Another Perspective on Huntington’s Disease: Disease Burden in Family Members and Pre-Manifest HD When Compared to Genotype-Negative Participants from ENROLL-HD
Abstract
:1. Introduction
2. Methods
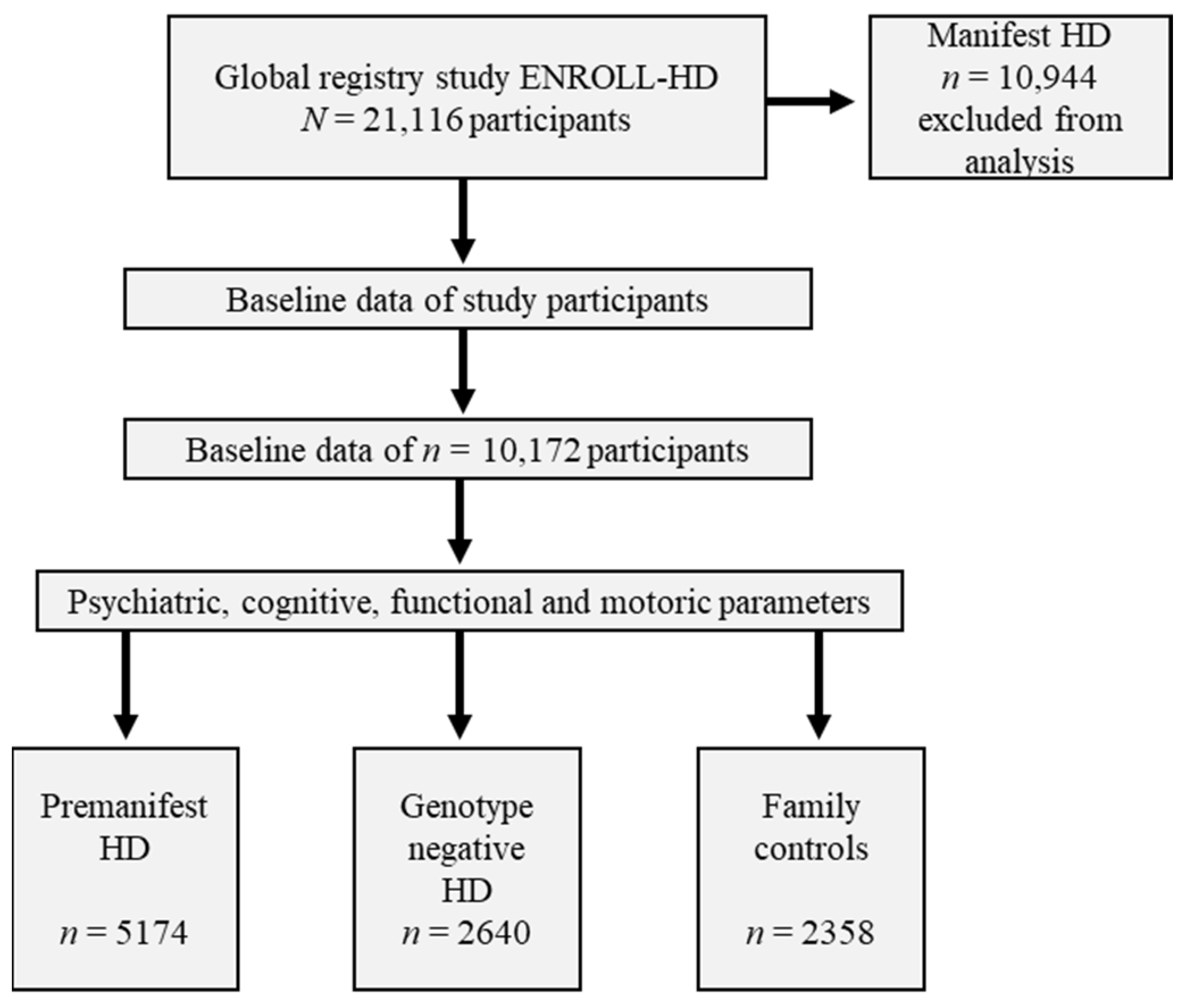
2.1. Participants from the Global Registry Study ENROLL-HD
2.2. Measures and Statistical Analyses
3. Results
3.1. Cohorts of Pre-Manifest, Family Controls and Genotype-Negative Participants in the ENROLL-HD Database
3.2. Baseline Characteristics of Premanifest, Genotype Negative and Family Controls
3.3. Pairwise Post Hoc Analysis between Groups
3.4. Exploration of Psychiatric Symptoms in Premanifest and Genotype Negative Participants in a Lifetime
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Ghosh, R.; Tabrizi, S.J. Huntington disease. Handb. Clin. Neurol. 2018, 147, 255–278. [Google Scholar] [CrossRef]
- Achenbach, J.; von Hein, S.M.; Saft, C. Functional and cognitive capacity differ in dystonic motor subtypes when compared to choreatic and hypokinetic-rigid motor subtypes in Huntington’s disease. Brain Behav. 2020, 10, e01704. [Google Scholar] [CrossRef]
- Saft, C.; Lauter, T.; Kraus, P.H.; Przuntek, H.; Andrich, J.E. Dose-dependent improvement of myoclonic hyperkinesia due to Valproic acid in eight Huntington’s Disease patients: A case series. BMC Neurol. 2006, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Roos, R.A.C. Huntington’s disease: A clinical review. Orphanet J. Rare Dis. 2010, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Epping, E.A.; Kim, J.-I.; Craufurd, D.; Brashers-Krug, T.M.; Anderson, K.E.; McCusker, E.; Luther, J.; Long, J.D.; Paulsen, J.S. Longitudinal Psychiatric Symptoms in Prodromal Huntington’s Disease: A Decade of Data. Am. J. Psychiatry 2016, 173, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Mestre, T.A.; Shannon, K. Huntington disease care: From the past to the present, to the future. Parkinsonism Relat. Disord. 2017, 44, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, K.; Sitek, E.J.; Rudzińska, M.; Sołtan, W.; Sławek, J.; Szczudlik, A. Huntington’s disease from the patient, caregiver and physician’s perspectives: Three sides of the same coin? J. Neural Transm. 2012, 119, 1361–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Røthing, M.; Malterud, K.; Frich, J.C. Caregiver roles in families affected by Huntington’s disease: A qualitative interview study. Scand. J. Caring Sci. 2014, 28, 700–705. [Google Scholar] [CrossRef] [Green Version]
- Hergert, D.C.; Cimino, C.R. Predictors of Caregiver Burden in Huntington’s Disease. Arch. Clin. Neuropsychol. 2021, 36, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Jona, C.M.H.; Labuschagne, I.; Mercieca, E.-C.; Fisher, F.; Gluyas, C.; Stout, J.C.; Andrews, S.C. Families Affected by Huntington’s Disease Report Difficulties in Communication, Emotional Involvement, and Problem Solving. J. Huntingtons. Dis. 2017, 6, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Sellers, J.; Ridner, S.H.; Claassen, D.O. A Systematic Review of Neuropsychiatric Symptoms and Functional Capacity in Huntington’s Disease. J. Neuropsychiatry Clin. Neurosci. 