An Analysis of Characteristics of Post-Stroke Fatigue in Patients without Depression: A Retrospective Chart Review
Abstract
:1. Introduction
2. Methods
2.1. Study Subjects
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.2. Study Design
2.3. Parameters
2.3.1. Demographic Characteristics
- (1)
- Age.
- (2)
- Sex.
- (3)
- Body mass index (BMI).
- (4)
- Religious status.
- (5)
- Smoking status.
- (6)
- Drinking status.
2.3.2. Stroke-Related Characteristics
- (1)
- Duration of stroke.
- (2)
- Type of stroke: ischemic stroke, hemorrhagic stroke.
- (3)
- Assessment of neurologic damage: National Institutes of Health Stroke Scale (NIHSS) score.
- (4)
- Assessment of cognitive function: Korean version of the Mini-Mental State Examination (MMSE-K) score.
- (5)
- Assessment of movement function: Modified Barthel Index (MBI), Manual Function Test (MFT) scores.
- (6)
- Medical history of stroke and various risk factors: medical histories of stroke, hypertension, dyslipidemia, diabetes mellitus, heart disease, and cancer were collected as listed on patients’ initial hospitalization records.
2.3.3. Fatigue Assessment
2.3.4. Laboratory Testing Results
2.3.5. Clinical Features and Pattern Identification
2.4. Statistical Analysis Methods
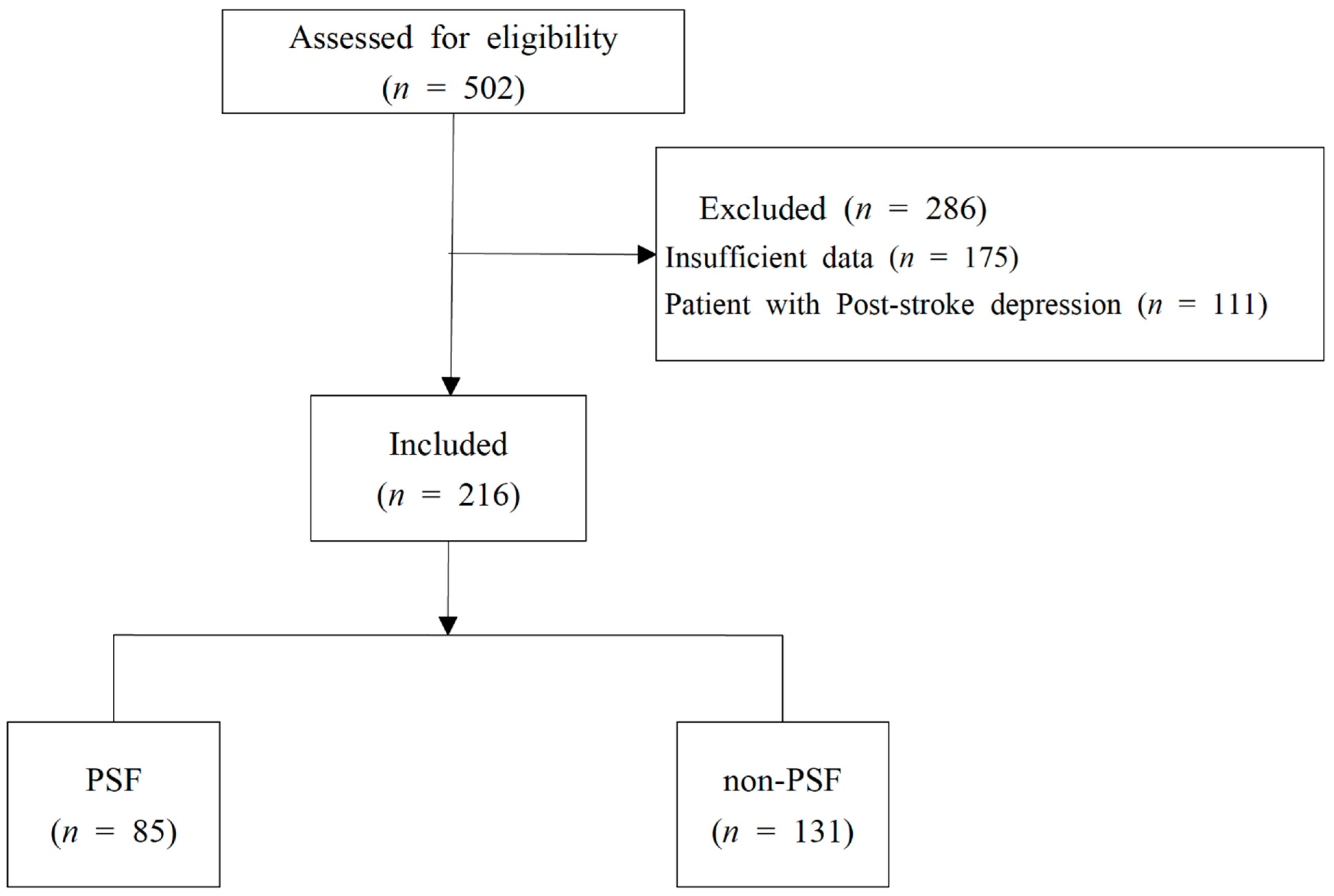
3. Results
3.1. Comparison of Demographic Characteristics
3.2. Comparison of Stroke-Related Characteristics
3.3. Comparison of Laboratory Examination Results
3.4. Comparison of Clinical Features and Pattern Identification
3.5. Multivariate Analysis on Factors Affecting PSF
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Causes of Death Statistics 2019; Statistics Korea: Daejeon, Korea, 2020.
- Ferro, J.; Caeiro, L.; Figueira, M.L. Neuropsychiatric sequelae of stroke. Nat. Rev. Neurol. 2016, 12, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Acciarresi, M.; Bogousslavsky, J.; Paciaroni, M. Post-Stroke Fatigue: Epidemiology, Clinical Characteristics and Treatment. Eur. Neurol. 2014, 72, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Cumming, T.B.; Packer, M.; Kramer, S.; English, C. The prevalence of fatigue after stroke: A systematic review and meta-analysis. Int. J. Stroke 2016, 11, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Egerton, T.; Hokstad, A.; Askim, T.; Bernhardt, J.; Indredavik, B. Prevalence of fatigue in pa-tients 3 months after stroke and association with early motor activity: A prospective study comparing stroke patients with a matched general population cohort. BMC Neurol. 2015, 15, 181. [Google Scholar] [CrossRef] [Green Version]
