Increased Likelihood of Falling in Older Cannabis Users vs. Non-Users
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Experimental Protocol
2.3. Measurements
2.3.1. Fall Risk
2.3.2. Arm and Hand Function
2.3.3. Cognitive Function
2.3.4. Static Posturography
2.3.5. Gait
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blazer, D.G.; Wu, L.T. The epidemiology of substance use and disorders among middle aged and elderly community adults: National survey on drug use and health. Am. J. Geriatr. Psychiatry 2009, 17, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Sherman, S.; Mauro, P.M.; Martins, S.S.; Rotenberg, J.; Palamar, J.J. Demographic trends among older cannabis users in the United States, 2006–2013. Addiction 2017, 112, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Kaskie, B.; Ayyagari, P.; Milavetz, G.; Shane, D.; Arora, K. The increasing use of cannabis among older Americans: A public health crisis or viable policy alternative? Gerontologist 2017, 57, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.J.; Efremova, I.; Frazier, A.; Saba, A. The emerging problems of alcohol and substance abuse in late life. J. Soc. Distress Homeless 1999, 8, 227–239. [Google Scholar] [CrossRef]
- Moore, A.A.; Endo, J.O.; Carter, M.K. Is there a relationship between excessive drinking and functional impairment in older persons? J. Am. Geriatr. Soc. 2003, 51, 44–49. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Kuller, L.H.; Fitzpatrick, A.L.; Longstreth, W.T., Jr.; Mittleman, M.A.; Siscovick, D.S. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA 2003, 289, 1405–1413. [Google Scholar] [CrossRef]
- National Institute on Aging. Alcohol Use in Older People. Available online: https://www.nia.nih.gov/health/publication/alcohol-use-older-people (accessed on 25 November 2016).
- Kuerbis, A.; Sacco, P.; Blazer, D.G.; Moore, A.A. Substance abuse among older adults. Clin. Geriatr. Med. 2014, 30, 629–654. [Google Scholar] [CrossRef]
- Deshpande, A.; Mailis-Gagnon, A.; Zoheiry, N.; Lakha, S.F. Efficacy and adverse effects of medical marijuana for chronic noncancer pain: Systematic review of randomized controlled trials. Can. Fam. Physician 2015, 61, e372–e381. [Google Scholar]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C.A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef]
- Wade, D.T.; Makela, P.; Robson, P.; House, H.; Bateman, C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult. Scler. 2004, 10, 434–441. [Google Scholar] [CrossRef]
- Cigolle, C.T.; Langa, K.M.; Kabeto, M.U.; Tian, Z.; Blaum, C.S. Geriatric conditions and disability: The health and retirement study. Ann. Intern. Med. 2007, 147, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Bergen, G.; Stevens, M.R.; Burns, E.R. Falls and fall injuries among adults aged ≥ 65 years—United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.J.; Hays, R.D.; Shapiro, M.F.; Wallace, S.P.; Ettner, S.L. The costs of fall-related injuries among older adults: Annual per-faller, service component, and patient out-of-pocket costs. Health Serv. Res. 2017, 52, 1794–1816. [Google Scholar] [CrossRef] [PubMed]