2020, 32, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Tan, K.; Koloms, K.; Bega, D. Assessment of Caregiver Burden in Huntington’s Disease. J. Huntingtons. Dis. 2019, 8, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Zarotti, N.; Dale, M.; Eccles, F.; Simpson, J. Psychological Interventions for People with Huntington’s Disease: A Call to Arms. J. Huntingtons Dis. 2020, 9, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Wibawa, P.; Zombor, R.; Dragovic, M.; Hayhow, B.; Lee, J.; Panegyres, P.K.; Rock, D.; Starkstein, S.E. Anosognosia Is Associated with Greater Caregiver Burden and Poorer Executive Function in Huntington Disease. J. Geriatr. Psychiatry Neurol. 2020, 33, 52–58. [Google Scholar] [CrossRef]
- Thompson, J.C.; Harris, J.; Sollom, A.C.; Stopford, C.L.; Howard, E.; Snowden, J.S.; Craufurd, D. Longitudinal evaluation of neuropsychiatric symptoms in Huntington’s disease. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; Snowden, J.S.; Craufurd, D.; Neary, D. Behavior in Huntington’s disease: Dissociating cognition-based and mood-based changes. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 37–43. [Google Scholar] [CrossRef]
- Migliore, S.; D’Aurizio, G.; Maffi, S.; Ceccarelli, C.; Ristori, G.; Romano, S.; Castaldo, A.; Mariotti, C.; Curcio, G.; Squitieri, F. Cognitive and behavioral associated changes in manifest Huntington disease: A retrospective cross-sectional study. Brain Behav. 2021, 11, e02151. [Google Scholar] [CrossRef]
- Simpson, J.; Dale, M.; Theed, R.; Gunn, S.; Zarotti, N.; Eccles, F.J.R. Validity of irritability in Huntington’s disease: A scoping review. Cortex 2019, 120, 353–374. [Google Scholar] [CrossRef]
- Brüne, M.; Blank, K.; Witthaus, H.; Saft, C. “Theory of mind” is impaired in Huntington’s disease. Mov. Disord. 2011, 26, 671–678. [Google Scholar] [CrossRef]
- Saft, C.; Lissek, S.; Hoffmann, R.; Nicolas, V.; Tegenthoff, M.; Juckel, G.; Brüne, M. Mentalizing in preclinical Huntington’s disease: An fMRI study using cartoon picture stories. Brain Imaging Behav. 2013, 7, 154–162. [Google Scholar] [CrossRef]
- Brüne, M.; von Hein, S.M.; Claassen, C.; Hoffmann, R.; Saft, C. Altered third-party punishment in Huntington’s disease: A study using neuroeconomic games. Brain Behav. 2021, 11, e01908. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Stout, J.C.; Solomon, A.C.; Langbehn, D.R.; Aylward, E.H.; Cruce, C.B.; Ross, C.A.; Nance, M.; Kayson, E.; Julian-Baros, E.; et al. Beyond disgust: Impaired recognition of negative emotions prior to diagnosis in Huntington’s disease. Brain 2007, 130, 1732–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallo, M.; Sergi, A.; Pagani, M. Cognitive and social cognition deficits in Huntington’s disease differ between the prodromal and the manifest stages of the condition: A scoping review of recent evidence. Br. J. Clin. Psychol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Horta, S.; Perez-Perez, J.; van Duijn, E.; Fernandez-Bobadilla, R.; Carceller, M.; Pagonabarraga, J.; Pascual-Sedano, B.; Campolongo, A.; Ruiz-Idiago, J.; Sampedro, F.; et al. Neuropsychiatric symptoms are very common in premanifest and early stage Huntington’s Disease. Parkinsonism Relat. Disord. 2016, 25, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, A.L.; Sills, T.; Anderson, K.E.; Bachoud-Lévi, A.-C.; Borowsky, B.; Craufurd, D.; Duff, K.; Giuliano, J.; Groves, M.; Guttman, M.; et al. Assessment of depression, anxiety and apathy in prodromal and early huntington disease. PLoS Curr. 2011, 3, RRN1242. [Google Scholar] [CrossRef]