- Hubacher, M.; Calabrese, P.; Bassetti, C.; Carota, A.; Stöcklin, M.; Penner, I.-K. Assessment of Post-Stroke Fatigue: The Fatigue Scale for Motor and Cognitive Functions. Eur. Neurol. 2012, 67, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Nadarajah, M.; Goh, H.-T. Post-stroke fatigue: A review on prevalence, correlates, measurement, and management. Top. Stroke Rehabil. 2015, 22, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Oyake, K.; Otaka, Y.; Matsuura, D.; Honaga, K.; Mori, N.; Kondo, K. Poststroke Fatigue at Admission is Associated With Independence Levels of Activities of Daily Living at Discharge From Subacute Rehabilitation Wards. Arch. Phys. Med. Rehabil. 2021, 102, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Tseng, B.Y.; Billinger, S.A.; Gajewski, B.J.; Kluding, P.M. Exertion fatigue and chronic fa-tigue are two distinct constructs in people post-stroke. Stroke 2010, 41, 2908–2912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radman, N.; Staub, F.; Aboulafia-Brakha, T.; Berney, A.; Bogousslavsky, J.; Annoni, J.-M. Poststroke fatigue following minor infarcts: A prospective study. Neurology 2012, 79, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Ponchel, A.; Bombois, S.; Bordet, R.; Hénon, H. Factors Associated with Poststroke Fatigue: A Systematic Review. Stroke Res. Treat. 2015, 2015, 347920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi-Kwon, S.; Han, S.W.; Kwon, S.U.; Kim, J.S. Poststroke fatigue: Characteristics and re-lated factors. Cerebrovasc. Dis. 2005, 19, 84–90. [Google Scholar] [CrossRef]
- Choi-Kwon, S.; Ko, M.; Jun, S.-E.; Kim, J.; Cho, K.-H.; Nah, H.-W.; Song, H.; Kim, J.S. Post-Stroke Fatigue May Be Associated with the Promoter Region of a Monoamine Oxidase A Gene Polymorphism. Cerebrovasc. Dis. 2017, 43, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Ormstad, H.; Aass, H.C.D.; Amthor, K.-F.; Lund-Sørensen, N.; Sandvik, L. Serum Levels of Cytokines, Glucose, and Hemoglobin as Possible Predictors of Poststroke Depression, and Association With Poststroke Fatigue. Int. J. Neurosci. 2012, 122, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Weymann, K.B.; Wood, L.; Wang, Q.M. Inflammatory signaling in post-stroke fa-tigue and depression. Eur. Neurol. 2018, 80, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Jin, C.; Cho, S.-Y.; Park, S.-U.; Jung, W.-S.; Moon, S.-K.; Park, J.-M.; Ko, C.-N.; Cho, K.-H. Analysis of Factors Affecting Post-Stroke Fatigue: An Observational, Cross-Sectional, Retrospective Chart Review Study. Healthcare 2021, 9, 1586. [Google Scholar] [CrossRef]
- Michielsen, H.J.; De Vries, J.; Van Heck, G.L. Psychometric qualities of a brief self-rated fatigue measure: The Fatigue Assessment Scale. J. Psychosom. Res. 2003, 54, 345–352. [Google Scholar] [CrossRef]
