Investigating the Relationships of P3b with Negative Symptoms and Neurocognition in Subjects with Chronic Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Clinical and Neurocognitive Assessments
2.3. EEG Recording Procedure
2.4. EEG Data Preprocessing
2.5. Statistical Analysis
3. Results
3.1. Participants Included
3.2. Demographic Characteristics, Neurocognitive Functions and Illness Related Variables
3.3. Group Comparison on P3b Amplitude and Latency
3.4. Correlation Analyses
3.5. Control Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galderisi, S.; Rucci, P.; Kirkpatrick, B.; Mucci, A.; Gibertoni, D.; Rocca, P.; Rossi, A.; Bertolino, A.; Strauss, G.P.; Aguglia, E.; et al. Interplay Among Psychopathologic Variables, Personal Resources, Context-Related Factors, and Real-life Functioning in Individuals With Schizophrenia: A Network Analysis. JAMA Psychiatry 2018, 75, 396–404. [Google Scholar] [CrossRef]
- Galderisi, S.; Rossi, A.; Rocca, P.; Bertolino, A.; Mucci, A.; Bucci, P.; Rucci, P.; Gibertoni, D.; Aguglia, E.; Amore, M.; et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry 2014, 13, 275–287. [Google Scholar] [CrossRef]
- Barch, D.M. Nonsocial and social cognitive function in psychosis: Interrelationships, specificity and innovative approaches. World Psychiatry 2019, 18, 117–118. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Rucci, P.; Mucci, A.; Rossi, A.; Rocca, P.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellomo, A.; Bozzatello, P.; et al. The interplay among psychopathology, personal resources, context-related factors and real-life functioning in schizophrenia: Stability in relationships after 4 years and differences in network structure between recovered and non-recovered patients. World Psychiatry 2020, 19, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Mucci, A.; Galderisi, S.; Gibertoni, D.; Rossi, A.; Rocca, P.; Bertolino, A.; Aguglia, E.; Amore, M.; Bellomo, A.; Biondi, M.; et al. Factors Associated with Real-Life Functioning in Persons with Schizophrenia in a 4-Year Follow-up Study of the Italian Network for Research on Psychoses. JAMA Psychiatry 2021, 78, 550–559. [Google Scholar] [CrossRef]
- Galderisi, S.; Kaiser, S.; Bitter, I.; Nordentoft, M.; Mucci, A.; Sabé, M.; Giordano, G.M.; Nielsen, M.; Glenthøj, L.B.; Pezzella, P.; et al. EPA guidance on treatment of negative symptoms in schizophrenia. Eur. Psychiatry 2021, 64, e21. [Google Scholar] [CrossRef] [PubMed]
- Milev, P.; Ho, B.C.; Arndt, S.; Andreasen, N.C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: A longitudinal first-episode study with 7-year follow-up. Am. J. Psychiatry 2005, 162, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Green, M.F.; Horan, W.P.; Lee, J. Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry 2019, 18, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Sahakian, B.J.; Savulich, G. Innovative methods for improving cognition, motivation and wellbeing in schizophrenia. World Psychiatry 2019, 18, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Falkai, P.; Schmitt, A. The need to develop personalized interventions to improve cognition in schizophrenia. World Psychiatry 2019, 18, 170. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M. Cognitive impairment as a diagnostic criterion and treatment target in schizophrenia. World Psychiatry 2019, 18, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Fleischhacker, W.W.; Uchida, H. Critical review of antipsychotic polypharmacy in the treatment of schizophrenia. Int. J. Neuropsychopharmacol. 2014, 17, 1083–1093. [Google Scholar] [CrossRef]
- Keefe, R.S.E. Why are there no approved treatments for cognitive impairment in schizophrenia? World Psychiatry 2019, 18, 167–168. [Google Scholar] [CrossRef]
- Reichenberg, A.; Velthorst, E.; Davidson, M. Cognitive impairment and psychosis in schizophrenia: Independent or linked conditions? World Psychiatry 2019, 18, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Bleuler, E. Dementia Praecox, or the Group of Schizophrenias; International Universities Press: New York, NY, USA, 1950. [Google Scholar]
- Heinrichs, R.W.; Zakzanis, K.K. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 1998, 12, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Mesholam-Gately, R.I.; Giuliano, A.J.; Goff, K.P.; Faraone, S.V.; Seidman, L.J. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology 2009, 23, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Reichenberg, A. The assessment of neuropsychological functioning in schizophrenia. Dialogues Clin. Neurosci. 2010, 12, 383–392. [Google Scholar] [CrossRef]
