Shaken Baby Syndrome: Magnetic Resonance Imaging Features in Abusive Head Trauma
Abstract
:1. Introduction
2. Imaging Features
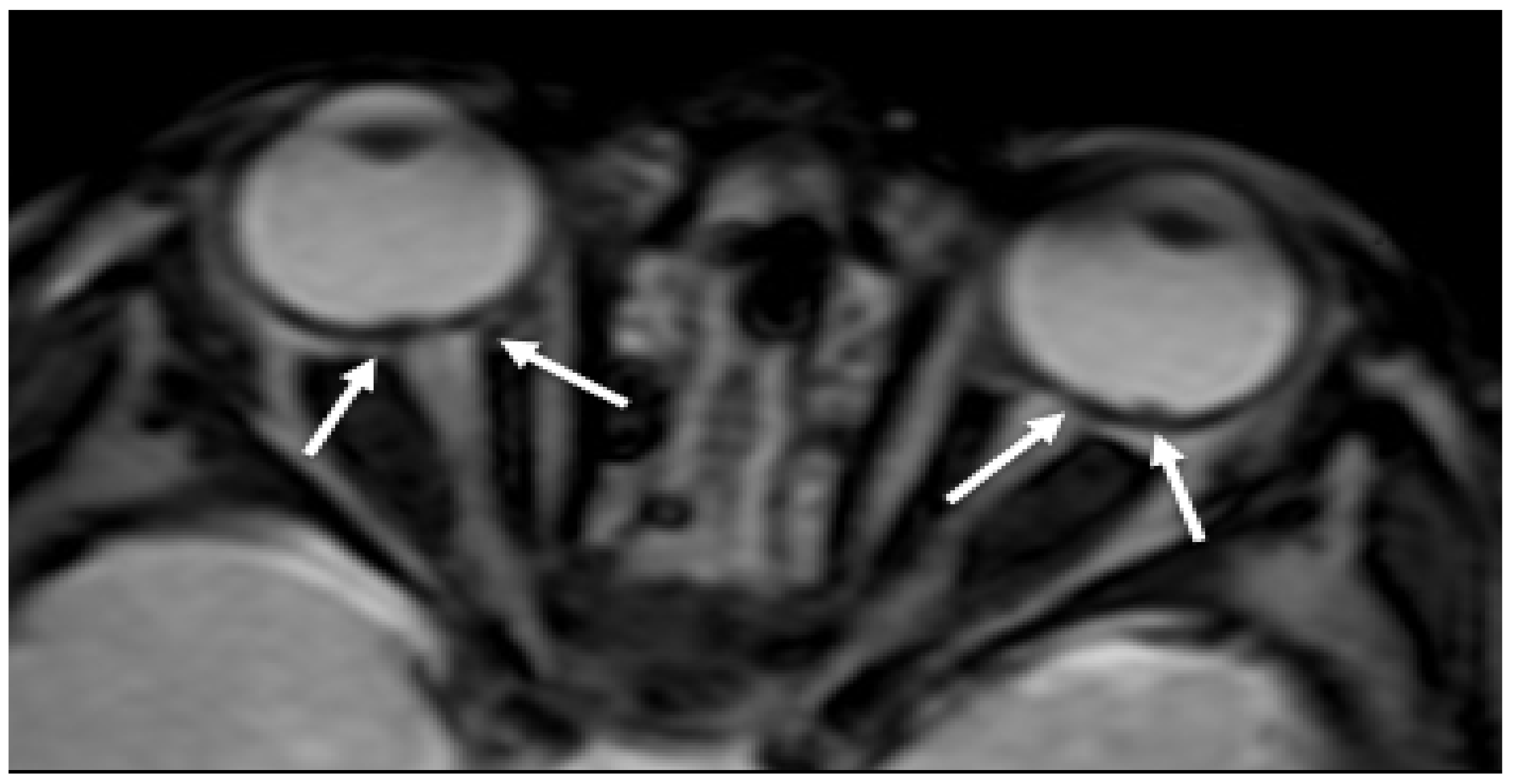
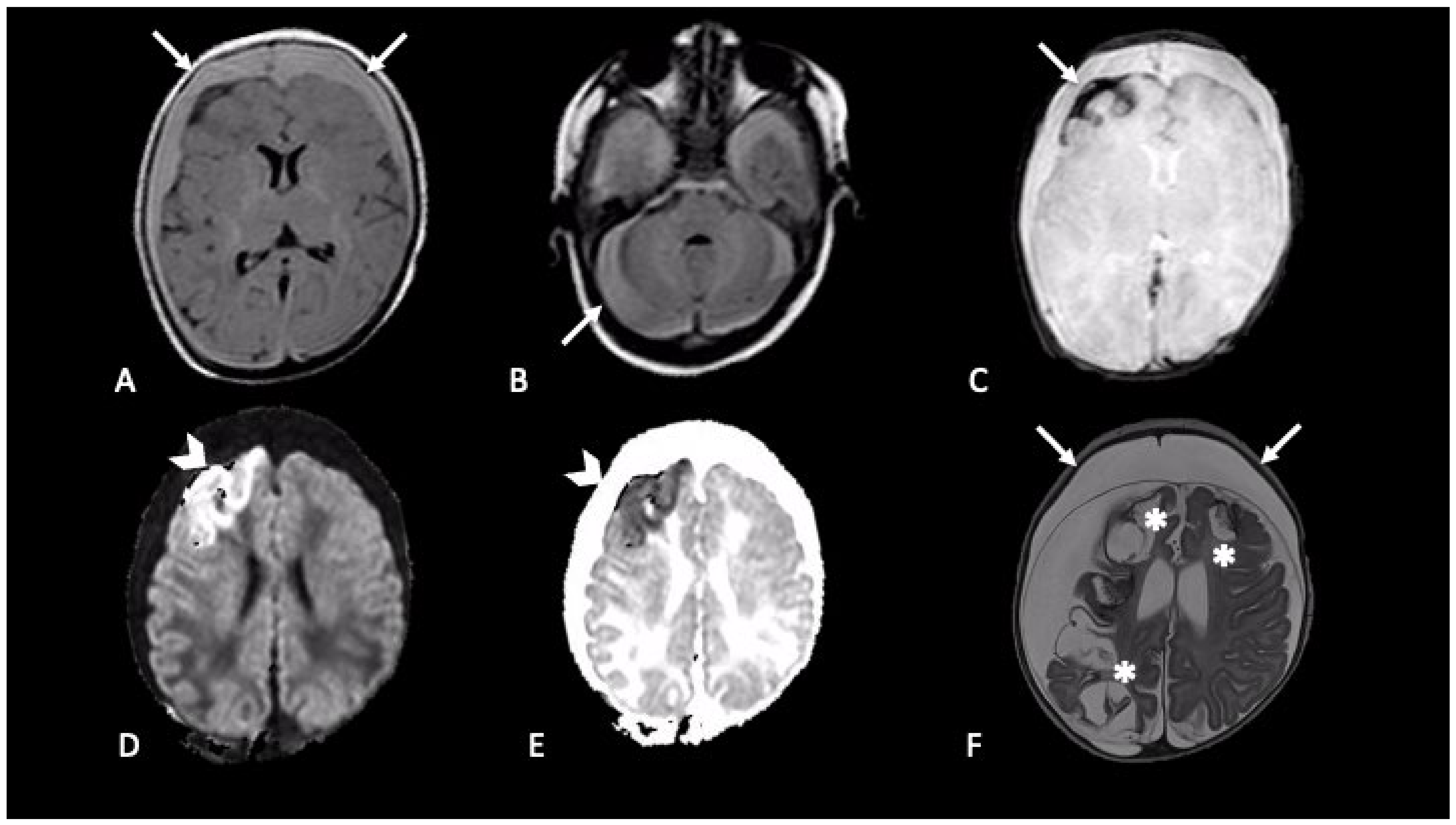
2.1. Hemorrhages
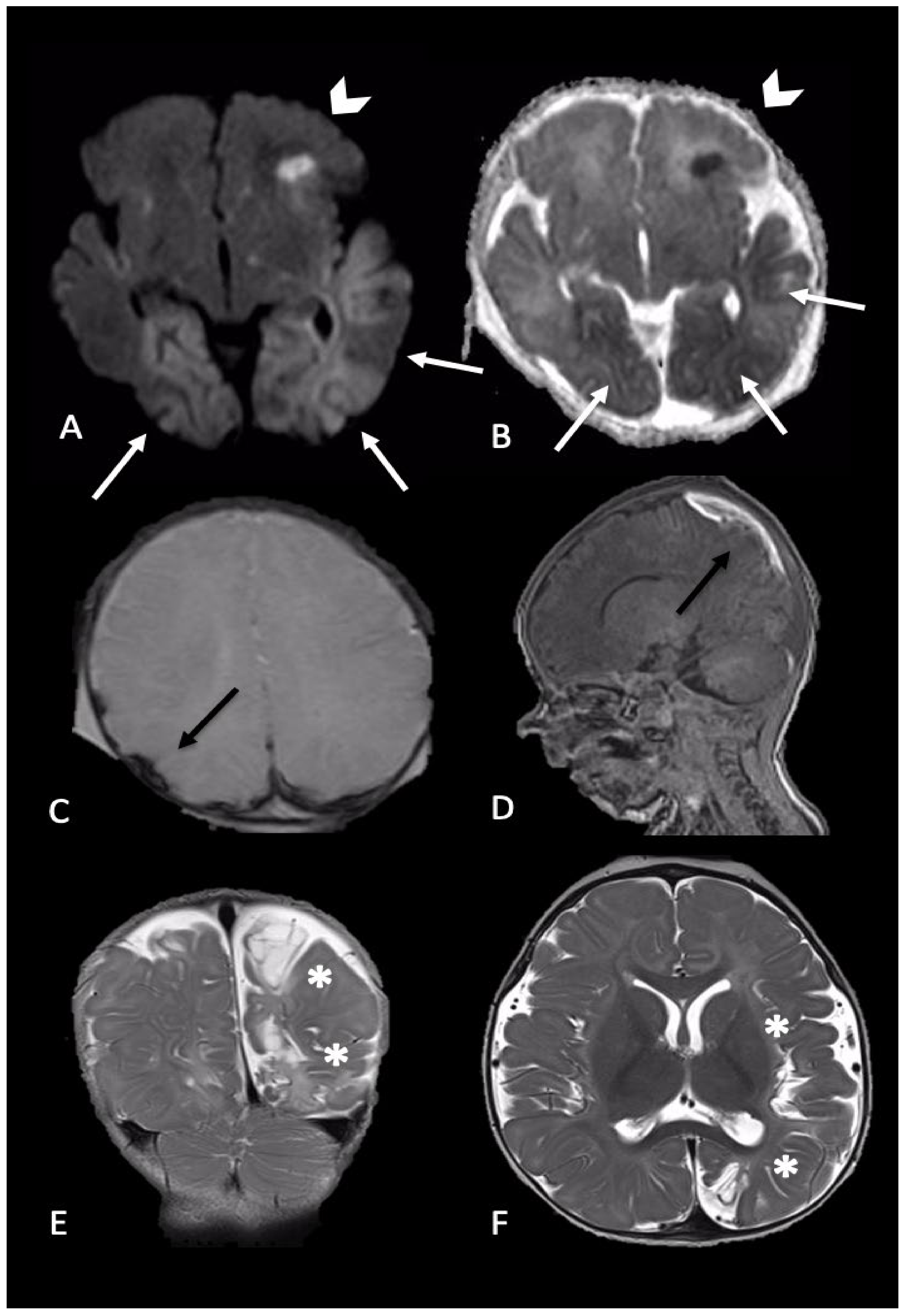
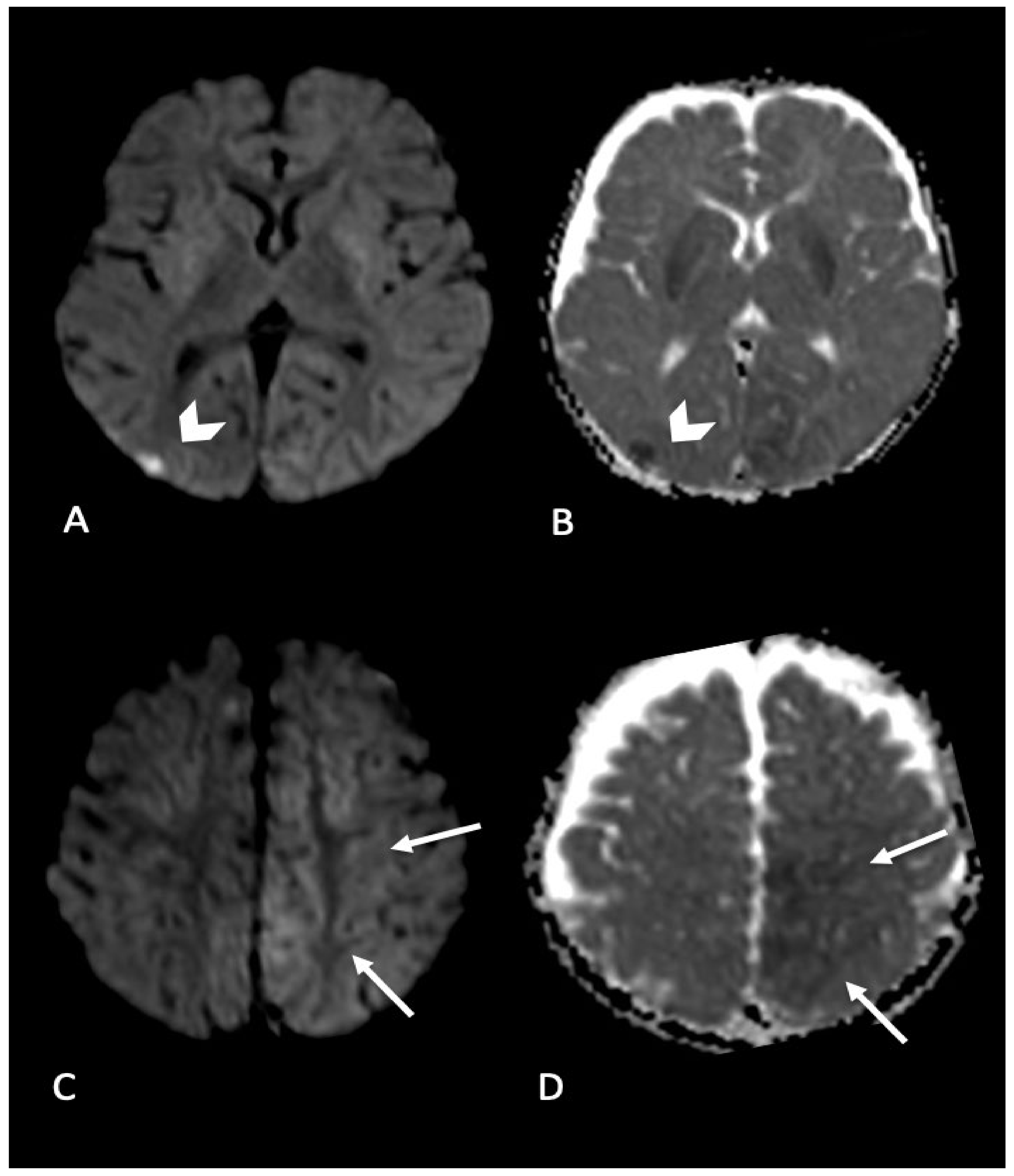
2.2. Parenchymal Injury
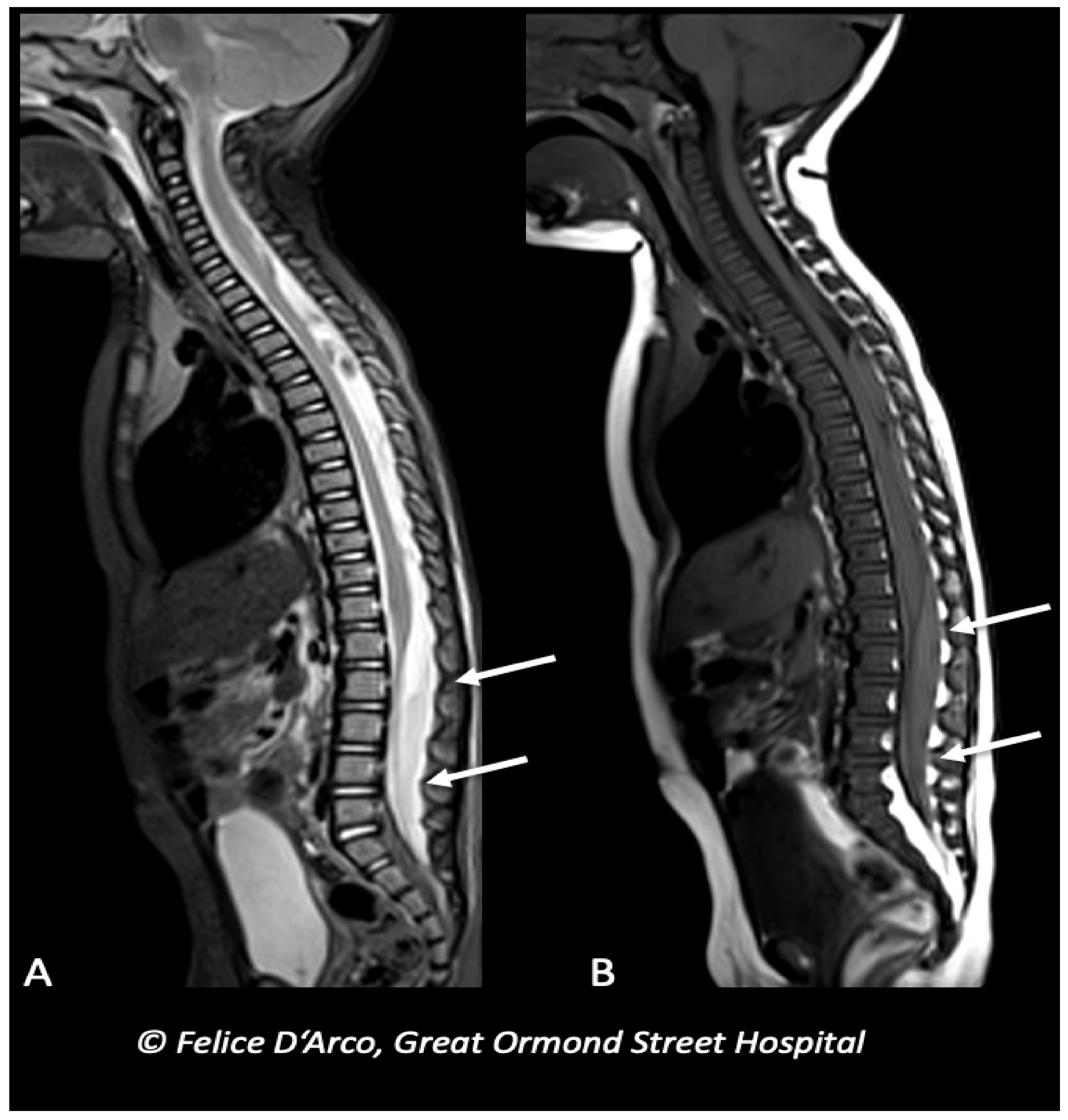
2.3. Spinal Injury
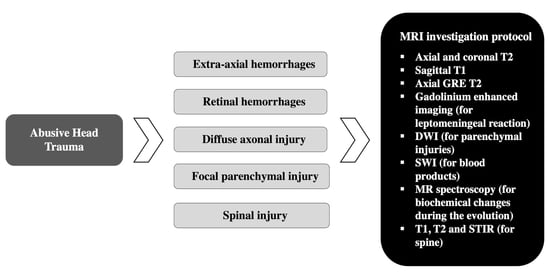
3. MRI Protocol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keenan, H.T.; Runyan, D.K.; Marshall, S.W.; Nocera, M.A.; Merten, D.F.; Sinal, S.H. A population-based study of inflicted traumatic brain injury in young children. JAMA 2003, 290, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, S.E.; Kegler, S.R.; Annest, J.L.; Mercy, J.A. Characteristics of fatal abusive head trauma among children in the USA: 2003–2007: An application of the CDC operational case definition to national vital statistics data. Inj. Prev. 2012, 18, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.; Shah, J.; Dalpiaz, A.; Schwamb, R.; Miao, Y.; Warren, K.; Khan, S. Shaken Baby Syndrome: A review. Fetal Pediatr. Pathol. 2015, 34, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Caffey, J. Multiple fractures in the long bones of infants suffering from chronic subdural hematoma. Am. J. Roentgenol. Radium Ther. 1946, 56, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Caffey, J. On the theory and practice of shaking infants. Its potential residual effects of permanent brain damage and mental retardation. Am. J. Dis. Child. 1972, 124, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Caffey, J. The whiplash shaken infant syndrome: Manual shaking by the extremities with whiplash-induced intracranial and intraocular bleedings, linked with residual permanent brain damage and mental retardation. Pediatrics 1974, 54, 396–403. [Google Scholar]
- Duhaime, A.C.; Gennarelli, T.A.; Thibault, L.E.; Bruce, D.A.; Margulies, S.S.; Wiser, R. The shaken baby syndrome. A clinical, pathological, and biomechanical study. J. Neurosurg. 1987, 66, 409–415. [Google Scholar] [CrossRef]
- Vinchon, M.; de Foort-Dhellemmes, S.; Desurmont, M.; Delestret, I. Confessed abuse versus witnessed accidents in infants: Comparison of clinical, radiological, and ophthalmological data in corroborated cases. Childs Nerv. Syst. 2010, 26, 637–645. [Google Scholar] [CrossRef]
- Narang, S.K.; Estrada, C.; Greenberg, S.; Lindberg, D. Acceptance of Shaken Baby Syndrome and Abusive Head Trauma as Medical Diagnoses. J. Pediatr. 2016, 177, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Christian, C.W.; Block, R.; Committee on Child Abuse and Neglect; American Academy of Pediatrics. Abusive head trauma in infants and children. Pediatrics 2009, 123, 1409–1411. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, A.K.; Servaes, S.; Slovis, T.L.; Palusci, V.J.; Hedlund, G.L.; Narang, S.K.; Moreno, J.A.; Dias, M.S.; Christian, C.W.; Nelson, M.D.; et al. Consensus statement on abusive head trauma in infants and young children. Pediatr. Radiol. 