- Houry, D.; Florence, C.; Baldwin, G.; Stevens, J.; McClure, R. The CDC injury center’s response to the growing public health problem of falls among older adults. Am. J. Lifestyle Med. 2016, 10, 74–77. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Verghese, J.; Beauchet, O.; Hausdorff, J.M. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012, 60, 2127–2136. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Solowij, N. Cannabis and Cognitive Functioning; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Grant, I.; Gonzalez, R.; Carey, C.L.; Natarajan, L.; Wolfson, T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. J. Int. Neuropsychol. Soc. 2003, 9, 679–689. [Google Scholar] [CrossRef]
- Solowij, N.; Stephens, R.S.; Roffman, R.A.; Babor, T.; Kadden, R.; Miller, M.; Christiansen, K.; McRee, B.; Vendetti, J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 2002, 287, 1123–1131. [Google Scholar] [CrossRef]
- Meier, M.H.; Caspi, A.; Ambler, A.; Harrington, H.; Houts, R.; Keefe, R.S.; McDonald, K.; Ward, A.; Poulton, R.; Moffitt, T.E. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. USA 2012, 109, e2657–e2664. [Google Scholar] [CrossRef]
- Auer, R.; Vittinghoff, E.; Yaffe, K.; Künzi, A.; Kertesz, S.G.; Levine, D.A.; Albanese, E.; Whitmer, R.A.; Jacobs, D.R., Jr.; Sidney, S.; et al. Association between lifetime marijuana use and cognitive function in middle age: The coronary artery risk development in young adults (CARDIA) study. JAMA Intern. Med. 2016, 176, 352–361. [Google Scholar] [CrossRef]
- Sakurai, R.; Fujiwara, Y.; Yasunaga, M.; Suzuki, H.; Kanosue, K.; Montero-Odasso, M.; Ishii, K. Association between hypometabolism in the supplementary motor area and fear of falling in older adults. Front. Aging Neurosci. 2017, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Zhang, H.; Sachdev, P.; Lord, S.R.; Brodaty, H.; Wen, W.; Delbaere, K. Regional gray matter volumes are related to concern about falling in older people: A voxel-based morphometric study. J. Gerontol. A Biol Sci. Med. Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.S.; Rogowska, J.; Kanayama, G.; Jon, D.I.; Gruber, S.; Simpson, N.; Cherayil, M.; Pope, H.G.; Yurgelun-Todd, D.A. Neurophysiology of motor function following cannabis discontinuation in chronic cannabis smokers: An fMRI study. Drug Alcohol Depend. 2004, 76, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.A.; Linden, D.J. Cannabinoid receptor modulation of synapses received by cerebellar Purkinje cells. J. Neurophysiol. 2000, 83, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; Mackie, K. A light and electron microscopic study of the CB1 cannabinoid receptor in the primate spinal cord. J. Neurocytol. 1999, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Seppala, L.J.; Wermelink, A.; de Vries, M.; Ploegmakers, K.J.; van de Glind, E.M.M.; Daams, J.G.; van der Velde, N. Fall-risk-increasing drugs: A systematic review and meta-analysis: II. Psychotropics. J. Am. Med. Dir. Assoc. 2018, 19, e311–e317. [Google Scholar] [CrossRef]
- Deary, I.J.; Liewald, D.; Nissan, J. A free, easy-to-use, computer-based simple and four-choice reaction time programme: The Deary-Liewald reaction time task. Behav. Res. Methods 2011, 43, 258–268. [Google Scholar] [CrossRef]
- Lajoie, Y.; Gallagher, S.P. Predicting falls within the elderly community: Comparison of postural sway, reaction time, the berg balance scale and the activities-specific balance confidence (ABC) scale for comparing fallers and non-fallers. Arch. Gerontol. Geriatr. 2004, 38, 11–26. [Google Scholar] [CrossRef]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83, S7–S11. [Google Scholar]