- Fatouros-Bergman, H.; Cervenka, S.; Flyckt, L.; Edman, G.; Farde, L. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr. Res. 2014, 158, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Hu, Y.; Zhu, Y.; Zhang, T.; Wang, J.; Ma, K.; Shi, C.; Yu, X.; Li, C. Meta-analysis of cognitive function in Chinese first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) profile of impairment. Gen. Psychiatry 2019, 32, e100043. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Murray, R.M. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: Do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr. Bull. 2014, 40, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Sitskoorn, M.M.; Aleman, A.; Ebisch, S.J.; Appels, M.C.; Kahn, R.S. Cognitive deficits in relatives of patients with schizophrenia: A meta-analysis. Schizophr. Res. 2004, 71, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Mucci, A.; Galderisi, S.; Green, M.F.; Nuechterlein, K.; Rucci, P.; Gibertoni, D.; Rossi, A.; Rocca, P.; Bertolino, A.; Bucci, P.; et al. Familial aggregation of MATRICS Consensus Cognitive Battery scores in a large sample of outpatients with schizophrenia and their unaffected relatives. Psychol. Med. 2017, 48, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- McCleery, A.; Ventura, J.; Kern, R.S.; Subotnik, K.L.; Gretchen-Doorly, D.; Green, M.F.; Hellemann, G.S.; Nuechterlein, K.H. Cognitive functioning in first-episode schizophrenia: MATRICS Consensus Cognitive Battery (MCCB) Profile of Impairment. Schizophr. Res. 2014, 157, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Kotov, R.; Jonas, K.G.; Carpenter, W.T.; Dretsch, M.N.; Eaton, N.R.; Forbes, M.K.; Forbush, K.T.; Hobbs, K.; Reininghaus, U.; Slade, T.; et al. Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): I. Psychosis superspectrum. World Psychiatry 2020, 19, 151–172. [Google Scholar] [CrossRef]
- Glenthøj, L.B.; Mariegaard, L.S.; Fagerlund, B.; Jepsen, J.R.M.; Kristensen, T.D.; Wenneberg, C.; Krakauer, K.; Medalia, A.; Roberts, D.L.; Hjorthøj, C.; et al. Effectiveness of cognitive remediation in the ultra-high risk state for psychosis. World Psychiatry 2020, 19, 401–402. [Google Scholar] [CrossRef]
- Heckers, S.; Kendler, K.S. The evolution of Kraepelin’s nosological principles. World Psychiatry 2020, 19, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Green, M.F.; Kern, R.S.; Heaton, R.K. Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophr. Res. 2004, 72, 41–51. [Google Scholar] [CrossRef]
- Thai, M.L.; Andreassen, A.K.; Bliksted, V. A meta-analysis of executive dysfunction in patients with schizophrenia: Different degree of impairment in the ecological subdomains of the Behavioural Assessment of the Dysexecutive Syndrome. Psychiatry Res. 2019, 272, 230–236. [Google Scholar] [CrossRef]
- Melle, I. Cognition in schizophrenia: A marker of underlying neurodevelopmental problems? World Psychiatry 2019, 18, 164–165. [Google Scholar] [CrossRef]
- Reininghaus, U.; Böhnke, J.R.; Chavez-Baldini, U.; Gibbons, R.; Ivleva, E.; Clementz, B.A.; Pearlson, G.D.; Keshavan, M.S.; Sweeney, J.A.; Tamminga, C.A. Transdiagnostic dimensions of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). World Psychiatry 2019, 18, 67–76. [Google Scholar] [CrossRef]
- Guloksuz, S.; Pries, L.K.; Delespaul, P.; Kenis, G.; Luykx, J.J.; Lin, B.D.; Richards, A.L.; Akdede, B.; Binbay, T.; Altınyazar, V.; et al. Examining the independent and joint effects of molecular genetic liability and environmental exposures in schizophrenia: Results from the EUGEI study. World Psychiatry 2019, 18, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Menon, V. Brain networks and cognitive impairment in psychiatric disorders. World Psychiatry 2020, 19, 309–310. [Google Scholar] [CrossRef]
- Insel, T.R. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef]
- Tripathi, A.; Kar, S.K.; Shukla, R. Cognitive Deficits in Schizophrenia: Understanding the Biological Correlates and Remediation Strategies. Clin. Psychopharmacol. Neurosci. 2018, 16, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Gourion, D.; Goldberger, C.; Olie, J.P.; Lôo, H.; Krebs, M.O. Neurological and morphological anomalies and the genetic liability to schizophrenia: A composite phenotype. Schizophr. Res. 2004, 67, 23–31. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Fischer, B. Subdomains Within the Negative Symptoms of Schizophrenia: Commentary. Schizophr. Bull. 2006, 32, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Mucci, A.; Dollfus, S.; Nordentoft, M.; Falkai, P.; Kaiser, S.; Giordano, G.M.; Vandevelde, A.; Nielsen, M.; Glenthøj, L.B.; et al. EPA guidance on assessment of negative symptoms in schizophrenia. Eur. Psychiatry 2021, 64, e23. [Google Scholar] [CrossRef]
- Galderisi, S.; Färden, A.; Kaiser, S. Dissecting negative symptoms of schizophrenia: History, assessment, pathophysiological mechanisms and treatment. Schizophr. Res. 2017, 186, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Mucci, A.; Buchanan, R.W.; Arango, C. Negative symptoms of schizophrenia: New developments and unanswered research questions. Lancet Psychiatry 2018, 5, 664–677. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Fenton, W.S.; Carpenter, W.T., Jr.; Marder, S.R. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr. Bull. 2006, 32, 214–219. [Google Scholar] [CrossRef]