2018, 48, 1048–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frasier, L.D. Abusive head trauma in infants and young children: A unique contributor to developmental disabilities. Pediatr. Clin. N. Am. 2008, 55, 1269–1285. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.R.L.; Eisenstein, E.; Williams, L.C.A. Abusive head trauma in children: A literature review. J. Pediatr. 2013, 89, 426–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosén, M.; Lynøe, N.; Elinder, G.; Hallberg, B.; Sundgren, P.; Eriksson, A. Shaken baby syndrome and the risk of losing scientific scrutiny. Acta Paediatr. 2017, 106, 1905–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieswerda-Hoogendoorn, T.; Boos, S.; Spivack, B.; Bilo, R.A.C.; van Rijn, R.R. Educational paper: Abusive Head Trauma part I. Clinical aspects. Eur. J. Pediatr. 2012, 171, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Petrak, M.; Holz, F.G.; Müller, A.; Krohne, T.U. Retinal Hemorrhages in Shaken Baby Syndrome. J. Pediatr. 2019, 207, 256. [Google Scholar] [CrossRef] [Green Version]
- Jenny, C.; Hymel, K.P.; Ritzen, A.; Reinert, S.E.; Hay, T.C. Analysis of missed cases of abusive head trauma. JAMA 1999, 281, 621–626. [Google Scholar] [CrossRef] [Green Version]
- Strouse, P.J. Shaken baby syndrome is real. Pediatr. Radiol. 2018, 48, 1043–1047. [Google Scholar] [CrossRef] [Green Version]
- Fanconi, M.; Lips, U. Shaken baby syndrome in Switzerland: Results of a prospective follow-up study, 2002–2007. Eur. J. Pediatr. 2010, 169, 1023–1028. [Google Scholar] [CrossRef] [Green Version]
- Chevignard, M.P.; Lind, K. Long-term outcome of abusive head trauma. Pediatr. Radiol. 2014, 44, 548–558. [Google Scholar] [CrossRef]
- Lonergan, G.J.; Baker, A.M.; Morey, M.K.; Boos, S.C. From the archives of the AFIP. Child abuse: Radiologic-pathologic correlation. Radiographics 2003, 23, 811–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodhi, K.S.; Krishna, S.; Saxena, A.K.; Sinha, A.; Khandelwal, N.; Lee, E.Y. Clinical application of ‘Justification’ and ‘Optimization’ principle of ALARA in pediatric CT imaging: “How many children can be protected from unnecessary radiation?”. Eur. J. Radiol. 2015, 84, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.J.; Kaste, S.C. The ALARA (as low as reasonably achievable) concept in pediatric interventional and fluoroscopic imaging: Striving to keep radiation doses as low as possible during fluoroscopy of pediatric patients—A white paper executive summary. Pediatr. Radiol. 2006, 36, 110–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, N.; Brunel, H.; Dory-Lautrec, P.; Chabrol, B. Neuroimaging differential diagnoses to abusive head trauma. Pediatr. Radiol. 2016, 46, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Slovis, T.L.; Strouse, P.J.; Strauss, K.J. Radiation Exposure in Imaging of Suspected Child Abuse: Benefits versus Risks. J. Pediatr. 2015, 167, 963–968. [Google Scholar] [CrossRef]