- Krupp, L.B.; LaRocca, N.G.; Muir-Nash, J.; Steinberg, A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989, 46, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-S.; Cho, S.-Y.; Park, S.-U.; Moon, S.-K.; Park, J.-M.; Ko, C.-N.; Cho, K.-H.; Kwon, S. Development of Standardized Predictive Models for Traditional Korean Medical Diagnostic Pattern Identification in Stroke Subjects: A Hospital-based Multi-center Trial. J. Korean Med. 2019, 40, 49–60. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Y.; Xu, W.; Dong, L.; Wang, Y.; Gao, B.; Li, G.; Zhang, W. Apolipoprotein A1-Unique Peptide as a Diagnostic Biomarker for Acute Ischemic Stroke. Int. J. Mol. Sci. 2016, 17, 458. [Google Scholar] [CrossRef] [Green Version]
- Paula-Lima, A.C.; Tricerri, M.A.; Brito-Moreira, J.; Bomfim, T.R.; Oliveira, F.F.; Magdesian, M.H.; Grinberg, L.T.; Panizzutti, R.; Ferreira, S.T. Human apolipoprotein A-I binds amyloid-beta and prevents Abeta-induced neurotoxicity. Int. J. Biochem. Cell Biol. 2009, 41, 1361–1370. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Gregory, J.; Kuchibhotla, K.V.; Fine, S.; Wei, Y.; Ayata, C.; Frosch, M.P.; Greenberg, S.M.; Bacskai, B.J. Cerebrovascular lesions induce transient beta-amyloid deposition. Brain 2011, 134, 3697–3707. [Google Scholar] [CrossRef] [PubMed]
- Hooper, C.; De Souto Barreto, P.; Coley, N.; Cesari, M.; Payoux, P.; Salabert, A.S.; Andrieu, S.; Vellas, B.; MAPT/DSA Study Group. Cross-sectional associations of fatigue with cerebral beta-amyloid in older adults at risk of dementia. Front. Med. 2017, 4, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, H.-H.; Lee, H.-J.; Jang, E.-S.; Choi, S.-M.; Lee, S.-G.; Lee, S.-W. Study on development of cold-heat pattern questionnaire. J. Physiol. Pathol. Korean Med. 2008, 22, 1410–1415. [Google Scholar]
- Joo, J.-C.; Lee, S.; Park, S.-J. Comparison of Health Status and Mibyeong Characteristics between Cold Syndrome and Heat Syndrome by Cold Heat Syndrome Differentiation Score. J. Korean Med. 2018, 39, 13–21. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, H.J.; Jang, E.S.; Jung, H.J.; Hwang, M.W.; Nam, D.H. Survey on Pattern Identification and Treatment of Chronic Fatigue in Korea Medicine. J. Physiol. Pathol. Korean Med. 2018, 32, 126–133. [Google Scholar] [CrossRef]
- Cho, K.H.; Park, J.Y. How to Approach Cold Syndrome in Korean Medicine: To Be Learned from Narrative Based Medicine; Koonja Publishing Inc.: Seoul, Korea, 2013. [Google Scholar]
- Treatment of ME/CFS. Centers Dis Control Prev. 2018. Available online: https://www.cdc.gov/me-cfs/treatment/index.html (accessed on 9 December 2021).