- Marder, S.R.; Galderisi, S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 2017, 16, 14–24. [Google Scholar] [CrossRef]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef]
- Reed, G.M.; First, M.B.; Kogan, C.S.; Hyman, S.E.; Gureje, O.; Gaebel, W.; Maj, M.; Stein, D.J.; Maercker, A.; Tyrer, P.; et al. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry 2019, 18, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Gaebel, W.; Falkai, P.; Hasan, A. The revised German evidence- and consensus-based schizophrenia guideline. World Psychiatry 2020, 19, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Gard, D.E.; Kring, A.M.; Gard, M.G.; Horan, W.P.; Green, M.F. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophr. Res. 2007, 93, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Heerey, E.A.; Gold, J.M. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J. Abnorm. Psychol. 2007, 116, 268–278. [Google Scholar] [CrossRef]
- Heerey, E.A.; Robinson, B.M.; McMahon, R.P.; Gold, J.M. Delay discounting in schizophrenia. Cogn. Neuropsychiatry 2007, 12, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Waltz, J.A.; Frank, M.J.; Robinson, B.M.; Gold, J.M. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol. Psychiatry 2007, 62, 756–764. [Google Scholar] [CrossRef]
- Kring, A.M.; Moran, E.K. Emotional response deficits in schizophrenia: Insights from affective science. Schizophr. Bull. 2008, 34, 819–834. [Google Scholar] [CrossRef]
- Barch, D.M.; Dowd, E.C. Goal representations and motivational drive in schizophrenia: The role of prefrontal-striatal interactions. Schizophr. Bull. 2010, 36, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.S.; Minor, K.S. Emotional experience in patients with schizophrenia revisited: Meta-analysis of laboratory studies. Schizophr. Bull. 2010, 36, 143–150. [Google Scholar] [CrossRef]
- Dowd, E.C.; Barch, D.M. Anhedonia and emotional experience in schizophrenia: Neural and behavioral indicators. Biol. Psychiatry 2010, 67, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Foussias, G.; Remington, G. Negative symptoms in schizophrenia: Avolition and Occam’s razor. Schizophr. Bull. 2010, 36, 359–369. [Google Scholar] [CrossRef]
- Pizzagalli, D.A. The “anhedonia paradox” in schizophrenia: Insights from affective neuroscience. Biol. Psychiatry 2010, 67, 899–901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simpson, E.H.; Waltz, J.A.; Kellendonk, C.; Balsam, P.D. Schizophrenia in translation: Dissecting motivation in schizophrenia and rodents. Schizophr. Bull. 2012, 38, 1111–1117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mann, C.L.; Footer, O.; Chung, Y.S.; Driscoll, L.L.; Barch, D.M. Spared and impaired aspects of motivated cognitive control in schizophrenia. J. Abnorm. Psychol. 2013, 122, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.P.; Cohen, A.S. A Transdiagnostic Review of Negative Symptom Phenomenology and Etiology. Schizophr. Bull. 2017, 43, 712–719. [Google Scholar] [CrossRef]
- Strauss, G.P.; Waltz, J.A.; Gold, J.M. A review of reward processing and motivational impairment in schizophrenia. Schizophr. Bull. 2014, 40, S107–S116. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.W.; Quail, S.; Griffiths, K.R.; Green, M.J.; Balleine, B.W. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biol. Psychiatry 2015, 77, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Mucci, A.; Dima, D.; Soricelli, A.; Volpe, U.; Bucci, P.; Frangou, S.; Prinster, A.; Salvatore, M.; Galderisi, S.; Maj, M. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol. Med. 2015, 45, 1765–1778. [Google Scholar] [CrossRef]
- Amodio, A.; Quarantelli, M.; Mucci, A.; Prinster, A.; Soricelli, A.; Vignapiano, A.; Giordano, G.M.; Merlotti, E.; Nicita, A.; Galderisi, S. Avolition-Apathy and White Matter Connectivity in Schizophrenia: Reduced Fractional Anisotropy Between Amygdala and Insular Cortex. Clin. EEG Neurosci. 2018, 49, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.M.; Stanziano, M.; Papa, M.; Mucci, A.; Prinster, A.; Soricelli, A.; Galderisi, S. Functional connectivity of the ventral tegmental area and avolition in subjects with schizophrenia: A resting state functional MRI study. Eur. Neuropsychopharmacol. 2018, 28, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Culbreth, A.J.; Moran, E.K.; Kandala, S.; Westbrook, A.; Barch, D.M. Effort, avolition and motivational experience in schizophrenia: Analysis of behavioral and neuroimaging data with relationships to daily motivational experience. Clin. Psychol. Sci. 2020, 8, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.; Lyne, J.; Agartz, I.; Clarke, M.; Mørch-Johnsen, L.; Faerden, A. Individual negative symptoms and domains –Relevance for assessment, pathomechanisms and treatment. Schizophr. Res. 2017, 186, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D.; Strassnig, M.T. Cognition and disability in schizophrenia: Cognition-related skills deficits and decision-making challenges add to morbidity. World Psychiatry 2019, 18, 165–167. [Google Scholar] [CrossRef]
