assessment of neuromuscular disease progression: A prospective observational cohort study. Lancet Neurol. 2016, 15, 65–77. [Google Scholar] [CrossRef]
- Bunschoten, C.; Jacobs, B.C.; Van den Bergh, P.Y.K.; Cornblath, D.R.; van Doorn, P.A. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. 2019, 18, 784–794. [Google Scholar] [CrossRef]
- Dortch, R.D.; Dethrage, L.M.; Gore, J.C.; Smith, S.A.; Li, J. Proximal nerve magnetization transfer MRI relates to disability in Charcot-Marie-Tooth diseases. Neurology 2014, 83, 1545–1553. [Google Scholar] [CrossRef]
- Sookhoo, S.; Mackinnon, I.; Bushby, K.; Chinnery, P.F.; Birchall, D. MRI for the demonstration of subclinical muscle involvement in muscular dystrophy. Clin. Radiol. 2007, 62, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Yang, G.; Gerig, G. ITK-SNAP: An interactive tool for semi-automatic segmentation of multi-modality biomedical images. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016, 2016, 3342–3345. [Google Scholar] [CrossRef]
- Bette, S.; Barz, M.; Ly Nham, H.; Huber, T.; Berndt, M.; Sales, A.; Schmidt-Graf, F.; Meyer, H.S.; Ryang, Y.M.; Meyer, B.; et al. Image Analysis Reveals Microstructural and Volumetric Differences in Glioblastoma Patients with and without Preoperative Seizures. Cancers 2020, 12, 994. [Google Scholar] [CrossRef]
- Uysal, G.; Ozturk, M. Hippocampal atrophy based Alzheimer’s disease diagnosis via machine learning methods. J. Neurosci. Methods 2020, 337, 108669. [Google Scholar] [CrossRef]
- Muller, M.; Dohrn, M.F.; Romanzetti, S.; Gadermayr, M.; Reetz, K.; Kramer, N.A.; Kuhl, C.; Schulz, J.B.; Gess, B. Semi-automated volumetry of MRI serves as a biomarker in neuromuscular patients. Muscle Nerve 2020, 61, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Medical Research Council. Aids to the Examination of the Peripheral Nervous System; Memorandum No. 45; Her Majesty’s Stationary Office: London, UK, 1976. [Google Scholar]
- Shy, M.E.; Blake, J.; Krajewski, K.; Fuerst, D.R.; Laura, M.; Hahn, A.F.; Li, J.; Lewis, R.A.; Reilly, M. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology 2005, 64, 1209–1214. [Google Scholar] [CrossRef]
- Murphy, S.M.; Herrmann, D.N.; McDermott, M.P.; Scherer, S.S.; Shy, M.E.; Reilly, M.M.; Pareyson, D. Reliability of the CMT neuropathy score (second version) in Charcot-Marie-Tooth disease. J. Peripher. Nerv. Syst. 2011, 16, 191–198. [Google Scholar] [CrossRef]
- Li, X.; Morgan, P.S.; Ashburner, J.; Smith, J.; Rorden, C. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods 2016, 264, 47–56. [Google Scholar] [CrossRef]
- Mahdi-Rogers, M.; Hughes, R.A. Epidemiology of chronic inflammatory neuropathies in southeast England. Eur. J. Neurol. 2014, 21, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Van den Bergh, P.Y.; Hadden, R.D.; Bouche, P.; Cornblath, D.R.; Hahn, A.; Illa, I.; Koski, C.L.; Leger, J.M.; Nobile-Orazio, E.; Pollard, J.; et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First revision. Eur. J. Neurol. 2010, 17, 356–363. [Google Scholar] [CrossRef]