- Butėnaitė, A.; Strumila, R.; Lengvenytė, A.; Pakutkaitė, I.K.; Morkūnienė, A.; Matulevičienė, A.; Dlugauskas, E.; Utkus, A. Significant Association Between Huntingtin Gene Mutation and Prevalence of Hopelessness, Depression and Anxiety Symptoms. Acta Med. Litu. 2021, 28, 77–85. [Google Scholar] [CrossRef]
- Van Duijn, E.; Giltay, E.J.; Zitman, F.G.; Roos, R.A.C.; van der Mast, R.C. Measurement of psychopathology in Huntington’s disease: The critical role of caregivers. J. Nerv. Ment. Dis. 2010, 198, 329–333. [Google Scholar] [CrossRef]
- Kingma, E.M.; van Duijn, E.; Timman, R.; van der Mast, R.C.; Roos, R.A.C. Behavioural problems in Huntington’s disease using the Problem Behaviours Assessment. Gen. Hosp. Psychiatry 2008, 30, 155–161. [Google Scholar] [CrossRef]
- Dale, M.; Maltby, J.; Martucci, R.; Shimozaki, S. Factor analysis of the hospital anxiety and depression scale among a Huntington’s disease population. Mov. Disord. 2015, 30, 1954–1960. [Google Scholar] [CrossRef]
- Callaghan, J.; Stopford, C.; Arran, N.; Boisse, M.-F.; Coleman, A.; Santos, R.D.; Dumas, E.M.; Hart, E.P.; Justo, D.; Owen, G.; et al. Reliability and factor structure of the Short Problem Behaviors Assessment for Huntington’s disease (PBA-s) in the TRACK-HD and REGISTRY studies. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 59–64. [Google Scholar] [CrossRef]
- McNally, G.; Rickards, H.; Horton, M.; Craufurd, D. Exploring the Validity of the Short Version of the Problem Behaviours Assessment (PBA-s) for Huntington’s disease: A Rasch Analysis. J. Huntingtons. Dis. 2015, 4, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, J.; Faissner, S.; Saft, C. Differential Diagnosis of Chorea-HIV Infection Delays Diagnosis of Huntington’s Disease by Years. Brain Sci. 2021, 11, 710. [Google Scholar] [CrossRef]
- Achenbach, J.; Saft, C.; Faissner, S. Longitudinal Evaluation of the Effect of Tricyclic Antidepressants and Neuroleptics on the Course of Huntington’s Disease—Data from a Real World Cohort. Brain Sci. 2021, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.; Ware, J.; Levey, J.; Neacy, E.; Blumenstein, R.; Noble, S.; Mühlbäck, A.; Rosser, A.; Landwehrmeyer, G.B.; Sampaio, C. Enroll-HD: An Integrated Clinical Research Platform and Worldwide Observational Study for Huntington’s Disease. Front. Neurol. 2021, 12, 667420. [Google Scholar] [CrossRef]
- Achenbach, J.; Saft, C. Data from ENROLL-HD: Is the prevalence of juvenile and pediatric Huntington’s disease overestimated? Parkinsonism Relat. Disord. 2021, 88, 1–2. [Google Scholar] [CrossRef]
- Crowell, V.; Houghton, R.; Tomar, A.; Fernandes, T.; Squitieri, F. Modeling Manifest Huntington’s Disease Prevalence Using Diagnosed Incidence and Survival Time. Neuroepidemiology 2021, 55, 361–368. [Google Scholar] [CrossRef]