- Grant, P.M.; Best, M.W.; Beck, A.T. The meaning of group differences in cognitive test performance. World Psychiatry 2019, 18, 163–164. [Google Scholar] [CrossRef]
- Moritz, S.; Silverstein, S.M.; Dietrichkeit, M.; Gallinat, J. Neurocognitive deficits in schizophrenia are likely to be less severe and less related to the disorder than previously thought. World Psychiatry 2020, 19, 254–255. [Google Scholar] [CrossRef]
- Bissonette, G.B.; Roesch, M.R. Development and function of the midbrain dopamine system: What we know and what we need to. Genes Brain Behav. 2016, 15, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.P. Multiple Systems for the Motivational Control of Behavior and Associated Neural Substrates in Humans. Curr. Top. Behav. Neurosci. 2016, 27, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R. Cognitive remediation for severe mental illness: State of the field and future directions. World Psychiatry 2019, 18, 274–275. [Google Scholar] [CrossRef]
- Levy, R.; Dubois, B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb. Cortex 2006, 16, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Faerden, A.; Vaskinn, A.; Finset, A.; Agartz, I.; Ann Barrett, E.; Friis, S.; Simonsen, C.; Andreassen, O.A.; Melle, I. Apathy is associated with executive functioning in first episode psychosis. BMC Psychiatry 2009, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Riemer, M.N.; Hager, O.M.; Kirschner, M.; Bischof, M.; Kluge, A.; Seifritz, E.; Kaiser, S. The association of neurocognitive impairment with diminished expression and apathy in schizophrenia. Schizophr. Res. 2015, 169, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Kring, A.M.; Elis, O. Emotion deficits in people with schizophrenia. Annu. Rev. Clin. Psychol. 2013, 9, 409–433. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.S.; Schwartz, E.; Le, T.P.; Fedechko, T.; Kirkpatrick, B.; Strauss, G.P. Using biobehavioral technologies to effectively advance research on negative symptoms. World Psychiatry 2019, 18, 103–104. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- İnce, E.; Üçok, A. Relationship Between Persistent Negative Symptoms and Findings of Neurocognition and Neuroimaging in Schizophrenia. Clin. EEG Neurosci. 2017, 49, 27–35. [Google Scholar] [CrossRef]
- Hasey, G.M.; Kiang, M. A review of recent literature employing electroencephalographic techniques to study the pathophysiology, phenomenology, and treatment response of schizophrenia. Curr. Psychiatry Rep. 2013, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.-Q.; Tang, Y.-X.; Chan, R.C.K.; Sun, X.-Y.; He, J. P300 aberration in first-episode schizophrenia patients: A meta-analysis. PLoS ONE 2014, 9, e97794. [Google Scholar] [CrossRef]
- Phillips, J.M.; Maxwell, C.R.; Ehrlichman, R.S.; Siegel, S.J. Event-Related Potentials (ERPs) in the Study of Schizophrenia: How Preclinical ERP Studies have Contributed to our Understanding of Schizophrenia. In Handbook of Neurochemistry and Molecular Neurobiology: Schizophrenia; Lajtha, A., Javitt, D., Kantrowitz, J., Eds.; Springer: Boston, MA, USA, 2009; pp. 525–543. [Google Scholar]
- Turetsky, B.I.; Bilker, W.B.; Siegel, S.J.; Kohler, C.G.; Gur, R.E. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Res. 2009, 165, 27–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, W.J.; Chen, W.W.; Zhang, X. The neurophysiology of P 300—An integrated review. Eur. Rev. Med Pharmacol. Sci. 2015, 19, 1480–1488. [Google Scholar] [PubMed]
- Wang, L.; Zheng, J.; Huang, S.; Sun, H. P300 and Decision Making under Risk and Ambiguity. Comput. Intell. Neurosci. 2015, 2015, 108417. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Zhou, L.; Zheng, Y. The time course of incentive processing in anticipatory and consummatory anhedonia. J. Affect. Disord. 2018, 238, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Broyd, S.J.; Richards, H.J.; Helps, S.K.; Chronaki, G.; Bamford, S.; Sonuga-Barke, E.J. An electrophysiological monetary incentive delay (e-MID) task: A way to decompose the different components of neural response to positive and negative monetary reinforcement. J. Neurosci. Methods 2012, 209, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Vignapiano, A.; Mucci, A.; Ford, J.; Montefusco, V.; Plescia, G.M.; Bucci, P.; Galderisi, S. Reward anticipation and trait anhedonia: An electrophysiological investigation in subjects with schizophrenia. Clin. Neurophysiol. 2016, 127, 2149–2160. [Google Scholar] [CrossRef] [PubMed]