- Pareyson, D.; Scaioli, V.; Laurà, M. Clinical and electrophysiological aspects of Charcot-Marie-Tooth disease. Neuromolecular. Med. 2006, 8, 3–22. [Google Scholar] [CrossRef]
- Banchs, I.; Casasnovas, C.; Albertí, A.; De Jorge, L.; Povedano, M.; Montero, J.; Martínez-Matos, J.A.; Volpini, V. Diagnosis of Charcot-Marie-Tooth disease. J. Biomed. Biotechnol. 2009, 2009, 985415. [Google Scholar] [CrossRef]
- Lehmann, H.C.; Burke, D.; Kuwabara, S. Chronic inflammatory demyelinating polyneuropathy: Update on diagnosis, immunopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2019, 90, 981–987. [Google Scholar] [CrossRef]
- Wattjes, M.P.; Kley, R.A.; Fischer, D. Neuromuscular imaging in inherited muscle diseases. Eur. Radiol. 2010, 20, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Pichiecchio, A.; Allsop, J.; Messina, S.; Pane, M.; Muntoni, F. Muscle MRI in inherited neuromuscular disorders: Past, present, and future. J. Magn. Reson. Imaging 2007, 25, 433–440. [Google Scholar] [CrossRef]
- Kugathasan, U.; Evans, M.R.B.; Morrow, J.M.; Sinclair, C.D.J.; Thornton, J.S.; Yousry, T.A.; Hornemann, T.; Suriyanarayanan, S.; Owusu-Ansah, K.; Lauria, G.; et al. Development of MRC Centre MRI calf muscle fat fraction protocol as a sensitive outcome measure in Hereditary Sensory Neuropathy Type 1. J. Neurol. Neurosurg. Psychiatry 2019, 90, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Chrzanowski, S.M.; Baligand, C.; Willcocks, R.J.; Deol, J.; Schmalfuss, I.; Lott, D.J.; Daniels, M.J.; Senesac, C.; Walter, G.A.; Vandenborne, K. Multi-slice MRI reveals heterogeneity in disease distribution along the length of muscle in Duchenne muscular dystrophy. Acta Myol. 2017, 36, 151–162. [Google Scholar]
- Forbes, S.C.; Willcocks, R.J.; Rooney, W.D.; Walter, G.A.; Vandenborne, K. MRI quantifies neuromuscular disease progression. Lancet Neurol. 2016, 15, 26–28. [Google Scholar] [CrossRef]
- Willis, T.A.; Hollingsworth, K.G.; Coombs, A.; Sveen, M.L.; Andersen, S.; Stojkovic, T.; Eagle, M.; Mayhew, A.; de Sousa, P.L.; Dewar, L.; et al. Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: A multicentre longitudinal study. PLoS ONE 2013, 8, e70993. [Google Scholar] [CrossRef]
- Gadermayr, M.; Disch, C.; Muller, M.; Merhof, D.; Gess, B. A comprehensive study on automated muscle segmentation for assessing fat infiltration in neuromuscular diseases. Magn. Reson. Imaging 2018, 48, 20–26. [Google Scholar] [CrossRef]
- Bas, J.; Ogier, A.C.; Le Troter, A.; Delmont, E.; Leporq, B.; Pini, L.; Guye, M.; Parlanti, A.; Lefebvre, M.N.; Bendahan, D.; et al. Fat fraction distribution in lower limb muscles of patients with CMT1A: A quantitative MRI study. Neurology 2020, 94, e1480–e1487. [Google Scholar] [CrossRef] [PubMed]
- Strijkers, G.J.; Mulder, W.J.; van Tilborg, G.A.; Nicolay, K. MRI contrast agents: Current status and future perspectives. Anticancer Agents Med. Chem. 2007, 7, 291–305. [Google Scholar] [CrossRef] [PubMed]