| PSF (n = 85) | Non-PSF (n = 131) | p-Value a | |
|---|---|---|---|
| Age, years | 68.0 ± 14.2 | 64.6 ± 11.9 | 0.024 |
| Sex, n (%) | |||
| Male | 46 (54.1) | 84 (64.1) | 0.142 |
| Female | 39 (45.9) | 47 (35.9) | |
| BMI, kg/m2 | 23.58 ± 3.75 | 24.20 ± 3.48 | 0.093 |
| Having religion, n (%) | |||
| Yes | 45 (52.9) | 55 (42.3) | 0.126 |
| No | 40 (47.1) | 75 (57.7) | |
| Smoking, n (%) | |||
| Smoker | 27 (31.8) | 47 (35.9) | 0.534 |
| Non-smoker | 58 (68.2) | 84 (64.1) | |
| Alcohol consumption, n (%) | |||
| Drinker | 22 (25.9) | 44 (33.6) | 0.230 |
| Non-drinker | 63 (74.1) | 87 (66.4) |
| PSF (n = 85) | Non-PSF (n = 131) | p-Value a | |
|---|---|---|---|
| Disease duration, d | 64.4 ± 118.0 | 190.7 ± 920.2 | 0.356 |
| Stroke subtype, n(%) | |||
| Ischemic | 71 (83.5) | 121 (92.4) | 0.043 |
| Hemorrhagic | 14 (16.5) | 10 (7.6) | |
| NIHSS | 4.2 ± 3.9 | 4.2 ± 4.3 | 0.918 |
| MMSE-K | 23.8 ± 5.7 | 25.3 ± 4.0 | 0.109 |
| MBI | 56.6 ± 27.9 | 60.3 ± 25.5 | 0.477 |
| MFT | 18.6 ± 9.6 | 17.9 ± 10.4 | 0.889 |
| Medical history, n(%) | |||
| Stroke | 20 (23.5) | 22 (16.8) | 0.222 |
| HTN | 49 (57.6) | 60 (45.8) | 0.089 |
| Dyslipidemia | 22 (25.9) | 42 (32.1) | 0.331 |
| DM | 28 (32.9) | 51 (38.9) | 0.372 |
| Heart Disease | 14 (16.5) | 23 (17.6) | 0.836 |
| Cancer | 7 (8.2) | 9 (6.9) | 0.708 |
| PSF (n = 85) | Non-PSF (n = 131) | p-Value a | |
|---|---|---|---|
| WBC, 103/μL | 6.89 ± 2.39 | 6.85 ± 2.15 | 0.666 |
| RBC, 106/μL | 4.32 ± 0.49 | 4.36 ± 0.54 | 0.563 |
| Hemoglobin, g/dL | 13.42 ± 1.61 | 13.96 ± 3.20 | 0.152 |
| Platelet, 103/μL | 241.1 ± 76.6 | 240.1 ± 81.5 | 0.927 |