- Pfabigan, D.M.; Seidel, E.-M.; Sladky, R.; Hahn, A.; Paul, K.; Grahl, A.; Küblböck, M.; Kraus, C.; Hummer, A.; Kranz, G.S.; et al. P300 amplitude variation is related to ventral striatum BOLD response during gain and loss anticipation: An EEG and fMRI experiment. NeuroImage 2014, 96, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Megías, A.; Gutiérrez-Cobo, M.J.; Gómez-Leal, R.; Cabello, R.; Fernández-Berrocal, P. Performance on emotional tasks engaging cognitive control depends on emotional intelligence abilities: An ERP study. Sci. Rep. 2017, 7, 16446. [Google Scholar] [CrossRef] [PubMed]
- Portnova, G.V.; Maslennikova, A.V.; Zakharova, N.V.; Martynova, O.V. The Deficit of Multimodal Perception of Congruent and Non-Congruent Fearful Expressions in Patients with Schizophrenia: The ERP Study. Brain Sci. 2021, 11, 96. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Bledowski, C.; Prvulovic, D.; Hoechstetter, K.; Scherg, M.; Wibral, M.; Goebel, R.; Linden, D.E.J. Localizing P300 Generators in Visual Target and Distractor Processing: A Combined Event-Related Potential and Functional Magnetic Resonance Imaging Study. J. Neurosci. 2004, 24, 9353. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.E. The p300: Where in the brain is it produced and what does it tell us? Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2005, 11, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Onitsuka, T.; Oribe, N.; Nakamura, I.; Kanba, S. Review of neurophysiological findings in patients with schizophrenia. Psychiatry Clin. Neurosci. 2013, 67, 461–470. [Google Scholar] [CrossRef]
- Galderisi, S.; Mucci, A.; Volpe, U.; Boutros, N. Evidence-based medicine and electrophysiology in schizophrenia. Clin. EEG Neurosci. 2009, 40, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.W.; Polich, J. Meta-analysis of P300 and schizophrenia: Patients, paradigms, and practical implications. Psychophysiology 2003, 40, 684–701. [Google Scholar] [CrossRef]
- Perrottelli, A.; Giordano, G.M.; Brando, F.; Giuliani, L.; Mucci, A. EEG-Based Measures in At-Risk Mental State and Early Stages of Schizophrenia: A Systematic Review. Front. Psychiatry 2021, 12, 582. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Rosquist, H.; Fjell, A.M. P300 amplitude age reductions are not caused by latency jitter. Psychophysiology 2008, 45, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Mott, K.K.; Alperin, B.R.; Holcomb, P.J.; Daffner, K.R. Age-related decline in differentiated neural responses to rare target versus frequent standard stimuli. Brain Res. 2014, 1587, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Porcaro, C.; Balsters, J.H.; Mantini, D.; Robertson, I.H.; Wenderoth, N. P3b amplitude as a signature of cognitive decline in the older population: An EEG study enhanced by Functional Source Separation. NeuroImage 2019, 184, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.H.; Campbell, A.M.; Schipul, S.E.; Bellion, C.M.; Donkers, F.C.; Evans, A.M.; Belger, A. Electrophysiological Correlates of Aberrant Motivated Attention and Salience Processing in Unaffected Relatives of Schizophrenia Patients. Clin. EEG Neurosci. 2016, 47, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, A.; Strik, W.K. Auditory P300 topography and neuropsychological test performance: Evidence for left hemispheric dysfunction in schizophrenia. Biol. Psychiatry 1997, 41, 327–335. [Google Scholar] [CrossRef]
- Schreiber, H.; Stolz-Born, G.; Kornhuber, H.H.; Born, J. Investigation of electrophysiological correlates of attention and information processing as vulnerability indicators for schizophrenia. J. Psychophysiol. 1997, 3, 286–300. [Google Scholar]
- Kruiper, C.; Fagerlund, B.; Nielsen, M.Ø.; Düring, S.; Jensen, M.H.; Ebdrup, B.H.; Glenthøj, B.Y.; Oranje, B. Associations between P3a and P3b amplitudes and cognition in antipsychotic-naïve first-episode schizophrenia patients. Psychol. Med. 2019, 49, 868–875. [Google Scholar] [CrossRef]
- Nagasawa, T.; Kamiya, T.; Kawasaki, Y.; Higashima, M.; Urata, K.; Sakai, N.; Koshino, Y. The relationship between auditory ERP and neuropsychological assessments in schizophrenia. Int. J. Psychophysiol. 1999, 34, 267–274. [Google Scholar] [CrossRef][Green Version]
- Sumich, A.; Kumari, V.; Dodd, P.; Ettinger, U.; Hughes, C.; Zachariah, E.; Sharma, T. N100 and P300 amplitude to Go and No-Go variants of the auditory oddball in siblings discordant for schizophrenia. Schizophr. Res. 2008, 98, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-H.; Chen, K.-C.; Yang, Y.-K.; Chen, P.-S.; Lu, R.-B.; Yeh, T.-L.; Wang, C.S.-M.; Lee, I.H. Association between auditory P300, psychopathology, and memory function in drug-naïve schizophrenia. Kaohsiung J. Med. Sci. 2014, 30, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Galletly, C.A.; MacFarlane, A.C.; Clark, C.R. Impaired updating of working memory in schizophrenia. Int. J. Psychophysiol. 2007, 63, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bruder, G.E.; Kayser, J.; Tenke, C.E.; Friedman, M.; Malaspina, D.; Gorman, J.M. Event-related potentials in schizophrenia during tonal and phonetic oddball tasks: Relations to diagnostic subtype, symptom features and verbal memory. Biol. Psychiatry 2001, 50, 447–452. [Google Scholar] [CrossRef]