| Diagnosis | n | n ♂ | n ♀ | Age (Years) | Muscle Volume Left Thigh (cm3) | Muscle Volume Left Lower Leg (cm3) | CMTNS-2 (x/36) | Foot Dorsiflexion Strength (x/5 by MRC) | Pallesthesia (Malleolar) (x/8) |
|---|---|---|---|---|---|---|---|---|---|
| Typical CIDP | 27 | 23 | 4 | 66.2 ± 8.8 | 4423.6 ± 1207.7 | 1442.6 ± 501.2 | 12.0 ± 5.2 | 5 ± 0.7 | 3.0 ± 2.2 |
| CIDP spectrum * | 3 | 2 | 1 | 54.7 ± 4.2 | 4291.3 ± 1626.9 | 1461.5 ± 437.8 | 10.0 ± 4.0 | 5 ± 0.3 | 2.0 ± 2.8 |
| CMT (demyelinating) | 13 | 6 | 7 | 48.5 ± 13.8 | 3565.2 ± 1041.3 | 1050.1 ± 451.9 | 15.9 ± 4.8 | 4 ± 0.9 | 2.7 ± 2.3 |
| PMP22duplication | 6 | 3 | 3 | 49.5 ± 11.3 | 3189.2 ± 1140.2 | 877.9 ± 484.6 | 18.5 ± 2.9 | 3 ± 1.2 | 3.3 ± 1.8 |
| MPZ mutation | 2 | 1 | 1 | 53.0 ± 24.0 | 4355.3 ± 1289.7 | 1443.2 ± 253.9 | 10.0 ± 2.8 | 5 ± 0.0 | 5.8 ± 1.1 |
| LITAF mutation | 2 | 0 | 2 | 43.5 ± 21.9 | 3767.6 ± 216.4 | 1366.4 ± 365.4 | 11.8 ± 5.3 | 5 ± 0.0 | 5.3 ± 2.3 |
| GJB1 mutation | 3 | 2 | 1 | 47.0 ± 15.4 | 3655.7 ± 1139.6 | 921.5 ± 413.3 | 17.3 ± 5.0 | 4 ± 0.3 | 1.7 ± 2.5 |
| ATTRv amyloidosis | 2 | 2 | 0 | 62.5 ± 13.4 | 3467.8 ± 87.6 | 1053.2 ± 282.7 | 17.5 ± 1.4 | 5 ± 0.0 | 0.0 ± 0.0 |
| Other hereditary NP | 5 | 4 | 1 | 51.8 ± 7.8 | 4780.8 ± 752.5 | 1390.6 ± 545.9 | 12.0 ± 7.5 | 4 ± 1.0 | 4.1 ± 2.6 |
| DSPN | 2 | 2 | 0 | 68.0 ± 11.3 | 4253.4 ± 2341.6 | 1477.2 ± 343.6 | 6.8 ± 3.9 | 5 ± 0.0 | 3.0 ± 2.8 |
| CIAP | 2 | 1 | 1 | 61.5 ± 3.5 | 4412.5 ± 1447.7 | 1362.9 ± 408.9 | 11.3 ± 3.9 | 4.5 ± 0.5 | 0.0 ± 0.0 |
| GBS-residual | 1 | 0 | 1 | 76.0 | 2296.2 | 824.4 | 22.5 | 0 | 2.5 |
| Unclear NP | 2 | 1 | 1 | 64.5 ± 6.4 | 3752.2 ± 1628.9 | 1545.8 ± 377.1 | 4.0 | 5 ± 0.0 | 4.0 ± 0.0 |
| All patients | 57 | 42 | 15 | 60.2 ± 12.3 | 4151.4 ± 1210.7 | 1327.0 ± 482.6 | 12.9 ± 5.6 | 5 ± 0.9 | 2.8 ± 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bähr, F.S.; Gess, B.; Müller, M.; Romanzetti, S.; Gadermayr, M.; Kuhl, C.; Nebelung, S.; Schulz, J.B.; Dohrn, M.F. Semi-Automatic MRI Muscle Volumetry to Diagnose and Monitor Hereditary and Acquired Polyneuropathies. Brain Sci. 2021, 11, 202. https://doi.org/10.3390/brainsci11020202
Bähr FS, Gess B, Müller M, Romanzetti S, Gadermayr M, Kuhl C, Nebelung S, Schulz JB, Dohrn MF. Semi-Automatic MRI Muscle Volumetry to Diagnose and Monitor Hereditary and Acquired Polyneuropathies. Brain Sciences. 2021; 11(2):202. https://doi.org/10.3390/brainsci11020202
Chicago/Turabian StyleBähr, Friederike S., Burkhard Gess, Madlaine Müller, Sandro Romanzetti, Michael Gadermayr, Christiane Kuhl, Sven Nebelung, Jörg B. Schulz, and Maike F. Dohrn. 2021. "Semi-Automatic MRI Muscle Volumetry to Diagnose and Monitor Hereditary and Acquired Polyneuropathies" Brain Sciences 11, no. 2: 202. https://doi.org/10.3390/brainsci11020202
APA StyleBähr, F. S., Gess, B., Müller, M., Romanzetti, S., Gadermayr, M., Kuhl, C., Nebelung, S., Schulz, J. B., & Dohrn, M. F. (2021). Semi-Automatic MRI Muscle Volumetry to Diagnose and Monitor Hereditary and Acquired Polyneuropathies. Brain Sciences, 11(2), 202. https://doi.org/10.3390/brainsci11020202