- Sprenger, G.P.; Roos, R.A.C.; van Zwet, E.; Reijntjes, R.H.; Achterberg, W.P.; de Bot, S.T. The prevalence of pain in Huntington’s disease in a large worldwide cohort. Parkinsonism Relat. Disord. 2021, 89, 73–78. [Google Scholar] [CrossRef]
- Tabrizi, S.J.; Scahill, R.I.; Owen, G.; Durr, A.; Leavitt, B.R.; Roos, R.A.; Borowsky, B.; Landwehrmeyer, B.; Frost, C.; Johnson, H.; et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: Analysis of 36-month observational data. Lancet Neurol. 2013, 12, 637–649. [Google Scholar] [CrossRef]
- Wijeratne, P.A.; Garbarino, S.; Gregory, S.; Johnson, E.B.; Scahill, R.I.; Paulsen, J.S.; Tabrizi, S.J.; Lorenzi, M.; Alexander, D.C. Revealing the Timeline of Structural MRI Changes in Premanifest to Manifest Huntington Disease. Neurol. Genet. 2021, 7, e617. [Google Scholar] [CrossRef] [PubMed]
- Winder, J.Y.; Roos, R.A.C. Premanifest Huntington’s disease: Examination of oculomotor abnormalities in clinical practice. PLoS ONE 2018, 13, e0193866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scahill, R.I.; Andre, R.; Tabrizi, S.J.; Aylward, E.H. Structural imaging in premanifest and manifest Huntington disease. Handb. Clin. Neurol. 2017, 144, 247–261. [Google Scholar] [CrossRef]
- Heim, B.; Peball, M.; Saft, C.; von Hein, S.M.; Ellmerer, P.; Piater, J.M.; Seppi, K.; Djamshidian, A. Time will tell: Decision making in premanifest and manifest Huntington’s disease. Brain Behav. 2020, 10, e01843. [Google Scholar] [CrossRef] [PubMed]
- You, S.C.; Geschwind, M.D.; Sha, S.J.; Apple, A.; Satris, G.; Wood, K.A.; Johnson, E.T.; Gooblar, J.; Feuerstein, J.S.; Finkbeiner, S.; et al. Executive functions in premanifest Huntington’s disease. Mov. Disord. 2014, 29, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, J.S.; Miller, A.C.; Hayes, T.; Shaw, E. Cognitive and behavioral changes in Huntington disease before diagnosis. Handb. Clin. Neurol. 2017, 144, 69–91. [Google Scholar] [CrossRef] [PubMed]
- Scahill, R.I.; Zeun, P.; Osborne-Crowley, K.; Johnson, E.B.; Gregory, S.; Parker, C.; Lowe, J.; Nair, A.; O’Callaghan, C.; Langley, C.; et al. Biological and clinical characteristics of gene carriers far from predicted onset in the Huntington’s disease Young Adult Study (HD-YAS): A cross-sectional analysis. Lancet Neurol. 2020, 19, 502–512. [Google Scholar] [CrossRef]
- Júlio, F.; Ribeiro, M.J.; Morgadinho, A.; Sousa, M.; van Asselen, M.; Simões, M.R.; Castelo-Branco, M.; Januário, C. Cognition, function and awareness of disease impact in early Parkinson’s and Huntington’s disease. Disabil. Rehabil. 2020, 1–19. [Google Scholar] [CrossRef]
- Hergert, D.C.; Sanchez-Ramos, J.; Cimino, C.R. Awareness of Chorea in Huntington’s Disease. J. Huntingtons. Dis. 2020, 9, 99–103. [Google Scholar] [CrossRef]
- Abreu, D.; Ware, J.; Georgiou-Karistianis, N.; Leavitt, B.R.; Fitzer-Attas, C.J.; Lobo, R.; Fernandes, A.R.; Handley, O.; Anderson, K.E.; Stout, J.C.; et al. Utility of Huntington’s Disease Assessments by Disease Stage: Floor/Ceiling Effects. Front. Neurol. 2021, 12, 595679. [Google Scholar] [CrossRef]