- Altinok, D.; Saleem, S.; Zhang, Z.; Markman, L.; Smith, W. MR imaging findings of retinal hemorrhage in a case of nonaccidental trauma. Pediatr. Radiol. 2009, 39, 290–292. [Google Scholar] [CrossRef]
- Yilmaz, U.; Körner, H.; Meyer, S.; Reith, W. Multifocal Signal Loss at Bridging Veins on Susceptibility-Weighted Imaging in Abusive Head Trauma. Clin. Neuroradiol. 2015, 25, 181–185. [Google Scholar] [CrossRef]
- Orman, G.; Kralik, S.F.; Meoded, A.; Desai, N.; Risen, S.; Huisman, T.A.G.M. MRI Findings in Pediatric Abusive Head Trauma: A Review. J. Neuroimaging 2020, 30, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Fernando, S.; Obaldo, R.E.; Walsh, I.R.; Lowe, L.H. Neuroimaging of nonaccidental head trauma: Pitfalls and controversies. Pediatr. Radiol. 2008, 38, 827–838. [Google Scholar] [CrossRef]
- Kemp, A.M. Abusive head trauma: Recognition and the essential investigation. Arch. Dis. Child. Educ. Pract. Ed. 2011, 96, 202–208. [Google Scholar] [CrossRef] [Green Version]
- American Academy of Pediatrics; Committee on Child Abuse and Neglect Shaken baby syndrome. Rotational cranial injuries-technical report. Pediatrics 2001, 108, 206–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamsbaum, C.; Rambaud, C. Abusive head trauma: Don’t overlook bridging vein thrombosis. Pediatr. Radiol. 2012, 42, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Barlow, K.M.; Gibson, R.J.; McPhillips, M.; Minns, R.A. Magnetic resonance imaging in acute non-accidental head injury. Acta Paediatr. 1999, 88, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Hahnemann, M.L.; Kinner, S.; Schweiger, B.; Bajanowski, T.; Karger, B.; Pfeiffer, H.; Wittschieber, D. Imaging of bridging vein thrombosis in infants with abusive head trauma: The “Tadpole Sign”. Eur. Radiol. 2015, 2, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Vezina, G. Assessment of the nature and age of subdural collections in nonaccidental head injury with CT and MRI. Pediatr. Radiol. 2009, 39, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Sukhbaatar, N.; Weichhart, T. Iron Regulation: Macrophages in Control. Pharmaceuticals 2018, 11, 137. [Google Scholar] [CrossRef] [Green Version]
- Bradley, W.G. MR appearance of hemorrhage in the brain. Radiology 1993, 189, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Gunda, D.; Cornwell, B.O.; Dahmoush, H.M.; Jazbeh, S.; Alleman, A.M. Pediatric Central Nervous System Imaging of Nonaccidental Trauma: Beyond Subdural Hematomas. RadioGraphics 2018, 39, 213–228. [Google Scholar] [CrossRef]
- Bradford, R.; Choudhary, A.K.; Dias, M.S. Serial neuroimaging in infants with abusive head trauma: Timing abusive injuries. J. Neurosurg. Pediatr. 2013, 12, 110–119. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.-L.; Chu, W.C.W.; Wong, G.W.K.; Yeung, D.K.W. Diffusion-weighted MRI in shaken baby syndrome. Pediatr. Radiol. 2003, 33, 574–577. [Google Scholar] [CrossRef]