- Kayser, J.; Bruder, G.E.; Friedman, D.; Tenke, C.E.; Amador, X.F.; Clark, S.C.; Malaspina, D.; Gorman, J.M. Brain event-related potentials (ERPs) in schizophrenia during a word recognition memory task. Int. J. Psychophysiol. 1999, 34, 249–265. [Google Scholar] [CrossRef]
- Nieman, D.H.; Koelman, J.H.T.M.; Linszen, D.H.; Bour, L.J.; Dingemans, P.M.; Ongerboer de Visser, B.W. Clinical and neuropsychological correlates of the P300 in schizophrenia. Schizophr. Res. 2002, 55, 105–113. [Google Scholar] [CrossRef]
- Shajahan, P.M.; O’Carroll, R.E.; Glabus, M.F.; Ebmeier, K.P.; Blackwood, D.H. Correlation of auditory ‘oddball’ P300 with verbal memory deficits in schizophrenia. Psychol. Med. 1997, 27, 579–586. [Google Scholar] [CrossRef]
- Kim, M.; Lee, T.H.; Kim, J.-H.; Hong, H.; Lee, T.Y.; Lee, Y.; Salisbury, D.F.; Kwon, J.S. Decomposing P300 into correlates of genetic risk and current symptoms in schizophrenia: An inter-trial variability analysis. Schizophr. Res. 2018, 192, 232–239. [Google Scholar] [CrossRef]
- Şevik, A.E.; Anıl Yağcıoğlu, A.E.; Yağcıoğlu, S.; Karahan, S.; Gürses, N.; Yıldız, M. Neuropsychological performance and auditory event related potentials in schizophrenia patients and their siblings: A family study. Schizophr. Res. 2011, 130, 195–202. [Google Scholar] [CrossRef]
- Ertekin, E.; Üçok, A.; Keskin-Ergen, Y.; Devrim-Üçok, M. Deficits in Go and NoGo P3 potentials in patients with schizophrenia. Psychiatry Res. 2017, 254, 126–132. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kang, S.-S.; Youn, T.; Kang, D.-H.; Kim, J.-J.; Kwon, J.S. Neuropsychological correlates of P300 abnormalities in patients with schizophrenia and obsessive–compulsive disorder. Psychiatry Res. Neuroimag. 2003, 123, 109–123. [Google Scholar] [CrossRef]
- Liu, Z.; Tam, W.C.; Xue, Z.; Yao, S.; Wu, D. Positive and negative symptom profile schizophrenia and abnormalities in the P300 component of the event-related potential: A longitudinal controlled study. Psychiatry Res. 2004, 132, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Mathalon, D.H.; Ford, J.M.; Pfefferbaum, A. Trait and state aspects of P300 amplitude reduction in schizophrenia: A retrospective longitudinal study. Biol. Psychiatry 2000, 47, 434–449. [Google Scholar] [CrossRef]
- Salgari, G.C.; Potts, G.F.; Schmidt, J.; Chan, C.C.; Spencer, C.C.; Bedwell, J.S. Event-related potentials to rare visual targets and negative symptom severity in a transdiagnostic psychiatric sample. Clin. Neurophysiol. 2021, 132, 1526–1536. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, H.K.; Woods, S.W.; Roach, B.J.; Llerena, K.; McGlashan, T.H.; Srihari, V.H.; Ford, J.M.; Mathalon, D.H. Auditory and Visual Oddball Stimulus Processing Deficits in Schizophrenia and the Psychosis Risk Syndrome: Forecasting Psychosis Risk With P300. Schizophr. Bull. 2019, 45, 1068–1080. [Google Scholar] [CrossRef]
- Van Tricht, M.J.; Nieman, D.H.; Koelman, J.H.T.M.; van der Meer, J.N.; Bour, L.J.; de Haan, L.; Linszen, D.H. Reduced Parietal P300 Amplitude is Associated with an Increased Risk for a First Psychotic Episode. Biol. Psychiatry 2010, 68, 642–648. [Google Scholar] [CrossRef]
- Lee, S.Y.; Namkoong, K.; Cho, H.H.; Song, D.-H.; An, S.K. Reduced visual P300 amplitudes in individuals at ultra-high risk for psychosis and first-episode schizophrenia. Neurosci. Lett. 2010, 486, 156–160. [Google Scholar] [CrossRef]
- Mori, K.; Morita, K.; Shoji, Y.; Matsuoka, T.; Fujiki, R.; Uchimura, N. State and trait markers of emotionally charged visual event-related potentials (P300) in drug-naïve schizophrenia. Psychiatry Clin. Neurosci. 2012, 66, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Sumich, A.; Harris, A.; Flynn, G.; Whitford, T.; Tunstall, N.; Kumari, V.; Brammer, M.; Gordon, E.; Williams, L.M. Event-related potential correlates of depression, insight and negative symptoms in males with recent-onset psychosis. Clin. Neurophysiol. 2006, 117, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Meisenzahl, E.M.; Frodl, T.; Müller, D.; Schmitt, G.; Gallinat, J.; Zetzsche, T.; Marcuse, A.; Juckel, G.; Leinsinger, G.; Hahn, K.; et al. Superior temporal gyrus and P300 in schizophrenia: A combined ERP/structural magnetic resonance imaging investigation. J. Psychiatr. Res. 2004, 38, 153–162. [Google Scholar] [CrossRef]
- Mucci, A.; Galderisi, S.; Kirkpatrick, B.; Bucci, P.; Volpe, U.; Merlotti, E.; Centanaro, F.; Catapano, F.; Maj, M. Double dissociation of N1 and P3 abnormalities in deficit and nondeficit schizophrenia. Schizophr. Res. 2007, 92, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.C. Scale for the Assessment of Negative Symptoms (SANS). Br. J. Psychiatry 1989, 155, 53–58. [Google Scholar] [CrossRef]