- Powell, L.E.; Myers, A.M. The activities-specific balance confidence (ABC) scale. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 28–34. [Google Scholar] [CrossRef]
- Reuben, D.B.; Magasi, S.; McCreath, H.E.; Bohannon, R.W.; Wang, Y.C.; Bubela, D.J.; Rymer, W.Z.; Beaumont, J.; Rine, R.M.; Lai, J.S.; et al. Motor assessment using the NIH toolbox. Neurology 2013, 80, S65–S75. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Bauer, P.J.; Carlozzi, N.E.; Slotkin, J.; Blitz, D.; Wallner-Allen, K.; et al. Cognition assessment using the NIH toolbox. Neurology 2013, 80, S54–S64. [Google Scholar] [CrossRef] [PubMed]
- McGraw, K.O.; Wong, S.P. A common language effect size statistic. Psychol. Bull. 1992, 111, 361–365. [Google Scholar] [CrossRef]
- Ruscio, J. A probability-based measure of effect size: Robustness to base rates and other factors. Psychol. Methods 2008, 13, 19–30. [Google Scholar] [CrossRef]
- El Manira, A.; Kyriakatos, A.; Nanou, E.; Mahmood, R. Endocannabinoid signaling in the spinal locomotor circuitry. Brain Res. Rev. 2008, 57, 29–36. [Google Scholar] [CrossRef]
- García-Morales, V.; Montero, F.; Moreno-López, B. Cannabinoid agonists rearrange synaptic vesicles at excitatory synapses and depress motoneuron activity in vivo. Neuropharmacology 2015, 92, 69–79. [Google Scholar] [CrossRef]
- Oliviero, A.; Arevalo-Martin, A.; Rotondi, M.; García-Ovejero, D.; Mordillo-Mateos, L.; Lozano-Sicilia, A.; Panyavin, I.; Chiovato, L.; Aguilar, J.; Foffani, G.; et al. CB1 receptor antagonism/inverse agonism increases motor system excitability in humans. Eur. Neuropsychopharmacol. 2012, 22, 27–35. [Google Scholar] [CrossRef]
- Romero, J.; Hillard, C.J.; Calero, M.; Rábano, A. Fatty acid amide hydrolase localization in the human central nervous system: An immunohistochemical study. Brain Res. Mol. Brain Res. 2002, 100, 85–93. [Google Scholar] [CrossRef]
- Ramaekers, J.G.; Kauert, G.; van Ruitenbeek, P.; Theunissen, E.L.; Schneider, E.; Moeller, M.R. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology 2006, 31, 2296–2303. [Google Scholar] [CrossRef]
- Kiplinger, G.F.; Manno, J.E.; Rodda, B.E.; Forney, R.B. Dose-response analysis of the effects of tetrahydrocannabinol in man. Clin. Pharmacol. Ther. 1971, 12, 650–657. [Google Scholar] [CrossRef]
- Zuurman, L.; Roy, C.; Schoemaker, R.C.; Hazekamp, A.; den Hartigh, J.; Bender, J.C.; Verpoorte, R.; Pinquier, J.L.; Cohen, A.F.; van Gerven, J.M. Effect of intrapulmonary tetrahydrocannabinol administration in humans. J. Psychopharmacol. 2008, 22, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Matochik, J.A.; Eldreth, D.A.; Cadet, J.L.; Bolla, K.I. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005, 77, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Solowij, N.; Pesa, N. Cannabis and cognition: Short and long-term effects. In Marijuana and Madness, 2nd ed.; Castle, D., Murray, R.M., D’Souza, D.C., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 91–102. [Google Scholar]