- Hsieh, K.L.-C.; Zimmerman, R.A.; Kao, H.W.; Chen, C.-Y. Revisiting neuroimaging of abusive head trauma in infants and young children. Am. J. Roentgenol. 2015, 204, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.S.; Poretti, A.; Meoded, A.; Tekes, A.; Huisman, T.A.G.M. The unique features of traumatic brain injury in children. Review of the characteristics of the pediatric skull and brain, mechanisms of trauma, patterns of injury, complications and their imaging findings—Part 1. J. Neuroimaging 2012, 22, e1–e17. [Google Scholar] [CrossRef] [PubMed]
- Wittschieber, D.; Karger, B.; Niederstadt, T.; Pfeiffer, H.; Hahnemann, M.L. Subdural hygromas in abusive head trauma: Pathogenesis, diagnosis, and forensic implications. Am. J. Neuroradiol. 2015, 36, 432–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zouros, A.; Bhargava, R.; Hoskinson, M.; Aronyk, K.E. Further characterization of traumatic subdural collections of infancy. Report of five cases. J. Neurosurg. 2004, 100, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.G.; Hegde, S.V.; Dineen, R.A.; Jaspan, T. Patterns of accidental craniocerebral injury occurring in early childhood. Arch. Dis. Child. 2013, 98, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.A.; Recker, M.J.; Lee, P.S.; Bell, M.J.; Tyler-Kabara, E.C. Factors associated with hemispheric hypodensity after subdural hematoma following abusive head trauma in children. J. Neurotrauma 2014, 31, 1625–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gencturk, M.; Tore, H.G.; Nascene, D.R.; Zhang, L.; Koksel, Y.; McKinney, A.M. Various Cranial and Orbital Imaging Findings in Pediatric Abusive and Non-abusive Head trauma, and Relation to Outcomes. Clin. Neuroradiol. 2019, 29, 253–261. [Google Scholar] [CrossRef]
- Ilves, P.; Lintrop, M.; Talvik, I.; Sisko, A.; Talvik, T. Predictive value of clinical and radiological findings in inflicted traumatic brain injury. Acta Paediatr. 2010, 99, 1329–1336. [Google Scholar] [CrossRef]
- Squier, W. The “Shaken Baby” syndrome: Pathology and mechanisms. Acta Neuropathol. 2011, 122, 519–542. [Google Scholar] [CrossRef]
- Matschke, J.; Büttner, A.; Bergmann, M.; Hagel, C.; Püschel, K.; Glatzel, M. Encephalopathy and death in infants with abusive head trauma is due to hypoxic-ischemic injury following local brain trauma to vital brainstem centers. Int. J. Legal. Med. 2015, 129, 105–114. [Google Scholar] [CrossRef]
- Calder, I.M.; Hill, I.; Scholtz, C.L. Primary brain trauma in non-accidental injury. J. Clin. Pathol. 1984, 37, 1095–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vowles, G.H.; Scholtz, C.L.; Cameron, J.M. Diffuse axonal injury in early infancy. J. Clin. Pathol. 1987, 40, 185–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geddes, J. Neuropathology of Inflicted Head Injury in Children. II. Microscopic Brain Injury in Infants—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/11408325/ (accessed on 13 June 2020).