- Overall, J.E.; Gorham, D.R. The Brief Psychiatric Rating Scale. Psychol. Rep. 1962, 10, 799–812. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Strauss, G.P.; Nguyen, L.; Fischer, B.A.; Daniel, D.G.; Cienfuegos, A.; Marder, S.R. The Brief Negative Symptom Scale: Psychometric Properties. Schizophr. Bull. 2010, 37, 300–305. [Google Scholar] [CrossRef]
- Mucci, A.; Galderisi, S.; Merlotti, E.; Rossi, A.; Rocca, P.; Bucci, P.; Piegari, G.; Chieffi, M.; Vignapiano, A.; Maj, M. The Brief Negative Symptom Scale (BNSS): Independent validation in a large sample of Italian patients with schizophrenia. Eur. Psychiatry 2015, 30, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Addington, D.; Addington, J.; Schissel, B. A depression rating scale for schizophrenics. Schizophr. Res. 1990, 3, 247–251. [Google Scholar] [CrossRef]
- Gerlach, J.; Korsgaard, S.; Clemmesen, P.; Lauersen, A.M.; Magelund, G.; Noring, U.; Povlsen, U.J.; Bech, P.; Casey, D.E. The St. Hans Rating Scale for extrapyramidal syndromes: Reliability and validity. Acta Psychiatr. Scand. 1993, 87, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Nuechterlein, K.H.; Green, M.F.; Kern, R.S.; Baade, L.E.; Barch, D.M.; Cohen, J.D.; Essock, S.; Fenton, W.S.; Frese, F.J., 3rd; Gold, J.M.; et al. The MATRICS Consensus Cognitive Battery, part 1: Test selection, reliability, and validity. Am. J. Psychiatry 2008, 165, 203–213. [Google Scholar] [CrossRef]
- Comerchero, M.D.; Polich, J. P3a and P3b from typical auditory and visual stimuli. Clin. Neurophysiol. 1999, 110, 24–30. [Google Scholar] [CrossRef]
- Bachiller, A.; Romero, S.; Molina, V.; Alonso, J.F.; Mañanas, M.A.; Poza, J.; Hornero, R. Auditory P3a and P3b neural generators in schizophrenia: An adaptive sLORETA P300 localization approach. Schizophr. Res. 2015, 169, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Shim, M.; Kim, J.-I.; Im, C.-H.; Lee, S.-H. Source Activation of P300 Correlates with Negative Symptom Severity in Patients with Schizophrenia. Brain Topogr. 2014, 27, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Molina, V.; Bachiller, A.; de Luis, R.; Lubeiro, A.; Poza, J.; Hornero, R.; Alonso, J.F.; Mañanas, M.A.; Marqués, P.; Romero, S. Topography of activation deficits in schizophrenia during P300 task related to cognition and structural connectivity. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Van der Stelt, O.; Lieberman, J.A.; Belger, A. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr. Res. 2005, 77, 309–320. [Google Scholar] [CrossRef]
- Morales-Muñoz, I.; Jurado-Barba, R.; Fernández-Guinea, S.; Álvarez-Alonso, M.J.; Rodríguez-Jiménez, R.; Jiménez-Arriero, M.A.; Rubio, G. Cognitive impairments in patients with first episode psychosis: The relationship between neurophysiological and neuropsychological assessments. J. Clin. Neurosci. 2017, 36, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Dosenbach, N.U.; Fair, D.A.; Cohen, A.L.; Schlaggar, B.L.; Petersen, S.E. A dual-networks architecture of top-down control. Trends Cogn. Sci. 2008, 12, 99–105. [Google Scholar] [CrossRef]
- Tam, A.; Luedke, A.C.; Walsh, J.J.; Fernandez-Ruiz, J.; Garcia, A. Effects of reaction time variability and age on brain activity during Stroop task performance. Brain Imaging Behav. 2015, 9, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.D.; Bizzell, J.; Kim, C.; Bellion, C.; Carpenter, K.L.H.; Dichter, G.; Belger, A. Attention deficits in schizophrenia—Preliminary evidence of dissociable transient and sustained deficits. Schizophr. Res. 2010, 122, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Dichter, G.S.; Bellion, C.; Casp, M.; Belger, A. Impaired Modulation of Attention and Emotion in Schizophrenia. Schizophr. Bull. 2008, 36, 595–606. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Jiang, Y.; Si, Y.; Peng, W.; Song, L.; Jiang, Y.; Zhang, Y.; Dong, W.; Yao, D.; et al. Top-Down Disconnectivity in Schizophrenia During P300 Tasks. Front. Comput. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.F.; McCarley, R.W.; Potts, G.F.; Salisbury, D.F.; Nestor, P.G.; Hirayasu, Y.; Niznikiewicz, M.A.; Barnard, J.; Shen, Z.J.; Weinstein, D.M.; et al. Identification of neural circuits underlying P300 abnormalities in schizophrenia. Psychophysiology 1999, 36, 388–398. [Google Scholar] [CrossRef]
- Sabeti, M.; Moradi, E.; Katebi, S. Analysis of Neural Sources of P300 Event-Related Potential in Normal and Schizophrenic Participants. In Software Tools and Algorithms for Biological Systems; Arabnia, H.R., Tran, Q.-N., Eds.; Springer: New York, NY, USA, 2011; pp. 589–597. [Google Scholar]