| ESR, mm/h | 29.1 ± 18.6 | 24.3 ± 23.2 | 0.004 |
| Segment of Lymphocyte, % | 28.35 ± 11.18 | 27.08 ± 7.52 | 0.359 |
| Monocyte, % | 5.57 ± 1.18 | 6.07 ± 1.55 | 0.123 |
| Eosinophil, % | 3.06 ± 2.37 | 2.62 ± 2.31 | 0.198 |
| Basophil, % | 0.48 ± 0.31 | 0.54 ± 0.70 | 0.173 |
| Neutrophil, % | 61.69 ± 10.62 | 61.75 ± 8.81 | 0.963 |
| PT INR | 1.07 ± 0.34 | 1.02 ± 0.16 | 0.411 |
| aPTT, s | 37.20 ± 5.55 | 35.41 ± 4.43 | 0.020 |
| PSF (n = 85) | Non-PSF (n = 131) | p-Value a | |
|---|---|---|---|
| Total protein, g/dL | 7.00 ± 0.61 | 6.99 ± 1.02 | 0.904 |
| Albumin, g/dL | 4.07 ± 0.4 | 4.47 ± 3.53 | 0.319 |
| Total bilirubin, mg/dL | 0.69 ± 0.27 | 1.05 ± 3.68 | 0.831 |
| Glucose, mg/dL | 127.6 ± 54.1 | 116.5 ± 41.2 | 0.608 |
| BUN, mg/dL | 17.3 ± 7.1 | 16.9 ± 7.2 | 0.497 |
| Creatinine, mg/dL | 0.84 ± 0.35 | 0.89 ± 0.66 | 0.705 |
| AST, U/L | 27.8 ± 15.7 | 28.8 ± 14.4 | 0.652 |
| ALT, U/L | 27.7 ± 35.5 | 27.8 ± 18.5 | 0.489 |
| ALP, U/L | 87.6 ± 35.7 | 80.5 ± 24.8 | 0.127 |
| Phosphorus, mg/dL | 3.69 ± 0.9 | 3.76 ± 0.64 | 0.548 |
| Calcium, mg/dL | 10.46 ± 9.62 | 9.45 ± 0.46 | 0.986 |
| Sodium, mmol/L | 139.0 ± 2.6 | 139.2 ± 2.4 | 0.585 |
| Potassium, mmol/L | 4.04 ± 0.4 | 4.06 ± 0.32 | 0.733 |
| Chloride, mmol/L | 105.1 ± 3.1 | 104.7 ± 2.8 | 0.376 |
| Uric acid, mg/dL | 5.0 ± 1.8 | 5.2 ± 1.6 | 0.375 |
| γ-GT, U/L | 29.2 ± 18.7 | 39.5 ± 47.6 | 0.414 |
| CK, U/L | 87.2 ± 61.1 | 106.2 ± 102.0 | 0.136 |
| CRP, mg/dL | 0.55 ± 1.58 | 1.28 ± 10.01 | 0.535 |
| hs-CRP, mg/dL | 0.89 ± 2.3 | 0.51 ± 1.17 | 0.143 |
| PSF (n = 85) | Non-PSF (n = 131) | p-Value a | |