- Tinetti, M.E.; Kumar, C. The patient who falls: “It’s always a trade-off”. JAMA 2010, 303, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kvelde, T.; McVeigh, C.; Toson, B.; Greenaway, M.; Lord, S.R.; Delbaere, K.; Close, J.C. Depressive symptomatology as a risk factor for falls in older people: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 2013, 61, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Kearney, F.C.; Harwood, R.H.; Gladman, J.R.; Lincoln, N.; Masud, T. The relationship between executive function and falls and gait abnormalities in older adults: A systematic review. Dement. Geriatr. Cogn. Disord. 2013, 36, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, K.; Close, J.C.; Brodaty, H.; Sachdev, P.; Lord, S.R. Determinants of disparities between perceived and physiological risk of falling among elderly people: Cohort study. BMJ 2010, 341, 4165. [Google Scholar] [CrossRef]
- Granholm, A.C.; Boger, H.; Emborg, M.E. Mood, memory and movement: An age-related neurodegenerative complex? Curr. Aging Sci. 2008, 1, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Kvelde, T.; Lord, S.R.; Close, J.C.; Reppermund, S.; Kochan, N.A.; Sachdev, P.; Brodaty, H.; Delbaere, K. Depressive symptoms increase fall risk in older people, independent of antidepressant use, and reduced executive and physical functioning. Arch. Gerontol. Geriatr. 2015, 60, 190–195. [Google Scholar] [CrossRef]
- Manchester, D.; Woollacott, M.; Zederbauer-Hylton, N.; Marin, O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J. Gerontol. 1989, 44, 118–127. [Google Scholar] [CrossRef]
- Choy, N.L.; Brauer, S.; Nitz, J. Changes in postural stability in women aged 20 to 80 years. J. Gerontol. A Biol. Sci. Med. Sci 2003, 58, 525–530. [Google Scholar] [CrossRef]
- Vellas, B.J.; Wayne, S.J.; Romero, L.; Baumgartner, R.N.; Rubenstein, L.Z.; Garry, P.J. One-leg balance is an important predictor of injurious falls in older persons. J. Am. Geriatr. Soc. 1997, 45, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Herssens, N.; Verbecque, E.; Hallemans, A.; Vereeck, L.; Van Rompaey, V.; Saeys, W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture 2018, 64, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Montero-Odasso, M.; Muir, S.W.; Speechley, M. Dual-task complexity affects gait in people with mild cognitive impairment: The interplay between gait variability, dual tasking, and risk of falls. Arch. Phys. Med. Rehabil. 2012, 93, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Woods, A.J.; Clark, L.A.; Criss, C.R.; Shadmehr, R.; Grooms, D.R. The aging brain & the dorsal basal ganglia: Implications for age-related limitations of mobility. Adv. Geriatr. Med. Res. 2019, 1. [Google Scholar] [CrossRef]
- Cruz-Jimenez, M. Normal changes in gait and mobility problems in the elderly. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 713–725. [Google Scholar] [CrossRef]
- Winter, D.A.; Patla, A.E.; Frank, J.S.; Walt, S.E. Biomechanical walking pattern changes in the fit and healthy elderly. Phys. Ther. 1990, 70, 340–347. [Google Scholar] [CrossRef]
- Ko, S.U.; Hausdorff, J.M.; Ferrucci, L. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: Results from the Baltimore longitudinal study of ageing. Age Ageing 2010, 39, 688–694. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cooper, R.; Shardell, M.; Simonsick, E.M.; Schrack, J.A.; Kuh, D. Age-related change in mobility: Perspectives from life course epidemiology and geroscience. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1184–1194. [Google Scholar] [CrossRef]