- Van Dinteren, R.; Arns, M.; Jongsma, M.L.; Kessels, R.P. Combined frontal and parietal P300 amplitudes indicate compensated cognitive processing across the lifespan. Front. Aging Neurosci. 2014, 6, 294. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. On the relationship between EEG and P300: Individual differences, aging, and ultradian rhythms. Int. J. Psychophysiol. 1997, 26, 299–317. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Fjell, A.M. Two- and three-stimuli auditory oddball ERP tasks and neuropsychological measures in aging. NeuroReport 2001, 12, 314–3153. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B.; Fischl, B.; Reinvang, I. Cognitive function, P3a/P3b brain potentials, and cortical thickness in aging. Hum. Brain Mapp. 2007, 28, 1098–1116. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Sumiyoshi, T.; Higuchi, Y.; Ito, T.; Takeuchi, M.; Kurachi, M. Voxel-based analysis of P300 electrophysiological topography associated with positive and negative symptoms of schizophrenia. Schizophr. Res. 2007, 94, 164–171. [Google Scholar] [CrossRef]

| SCZ (n = 114) | HCs (n = 63) | Statistics | ||
|---|---|---|---|---|
| Gender | 81 M–33 W | 32 M–31 W | χ2 = 7.214; p = 0.007 | |
| Mean ± SD | Mean ± SD | t/U | p | |
| Age | 36.86 ± 9.39 | 34.44 ± 12.48 | U = 2982.00 | 0.062 |
| Educational level (years) | 12.35 ± 3.02 | 13.98 ± 4.04 | U = 2759.00 | 0.0083 |
| BNSS Expressive Deficit Domain | 11.35 ± 7.27 | - | - | - |
| BNSS Experiential Domain | 21.11 ± 9.25 | - | - | - |
| PANSS Positive | 8.33 ± 4.74 | - | - | - |
| PANSS Disorganization | 8.60 ± 3.49 | - | - | - |
| CDSS Total score | 3.24 ± 3.92 | - | - | - |
| SHRS Global Parkinsonism | 0.86 ± 1.15 | - | - | - |
| MCCB SoP | 32.79 ± 10.42 | 48.79 ± 9.94 | t = −9.774 | <0.0001 |
| MCCB AV | 40.20 ± 10.27 | 51.67 ± 10.22 | t = −6.420 | <0.0001 |
| MCCB WM | 36.33 ± 11.78 | 50.60 ± 10.12 | t = −7.189 | <0.0001 |
| MCCB VrbLrn | 37.02 ± 11.03 | 52.13 ± 7.30 | t = −10.489 | <0.0001 |
| MCCB VisLrn | 31.86 ± 13.20 | 47.76 ± 11.19 | U = 1033.50 | <0.0001 |
| MCCB RPS | 38.53 ± 11.33 | 51.03 ± 8.75 | U = 1262.00 | <0.0001 |
| SCZ | HCs | |||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | U | p | |
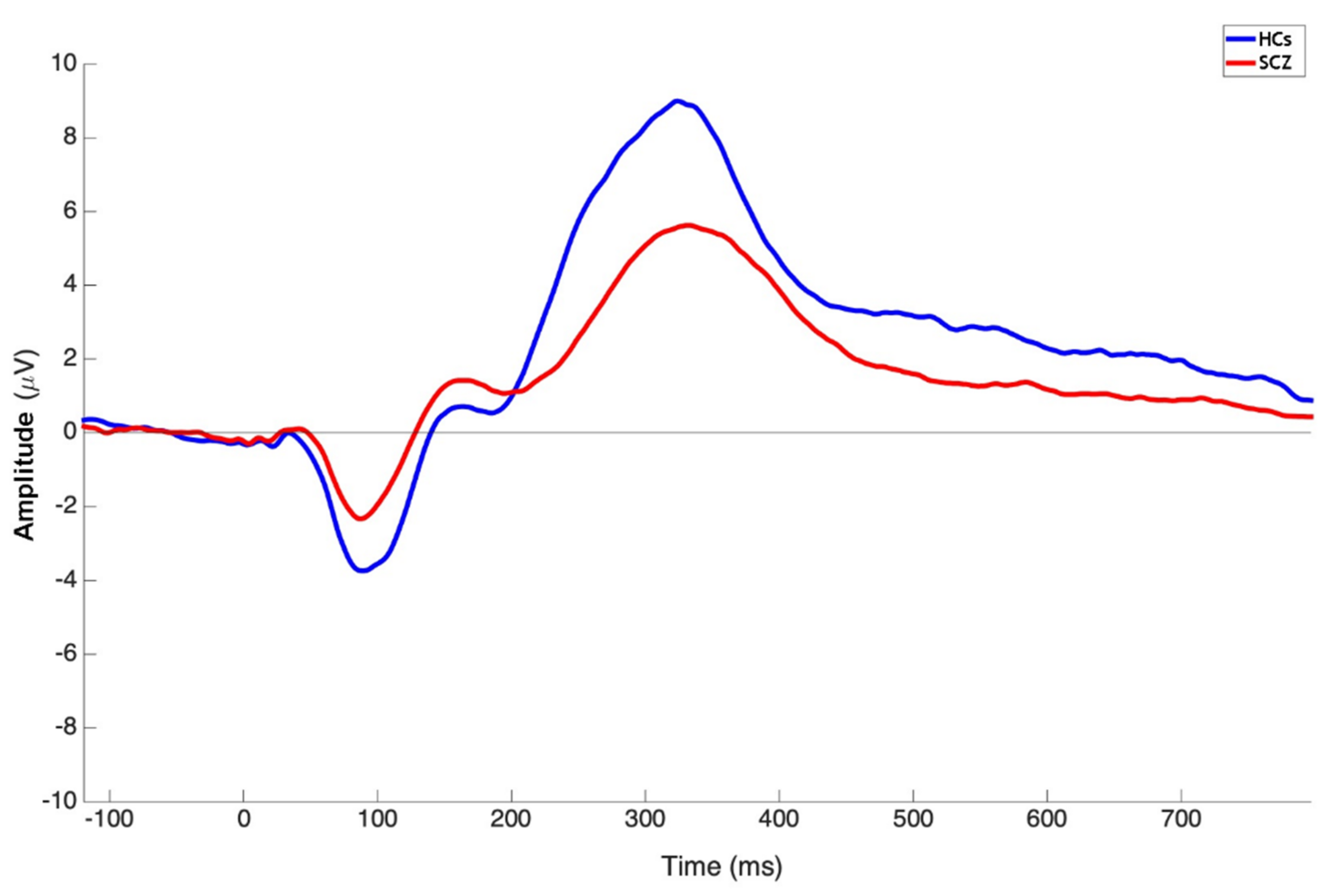
| P3b Pz Amplitude | 9.60 ± 5.09 | 14.05 ± 6.10 | 2023.00 | <0.0001 |
| P3b Pz Latency | 347.36 ± 44.83 | 327.26 ± 33.25 | 2683.50 | 0.0054 |
| SoP | AV | WM | VrbLrn | VisLrn | RPS | Age | ED | Exp | ||
|---|---|---|---|---|---|---|---|---|---|---|
| P3b Pz Amplitude | Spearman’s correlation coefficient | 0.140 | 0.259 | 0.114 | 0.148 | 0.044 | 0.067 | −0.0170 | −0.060 | −0.053 |
| p value | 0.144 | 0.0076 * | 0.238 | 0.124 | 0.650 | 0.490 | 0.070 | 0.533 | 0.577 | |
| P3b Pz Latency | Spearman’s correlation coefficient | −0.027 | −0.107 | −0.119 | −0.223 | −0.195 | −0.093 | 0.320 | −0.083 | −0.037 |
| p value | 0.783 | 0.278 | 0.216 | 0.019 | 0.043 | 0.338 | 0.00052 | 0.387 | 0.701 | |
| SoP | AV | WM | VrbLrn | VisLrn | RPS | Age | ||
|---|---|---|---|---|---|---|---|---|
| P3b Pz Amplitude | Spearman’s correlation coefficient | −0.028 | 0.151 | 0.042 | −0.086 | −0.017 | 0.064 | −0.470 |
| p value | 0.827 | 0.305 | 0.757 | 0.532 | 0.904 | 0.633 | 0.00010 | |
| P3b Pz Latency | Spearman’s correlation coefficient | 0.030 | 0.021 | −0.154 | −0.154 | −0.159 | −0.067 | 0.309 |
| p value | 0.819 | 0.886 | 0.249 | 0.262 | 0.266 | 0.620 | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, G.M.; Perrottelli, A.; Mucci, A.; Di Lorenzo, G.; Altamura, M.; Bellomo, A.; Brugnoli, R.; Corrivetti, G.; Girardi, P.; Monteleone, P.; et al. Investigating the Relationships of P3b with Negative Symptoms and Neurocognition in Subjects with Chronic Schizophrenia. Brain Sci. 2021, 11, 1632. https://doi.org/10.3390/brainsci11121632
Giordano GM, Perrottelli A, Mucci A, Di Lorenzo G, Altamura M, Bellomo A, Brugnoli R, Corrivetti G, Girardi P, Monteleone P, et al. Investigating the Relationships of P3b with Negative Symptoms and Neurocognition in Subjects with Chronic Schizophrenia. Brain Sciences. 2021; 11(12):1632. https://doi.org/10.3390/brainsci11121632
Chicago/Turabian StyleGiordano, Giulia M., Andrea Perrottelli, Armida Mucci, Giorgio Di Lorenzo, Mario Altamura, Antonello Bellomo, Roberto Brugnoli, Giulio Corrivetti, Paolo Girardi, Palmiero Monteleone, and et al. 2021. "Investigating the Relationships of P3b with Negative Symptoms and Neurocognition in Subjects with Chronic Schizophrenia" Brain Sciences 11, no. 12: 1632. https://doi.org/10.3390/brainsci11121632
APA StyleGiordano, G. M., Perrottelli, A., Mucci, A., Di Lorenzo, G., Altamura, M., Bellomo, A., Brugnoli, R., Corrivetti, G., Girardi, P., Monteleone, P., Niolu, C., Galderisi, S., Maj, M., & The Italian Network for Research on Psychoses. (2021). Investigating the Relationships of P3b with Negative Symptoms and Neurocognition in Subjects with Chronic Schizophrenia. Brain Sciences, 11(12), 1632. https://doi.org/10.3390/brainsci11121632