|---|---|---|---|
| Total Cholesterol, mg/dL | 151.4 ± 47.0 | 150.0 ± 55.8 | 0.457 |
| Triglyceride, mg/dL | 139.2 ± 107.9 | 126.6 ± 79.4 | 0.529 |
| LDL-Cholesterol, mg/dL | 75.8 ± 34.9 | 74.4 ± 36.7 | 0.605 |
| HDL-Cholesterol, mg/dL | 55.9 ± 31.8 | 59.0 ± 28.7 | 0.063 |
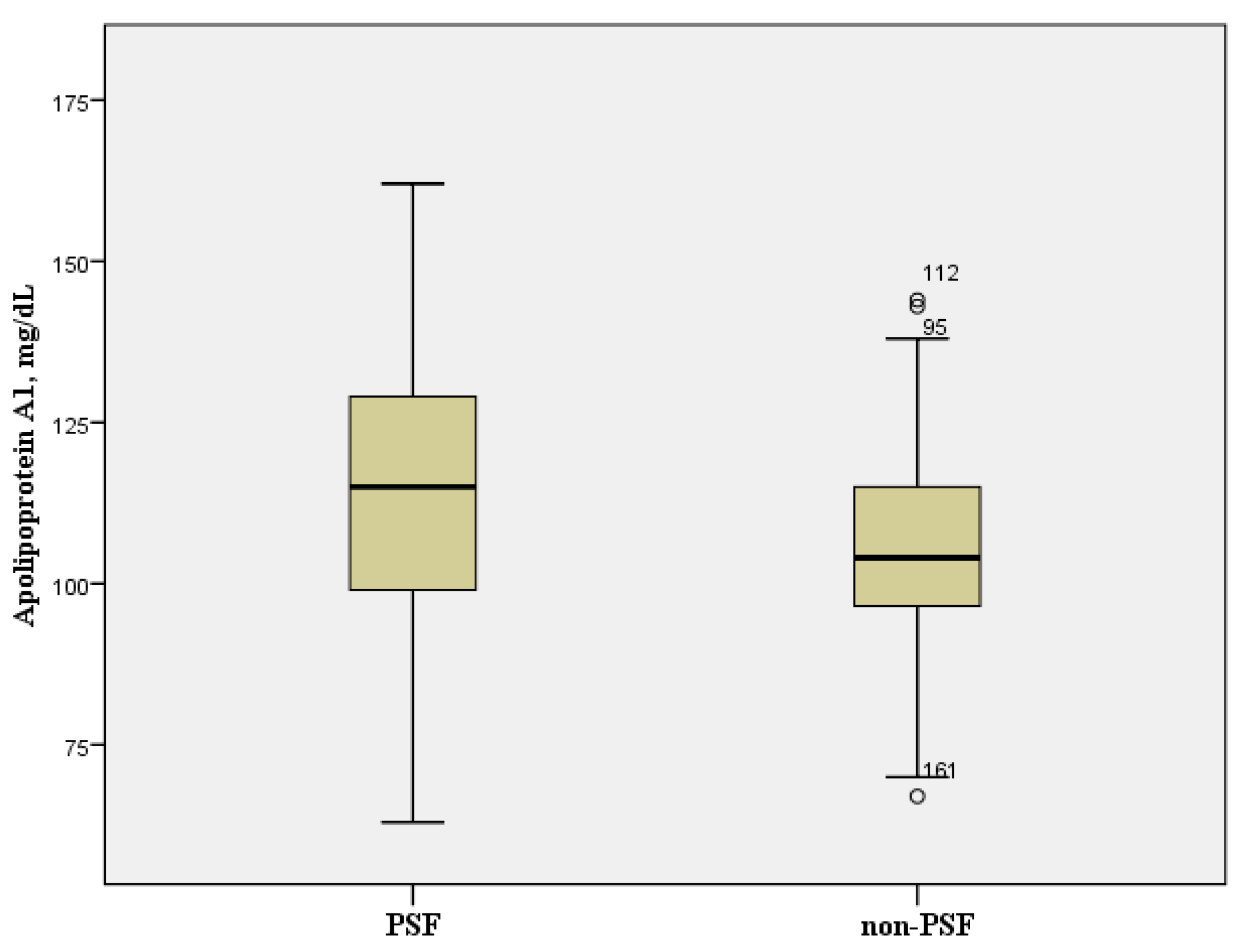
| Apolipoprotein A1, mg/dL | 105.6 ± 16.5 | 116.2 ± 21.8 | <0.001 |
| Apolipoprotein B, mg/dL | 75.6 ± 27.0 | 73.5 ± 25.4 | 0.665 |
| Total lipid, mg/dL | 456.9 ± 166.3 | 440.5 ± 117.5 | 0.884 |
| Phospholipid, mg/dL | 170.2 ± 36.7 | 172.3 ± 36.8 | 0.649 |
| Homocysteine, μmol/L | 12.31 ± 7.49 | 11.66 ± 4.99 | 0.884 |
| HbA1c, % | 6.26 ± 1.38 | 6.48 ± 4.60 | 0.761 |
| TSH, mIU/L | 2.21 ± 2.05 | 2.38 ± 1.83 | 0.314 |
| PSF (n = 85) | Non-PSF (n = 131) | p-Value a | |
|---|---|---|---|
| Insomnia, n (%) | 20 (23.5) | 32 (24.4) | 0.880 |
| Complexion, n (%) | |||
| Pale | 11 (12.9) | 21 (16.0) | 0.532 |
| Reddened | 5 (2.3) | 24 (18.3) | 0.880 |
| Faint low voice, n (%) | 17 (20.0) | 25 (19.1) | 0.868 |
| Thirst, n(%) | 10 (11.8) | 18 (13.7) | 0.673 |
| Reversal cold of the extremities, n(%) | 17 (20.0) | 13 (9.9) | 0.036 |
| Pulse, n(%) | |||
| Floating pulse | 30 (35.3) | 45 (34.4) | 0.887 |
| Deep pulse | 11 (12.9) | 8 (6.1) | 0.083 |
| Slow pulse | 3 (3.5) | 2 (1.2) | 0.339 |
| Rapid pulse | 2 (2.4) | 6 (4.6) | 0.397 |
| Fire-heat pattern, % | 9.0 ± 18.7 | 16.7 ± 26.8 | 0.014 |
| Phlegm dampness, % | 3.8 ± 11.3 | 3.4 ± 10.3 | 0.775 |
| Qi deficiency, % | 27.5 ± 33.5 | 26.6 ± 35.8 | 0.856 |
| Yin deficiency, % | 4.9 ± 6.3 | 5.4 ± 7.7 | 0.659 |