- Shannon, P.; Smith, C.R.; Deck, J.; Ang, L.C.; Ho, M.; Becker, L. Axonal injury and the neuropathology of shaken baby syndrome. Acta Neuropathol. 1998, 95, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; John, S.; Vincent, A.L.; Reed, P. Abusive head trauma and accidental head injury: A 20-year comparative study of referrals to a hospital child protection team. Arch. Dis. Child. 2015, 100, 1123–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiner, M.V.; Greiner, H.M.; Caré, M.M.; Owens, D.; Shapiro, R.; Holland, K. Adding Insult to Injury: Nonconvulsive Seizures in Abusive Head Trauma. J. Child. Neurol. 2015, 30, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Ichord, R.N.; Naim, M.; Pollock, A.N.; Nance, M.L.; Margulies, S.S.; Christian, C.W. Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: The role of diffusion-weighted imaging. J. Neurotrauma 2007, 24, 106–118. [Google Scholar] [CrossRef]
- Kemp, A.M.; Jaspan, T.; Griffiths, J.; Stoodley, N.; Mann, M.K.; Tempest, V.; Maguire, S.A. Neuroimaging: What neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Arch. Dis. Child. 2011, 96, 1103–1112. [Google Scholar] [CrossRef]
- McKinney, A.M.; Thompson, L.R.; Truwit, C.L.; Velders, S.; Karagulle, A.; Kiragu, A. Unilateral hypoxic-ischemic injury in young children from abusive head trauma, lacking craniocervical vascular dissection or cord injury. Pediatr. Radiol. 2008, 38, 164–174. [Google Scholar] [CrossRef]
- Cantu, R.C.; Gean, A.D. Second-Impact Syndrome and a Small Subdural Hematoma: An Uncommon Catastrophic Result of Repetitive Head Injury with a Characteristic Imaging Appearance. J. Neurotrauma 2010, 27, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- Bonnier, C.; Nassogne, M.-C.; Saint-Martin, C.; Mesples, B.; Kadhim, H.; Sébire, G. Neuroimaging of intraparenchymal lesions predicts outcome in shaken baby syndrome. Pediatrics 2003, 112, 808–814. [Google Scholar] [CrossRef]
- Foerster, B.R.; Petrou, M.; Lin, D.; Thurnher, M.M.; Carlson, M.D.; Strouse, P.J.; Sundgren, P.C. Neuroimaging evaluation of non-accidental head trauma with correlation to clinical outcomes: A review of 57 cases. J. Pediatr. 2009, 154, 573–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, R.C.; Schor, D.P.; Smith, W.L. Magnetic resonance imaging of intracranial injuries from child abuse. J. Pediatr. 1986, 109, 975–979. [Google Scholar] [CrossRef]
- Suh, D.Y.; Davis, P.C.; Hopkins, K.L.; Fajman, N.N.; Mapstone, T.B. Nonaccidental pediatric head injury: Diffusion-weighted imaging findings. Neurosurgery 2001, 49, 309–318; discussion 318–320. [Google Scholar] [CrossRef] [PubMed]
- Huisman, T.A.G.M. Diffusion-weighted imaging: Basic concepts and application in cerebral stroke and head trauma. Eur. Radiol. 2003, 13, 2283–2297. [Google Scholar] [CrossRef] [PubMed]