- Callisaya, M.L.; Launay, C.P.; Srikanth, V.K.; Verghese, J.; Allali, G.; Beauchet, O. Cognitive status, fast walking speed and walking speed reserve-the gait and Alzheimer interactions tracking (gait) study. Geroscience 2017, 39, 231–239. [Google Scholar] [CrossRef]
- Donoghue, O.A.; Setti, A.; O’Leary, N.; Kenny, R.A. Self-reported unsteadiness predicts fear of falling, activity restriction, falls, and disability. J. Am. Med. Dir. Assoc. 2017, 18, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Eggenberger, P.; Tomovic, S.; Münzer, T.; de Bruin, E.D. Older adults must hurry at pedestrian lights! A cross-sectional analysis of preferred and fast walking speed under single- and dual-task conditions. PLoS ONE 2017, 12, e0182180. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.C.; Slomiak, S.T.; Jones, J.D.; Rosen, A.F.G.; Moore, T.M.; Gur, R.C. Association of cannabis with cognitive functioning in adolescents and young adults: A systematic review and meta-analysis. JAMA Psychiatry 2018, 75, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Gorey, C.; Kuhns, L.; Smaragdi, E.; Kroon, E.; Cousijn, J. Age-related differences in the impact of cannabis use on the brain and cognition: A systematic review. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Crean, R.D.; Crane, N.A.; Mason, B.J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict. Med. 2011, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, A.M.; Dunn, M.E. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: A meta-analysis. Exp. Clin. Psychopharmacol. 2012, 20, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Blest-Hopley, G.; Giampietro, V.; Bhattacharyya, S. A systematic review of human neuroimaging evidence of memory-related functional alterations associated with cannabis use complemented with preclinical and human evidence of memory performance alterations. Brain Sci. 2020, 10, 102. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef]
- Tangestani Fard, M.; Stough, C. A review and hypothesized model of the mechanisms that underpin the relationship between inflammation and cognition in the elderly. Front. Aging Neurosci. 2019, 11, 56. [Google Scholar] [CrossRef]
- Patterson, S.L. Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1β, BDNF and synaptic plasticity. Neuropharmacology 2015, 96, 11–18. [Google Scholar] [CrossRef]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Karrer, T.M.; Josef, A.K.; Mata, R.; Morris, E.D.; Samanez-Larkin, G.R. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: A meta-analysis. Neurobiol. Aging 2017, 57, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, M.A.; Ashok, A.H.; Volkow, N.D.; Howes, O.D. The effects of δ(9)-tetrahydrocannabinol on the dopamine system. Nature 2016, 539, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L.; Bäckman, L. Cognitive aging: A view from brain imaging. In New Frontiers in Cognitive Aging; Dixon, R., Baeckman, L., Nilsson, L., Eds.; Oxford University Press: Oxford, UK, 2004; pp. 135–160. [Google Scholar]
- Mukherjee, J.; Christian, B.T.; Dunigan, K.A.; Shi, B.; Narayanan, T.K.; Satter, M.; Mantil, J. Brain imaging of 18F-fallypride in normal volunteers: Blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse 2002, 46, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Thayer, R.E.; YorkWilliams, S.L.; Hutchison, K.E.; Bryan, A.D. Preliminary results from a pilot study examining brain structure in older adult cannabis users and nonusers. Psychiatry Res. Neuroimaging 2019, 285, 58–63. [Google Scholar] [CrossRef]