- Carlozzi, N.E.; Boileau, N.R.; Perlmutter, J.S.; Chou, K.L.; Stout, J.C.; Paulsen, J.S.; McCormack, M.K.; Cella, D.; Nance, M.A.; Lai, J.-S.; et al. Agreement between clinician-rated versus patient-reported outcomes in Huntington disease. J. Neurol. 2018, 265, 1443–1453. [Google Scholar] [CrossRef]
- Winder, J.Y.; Roos, R.A.C.; Burgunder, J.-M.; Marinus, J.; Reilmann, R. Interrater Reliability of the Unified Huntington’s Disease Rating Scale-Total Motor Score Certification. Mov. Disord. Clin. Pract. 2018, 5, 290–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Duijn, E.; Kingma, E.M.; van der Mast, R.C. Psychopathology in verified Huntington’s disease gene carriers. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 441–448. [Google Scholar] [CrossRef]
- Hinz, A.; Schwarz, R. Angst und Depression in der Allgemeinbevölkerung. Eine Normierungsstudie zur Hospital Anxiety and Depression Scale. Psychother. Psychosom. Med. Psychol. 2001, 51, 193–200. [Google Scholar] [CrossRef]
- Herrmann, C. International experiences with the Hospital Anxiety and Depression Scale—A review of validation data and clinical results. J. Psychosom. Res. 1997, 42, 17–41. [Google Scholar] [CrossRef]
- Luppa, M.; Sikorski, C.; Luck, T.; Ehreke, L.; Konnopka, A.; Wiese, B.; Weyerer, S.; König, H.-H.; Riedel-Heller, S.G. Age- and gender-specific prevalence of depression in latest-life—Systematic review and meta-analysis. J. Affect. Disord. 2012, 136, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Kringlen, G.; Kinsley, L.; Aufox, S.; Rouleau, G.; Bega, D. The Impact of Family History on the Clinical Features of Huntington’s Disease. J. Huntingtons. Dis. 2017, 6, 327–335. [Google Scholar] [CrossRef]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993, 269, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, S.; Marwaha, R. StatPearls: Depressive Cognitive Disorders; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
| Premanifest HD (n = 5174) | Family Controls (n = 2358) | Genotype Negative (n = 2640) | F | p | Part. Eta2 | |
|---|---|---|---|---|---|---|
| Age (y); M (SD) | 39.8 (12.1) | 52.7 (12.9) | 41.3 (14.2) | 858.832 | <0.001 | 0.145 |
| Sex (f/m) (%f) | 3099/2074 (59.9) | 1339/1018 (56.8) | 1717/922 (65.1) | 37.395 | <0.001 | 0.001 |
| CAG high | 42.4 (2.8) | 20.0 (3.4) | 20.3 (3.8) | 60,637.636 | <0.001 | 0.923 |
| ISCED+ | 3.9 (1.1) (n = 5157) | 3.8 (1.2) (n = 2351) | 3.9 (1.2) (n = 2624) | 14.737 | <0.001 | 0.003 |
| Motoric UHDRS TMS # | 3.0 (4.5) | 1.5 (2.9) | 1.7 (3.6) | 167.799 | <0.001 | 0.031 |
| TFC+ | 12.7 (0.9) | 12.9 (0.6) | 12.9 (0.7) | 43.321 | <0.001 | 0.008 |
| IS+ | 98.9 (3.8) | 99.6 (2.1) | 99.6 (2.8) | 43.639 | <0.001 | 0.009 |
| SDMT+ | 49.3 (12.1) (n = 5139) | 47.8 (12.0) (n = 2342) | 51.7 (11.9) (n = 2610) | 105.016 | <0.001 | 0.020 |
| Verfct+ | 21.2 (5.8) (n = 5133) | 21.7 (5.5) (n = 2335) | 22.1 (5.7) (n = 2618) | 58.897 | <0.001 | 0.012 |
| SCNT+ | 72.4 (14.8) (n = 5126) | 73.1 (14.1) (n = 2324) | 75.4 (14.3) (n = 2606) | 91.273 | <0.001 | 0.018 |
| SWRT+ | 92.7 (18.4) (n = 5130) | 94.3 (17.3) (n = 2326) | 96.6 (17.3) (n = 2611) | 92.996 | <0.001 | 0.018 |
| MMSE+ | 28.7 (1.7) (n = 3653) | 28.8 (1.6) (n = 1612) | 29.0 (1.5) (n = 2031) | 56.640 | <0.001 | 0.015 |