- Galloway, N.R.; Tong, K.A.; Ashwal, S.; Oyoyo, U.; Obenaus, A. Diffusion-weighted imaging improves outcome prediction in pediatric traumatic brain injury. J. Neurotrauma 2008, 25, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, P.W.; Huisman, T.A.G.M.; Sorensen, A.G.; Gonzalez, R.G.; Schwamm, L.H. Diffusion-weighted MR imaging in closed head injury: High correlation with initial glasgow coma scale score and score on modified Rankin scale at discharge. Radiology 2004, 233, 58–66. [Google Scholar] [CrossRef]
- Zimmerman, R.A.; Bilaniuk, L.T.; Farina, L. Non-accidental brain trauma in infants: Diffusion imaging, contributions to understanding the injury process. J. Neuroradiol. 2007, 34, 109–114. [Google Scholar] [CrossRef]
- Orru’, E.; Huisman, T.A.G.M.; Izbudak, I. Prevalence, Patterns, and Clinical Relevance of Hypoxic-Ischemic Injuries in Children Exposed to Abusive Head Trauma. J. Neuroimaging 2018, 28, 608–614. [Google Scholar] [CrossRef]
- Khan, N.R.; Fraser, B.D.; Nguyen, V.; Moore, K.; Boop, S.; Vaughn, B.N.; Klimo, P. Pediatric abusive head trauma and stroke. J. Neurosurg. Pediatr. 2017, 20, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Valdez, R.; Martin, L.J.; Northington, F.J. Programmed Necrosis: A Prominent Mechanism of Cell Death following Neonatal Brain Injury. Neurol. Res. Int. 2012, 2012, 257563. [Google Scholar] [CrossRef]
- Imagawa, K.K.; Hamilton, A.; Ceschin, R.; Tokar, E.; Pham, P.; Bluml, S.; Wisnowski, J.; Panigrahy, A. Characterization of microstructural injury: A novel approach in infant abusive head trauma-initial experience. J. Neurotrauma 2014, 31, 1632–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Case, M.E.; Graham, M.A.; Handy, T.C.; Jentzen, J.M.; Monteleone, J.A.; National Association of Medical Examiners Ad Hoc Committee on Shaken Baby Syndrome. Position paper on fatal abusive head injuries in infants and young children. Am. J. Forensic Med. Pathol. 2001, 22, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roach, J.P.; Acker, S.N.; Bensard, D.D.; Sirotnak, A.P.; Karrer, F.M.; Partrick, D.A. Head injury pattern in children can help differentiate accidental from non-accidental trauma. Pediatr. Surg. Int. 2014, 30, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.R.; Gonçalves, F.G.; Servin, C.A.; Mankad, K.; Zuccoli, G. Ocular and Intracranial MR Imaging Findings in Abusive Head Trauma. Top. Magn. Reson. Imaging 2018, 27, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Ashwal, S.; Wycliffe, N.D.; Holshouser, B.A. Advanced neuroimaging in children with nonaccidental trauma. Dev. Neurosci. 2010, 32, 343–360. [Google Scholar] [CrossRef] [PubMed]
- Colbert, C.A.; Holshouser, B.A.; Aaen, G.S.; Sheridan, C.; Oyoyo, U.; Kido, D.; Ashwal, S. Value of cerebral microhemorrhages detected with susceptibility-weighted MR Imaging for prediction of long-term outcome in children with nonaccidental trauma. Radiology 2010, 256, 898–905. [Google Scholar] [CrossRef]