- Burggren, A.C.; Siddarth, P.; Mahmood, Z.; London, E.D.; Harrison, T.M.; Merrill, D.A.; Small, G.W.; Bookheimer, S.Y. Subregional hippocampal thickness abnormalities in older adults with a history of heavy cannabis use. Cannabis Cannabinoid Res. 2018, 3, 242–251. [Google Scholar] [CrossRef]
- Fattore, L.; Marti, M.; Mostallino, R.; Castelli, M.P. Sex and gender differences in the effects of novel psychoactive substances. Brain Sci. 2020, 10, 606. [Google Scholar] [CrossRef]
- Colizzi, M.; Bhattacharyya, S. Cannabis: Neuropsychiatry and its effects on brain and behavior. Brain Sci. 2020, 10, 834. [Google Scholar] [CrossRef]
| Demographic | Non-Users | Users |
|---|---|---|
| Sex (M/F) | 3/5 | 3/5 |
| Age (years) | 60.5 ± 4.7 | 59.6 ± 4.8 |
| Height (cm) | 168.9 ± 10.6 | 169.5 ± 11.4 |
| Weight (kg) | 82.5 ± 20.7 | 92.8 ± 24.9 |
| Duration of cannabis use | n/a | 10.40 ± 12.6 |
| Uses per week (days) | n/a | 4.9 ± 2.5 |
| Uses per day (times) | n/a | 1.4 ± 0.7 |
| THC dominant (n) | n/a | 4 |
| THC = CBD (n) | n/a | 2 |
| CBD dominant (n) | n/a | 1 |
| Multiple types (n) | n/a | 1 |
| Medical reasons for use (n) | n/a | Pain (7), PD (1) |
| Variable Name | Users | Non-Users | p-Value | Effect Size |
|---|---|---|---|---|
| ABC-1 (%) * | 83.3 ± 15.4 | 85.6 ± 14.5 | 0.76 | d = 0.2 |
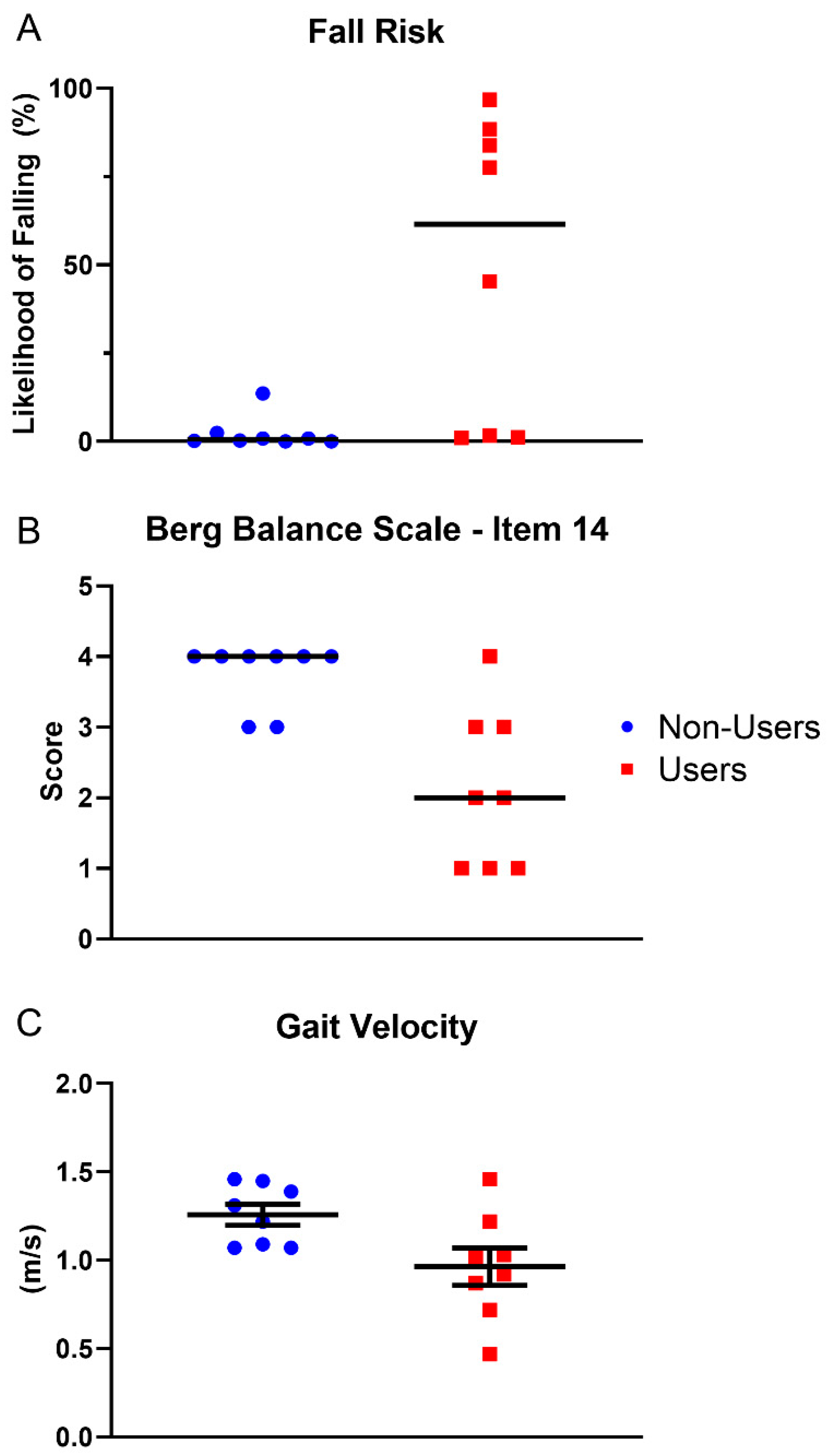
| BBS-14 (score) * | 2 (1–4) | 4 (3–4) | 0.008 | A = 0.89 |
| Simple RT (ms) * | 629.4 ± 38.7 | 668.1 ± 65.0 | 0.17 | d = 0.7 |
| Choice RT (ms) | 628.3 ± 204.4 | 623.9 ± 88.4 | 0.96 | d = 0.0 |
| Likelihood of Falling (%) | 61.5 (1.1–96.8) | 0.5 ± (0.05–13.6) | 0.005 | A =0.91 |
| Flanker-Compatible (ms) | 959.3 ± 189.3 | 963.0 ± 63.8 | 0.96 | d = 0.0 |
| Flanker-Incompatible (ms) | 1100.6 ± 224.5 | 1031.1 ± 59.0 | 0.44 | d = 0.4 |
| Flanker Effect (ms) | 140 (41–333) | 72 (2–112) | 0.07 | A = 0.71 |
| 9-HPT, D (s) | 24.4 ± 4.5 | 22.0 ± 4.0 | 0.30 | d = 0.6 |
| 9-HPT, ND (s) | 26.4 ± 4.1 | 23.2 ± 3.7 | 0.13 | d = 0.8 |
| Gait Velocity (m/s) | 0.96 ± 0.30 | 1.26 ± 0.17 | 0.03 | d = 1.2 |
| Stride Length (m) | 1.1 ± 0.2 | 1.3 ± 0.2 | 0.06 | d = 1.01 |
| AP-Pathlength (cm) | 2.5 ± 0.8 | 2.2 ± 0.6 | 0.47 | d = 0.4 |
| ML-Pathlength (cm) | 1.1 ± 0.4 | 0.9 ± 0.3 | 0.28 | d = 0.6 |
| COParea (cm2) | 1.6 (0.9–3.8) | 1.1 (0.4–6.6) | 0.38 | A = 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Workman, C.D.; Fietsam, A.C.; Sosnoff, J.; Rudroff, T. Increased Likelihood of Falling in Older Cannabis Users vs. Non-Users. Brain Sci. 2021, 11, 134. https://doi.org/10.3390/brainsci11020134
Workman CD, Fietsam AC, Sosnoff J, Rudroff T. Increased Likelihood of Falling in Older Cannabis Users vs. Non-Users. Brain Sciences. 2021; 11(2):134. https://doi.org/10.3390/brainsci11020134
Chicago/Turabian StyleWorkman, Craig D., Alexandra C. Fietsam, Jacob Sosnoff, and Thorsten Rudroff. 2021. "Increased Likelihood of Falling in Older Cannabis Users vs. Non-Users" Brain Sciences 11, no. 2: 134. https://doi.org/10.3390/brainsci11020134
APA StyleWorkman, C. D., Fietsam, A. C., Sosnoff, J., & Rudroff, T. (2021). Increased Likelihood of Falling in Older Cannabis Users vs. Non-Users. Brain Sciences, 11(2), 134. https://doi.org/10.3390/brainsci11020134