| Patient Characteristic | β Coefficient | 95% CI | p-Value | |
|---|---|---|---|---|
| Fire-heat pattern | −0.003 | −0.006 | 0.000 | 0.028 |
| Apolipoprotein A1 | −0.005 | −0.009 | −0.002 | 0.003 |
| aPTT | 0.015 | −0.001 | 0.030 | 0.066 |
| Age | 0.005 | −0.001 | 0.010 | 0.088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.J.; Jung, W.-S.; Kwon, S.; Jin, C.; Cho, S.-Y.; Park, S.-U.; Moon, S.-K.; Park, J.-M.; Ko, C.-N.; Cho, K.-H. An Analysis of Characteristics of Post-Stroke Fatigue in Patients without Depression: A Retrospective Chart Review. Brain Sci. 2021, 11, 1642. https://doi.org/10.3390/brainsci11121642
Lee YJ, Jung W-S, Kwon S, Jin C, Cho S-Y, Park S-U, Moon S-K, Park J-M, Ko C-N, Cho K-H. An Analysis of Characteristics of Post-Stroke Fatigue in Patients without Depression: A Retrospective Chart Review. Brain Sciences. 2021; 11(12):1642. https://doi.org/10.3390/brainsci11121642
Chicago/Turabian StyleLee, Yu Jin, Woo-Sang Jung, Seungwon Kwon, Chul Jin, Seung-Yeon Cho, Seong-Uk Park, Sang-Kwan Moon, Jung-Mi Park, Chang-Nam Ko, and Ki-Ho Cho. 2021. "An Analysis of Characteristics of Post-Stroke Fatigue in Patients without Depression: A Retrospective Chart Review" Brain Sciences 11, no. 12: 1642. https://doi.org/10.3390/brainsci11121642
APA StyleLee, Y. J., Jung, W.-S., Kwon, S., Jin, C., Cho, S.-Y., Park, S.-U., Moon, S.-K., Park, J.-M., Ko, C.-N., & Cho, K.-H. (2021). An Analysis of Characteristics of Post-Stroke Fatigue in Patients without Depression: A Retrospective Chart Review. Brain Sciences, 11(12), 1642. https://doi.org/10.3390/brainsci11121642