- Wright, J.N. CNS Injuries in Abusive Head Trauma. Am. J. Roentgenol. 2017, 208, 991–1001. [Google Scholar] [CrossRef]
- Palifka, L.A.; Frasier, L.D.; Metzger, R.R.; Hedlund, G.L. Parenchymal Brain Laceration as a Predictor of Abusive Head Trauma. Am. J. Neuroradiol. 2016, 37, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Jaspan, T.; Narborough, G.; Punt, J.A.; Lowe, J. Cerebral contusional tears as a marker of child abuse--detection by cranial sonography. Pediatr. Radiol. 1992, 22, 237–245. [Google Scholar] [CrossRef]
- Beauchamp, M.H.; Ditchfield, M.; Babl, F.E.; Kean, M.; Catroppa, C.; Yeates, K.O.; Anderson, V. Detecting traumatic brain lesions in children: CT versus MRI versus susceptibility weighted imaging (SWI). J. Neurotrauma 2011, 28, 915–927. [Google Scholar] [CrossRef]
- Bandak, F.A. Shaken baby syndrome: A biomechanics analysis of injury mechanisms. Forensic Sci. Int. 2005, 151, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.; Cowley, L.; Maguire, S. Spinal injuries in abusive head trauma: Patterns and recommendations. Pediatr. Radiol. 2014, 44, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Koumellis, P.; McConachie, N.S.; Jaspan, T. Spinal subdural haematomas in children with non- Accidental head injury. Arch. Dis. Child. 2009, 94, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Bradford, R.K.; Dias, M.S.; Moore, G.J.; Boal, D.K.B. Spinal subdural hemorrhage in abusive head trauma: A retrospective study. Radiology 2012, 262, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, A.K.; Ishak, R.; Zacharia, T.T.; Dias, M.S. Imaging of spinal injury in abusive head trauma: A retrospective study. Pediatr. Radiol. 2014, 44, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.; Sawvel, M.; Heaner, D.; Bhatia, A.; Reisner, A.; Tubbs, R.S.; Chern, J.J. Changes in use of cervical spine magnetic resonance imaging for pediatric patients with nonaccidental trauma. J. Neurosurg. Pediatr. 2017, 20, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Brennan, L.K.; Rubin, D.; Christian, C.W.; Duhaime, A.C.; Mirchandani, H.G.; Rorke-Adams, L.B. Neck injuries in young pediatric homicide victims: Clinical article. J. Neurosurg. Pediatr. 2009, 3, 232–239. [Google Scholar] [CrossRef]
- Barber, I.; Perez-Rossello, J.M.; Wilson, C.R.; Silvera, M.V.; Kleinman, P.K. Prevalence and relevance of pediatric spinal fractures in suspected child abuse. Pediatr. Radiol. 2013, 43, 1507–1515. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, K.S.; Hwang, D.H.; Lee, I.J.; Kim, H.B.; Lee, J.Y. MR Imaging of Shaken Baby Syndrome Manifested as Chronic Subdural Hematoma. Korean J. Radiol. 2001, 2, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Section on Radiology; American Academy of Pediatrics. Diagnostic imaging of child abuse. Pediatrics 2009, 123, 1430–1435. [Google Scholar] [CrossRef] [Green Version]
- Tanoue, K.; Aida, N.; Matsui, K. Apparent diffusion coefficient values predict outcomes of abusive head trauma. Acta Paediatr. 2013, 102, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Parizel, P.M.; Ceulemans, B.; Laridon, A.; Ozsarlak, O.; Van Goethem, J.W.; Jorens, P.G. Cortical hypoxic-ischemic brain damage in shaken-baby (shaken impact) syndrome: Value of diffusion-weighted MRI. Pediatr. Radiol. 2003, 33, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Duyn, J.H.; van Gelderen, P.; Li, T.Q.; de Zwart, J.A.; Koretsky, A.P.; Fukunaga, M. High-field MRI of brain cortical substructure based on signal phase. Proc. Natl. Acad. Sci. USA 2007, 104, 11796–11801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, M.E.; Jaju, A.; Ciolino, J.D.; Alden, T. Rapid MRI evaluation of acute intracranial hemorrhage in pediatric head trauma. Neuroradiology 2016, 58, 793–799. [Google Scholar] [CrossRef]
- Young, J.Y.; Duhaime, A.-C.; Caruso, P.A.; Rincon, S.P. Comparison of non-sedated brain MRI and CT for the detection of acute traumatic injury in children 6 years of age or less. Emerg. Radiol. 2016, 23, 325–331. [Google Scholar] [CrossRef]
- Haseler, L.J.; Arcinue, E.; Danielsen, E.R.; Bluml, S.; Ross, B.D. Evidence from proton magnetic resonance spectroscopy for a metabolic cascade of neuronal damage in shaken baby syndrome. Pediatrics 1997, 99, 4–14. [Google Scholar] [CrossRef]
- Mehta, H.; Acharya, J.; Mohan, A.L.; Tobias, M.E.; LeCompte, L.; Jeevan, D. Minimizing Radiation Exposure in Evaluation of Pediatric Head Trauma: Use of Rapid MR Imaging. Am. J. Neuroradiol. 2016, 37, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Flom, L.; Fromkin, J.; Panigrahy, A.; Tyler-Kabara, E.; Berger, R.P. Development of a screening MRI for infants at risk for abusive head trauma. Pediatr. Radiol. 2016, 46, 519–526. [Google Scholar] [CrossRef]
- Ryan, M.E. Rapid magnetic resonance imaging screening for abusive head trauma. Pediatr. Radiol. 2020, 50, 13–14. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartocci, G.; Fineschi, V.; Padovano, M.; Scopetti, M.; Rossi-Espagnet, M.C.; Giannì, C. Shaken Baby Syndrome: Magnetic Resonance Imaging Features in Abusive Head Trauma. Brain Sci. 2021, 11, 179. https://doi.org/10.3390/brainsci11020179
Cartocci G, Fineschi V, Padovano M, Scopetti M, Rossi-Espagnet MC, Giannì C. Shaken Baby Syndrome: Magnetic Resonance Imaging Features in Abusive Head Trauma. Brain Sciences. 2021; 11(2):179. https://doi.org/10.3390/brainsci11020179
Chicago/Turabian StyleCartocci, Gaia, Vittorio Fineschi, Martina Padovano, Matteo Scopetti, Maria Camilla Rossi-Espagnet, and Costanza Giannì. 2021. "Shaken Baby Syndrome: Magnetic Resonance Imaging Features in Abusive Head Trauma" Brain Sciences 11, no. 2: 179. https://doi.org/10.3390/brainsci11020179
APA StyleCartocci, G., Fineschi, V., Padovano, M., Scopetti, M., Rossi-Espagnet, M. C., & Giannì, C. (2021). Shaken Baby Syndrome: Magnetic Resonance Imaging Features in Abusive Head Trauma. Brain Sciences, 11(2), 179. https://doi.org/10.3390/brainsci11020179