| PBA_depscore # | 4.3 (5.8) (n = 5155) | 3.5 (4.9) (n = 2352) | 3.2 (5.2) (n = 2631) | 39.697 | <0.001 | 0.008 |
| Irascore # | 2.0 (3.6) | 1.4 (2.7) | 1.2 (2.8) | 54.574 | <0.001 | 0.011 |
| Psyscore # | 0.12 (1.0) | 0.02 (0.3) | 0.11 (0.9) | 5.531 | 0.004 | 0.001 |
| Aptscore # | 0.99 (2.4) | 0.43 (1.4) | 0.51 (1.7) | 71.966 | <0.001 | 0.014 |
| Exfscore # | 1.3 (3.1) | 0.6 (2.1) | 0.9 (2.4) | 37.343 | <0.001 | 0.007 |
| Hads_anxscore # | 5.6 (4.0) (n = 3552) | 5.3 (3.7) (n = 1755) | 5.2 (3.9) (n = 1988) | 6.669 | <0.001 | 0.002 |
| Depscore # | 3.6 (3.5) | 3.9 (3.3) | 3.1 (3.1) | 39.697 | <0.001 | 0.008 |
| Irrascore # | 5.1 (4.0) | 4.3 (3.1) | 4.3 (3.7) | 32.927 | <0.001 | 0.009 |
| Outscore # | 3.2 (2.5) | 2.8 (2.1) | 2.7 (2.3) | 28.648 | <0.001 | 0.008 |
| Inwscore # | 1.9 (2.1) | 1.5 (1.7) | 1.6 (2.0) | 20.130 | <0.001 | 0.005 |
| Post-Hoc Tukey-HSD | Participant Category 1 | vs. Category 2 | Sign. (p) |
|---|---|---|---|
| Motoric UHDRS TMS# | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | 0.325 | |
| ISCED+ | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | 0.325 | |
| TFC+ | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | 0.520 | |
| IS+ | Premanifest HD | Genotype negative | 0.333 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | 0.060 | |
| SDMT+ | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | <0.001 | |
| Verfct+ | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.005 | ||
| Genotype negative | Family controls | <0.050 | |
| SCNT+ | Premanifest HD | Genotype negative | <0.001 |
| Family controls | 0.096 | ||
| Genotype negative | Family controls | <0.001 | |
| SWRT+ | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.005 | ||
| Genotype negative | Family controls | <0.001 | |
| MMSE+ | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.05 | ||
| Genotype negative | Family controls | <0.001 | |
| PBA_depscore # | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | 0.233 | |
| Irascore# | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | 0.167 | |
| Psyscore# | Premanifest HD | Genotype negative | 0.756 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | <0.005 | |
| Aptscore# | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | <0.001 | |
| Exfscore# | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | <0.001 | |
| Hads_anxscore# | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.05 | ||
| Genotype negative | Family controls | 0.842 | |
| Depscore# | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.05 | ||
| Genotype negative | Family controls | <0.001 | |
| Irrascore# | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | 0.960 | |
| Outscore# | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | 0.874 | |
| Inwscore# | Premanifest HD | Genotype negative | <0.001 |
| Family controls | <0.001 | ||
| Genotype negative | Family controls | 0.534 |
| Premanifest HD | Genotype Negative | |
|---|---|---|
| (n = 5174) | (n = 2640) | |
| Depression | ||
| Yes (%) | 2797 (54.1) | 1016 (38.5) |
| No (%) | 2375 (45.9) | 1622 (61.5) |
| Irritability | ||
| Yes (%) | 2074 (40.1) | 922 (34.9) |
| No (%) | 3099 (59.9) | 1717 (65.1) |
| Aggressive behavior | ||
| Yes (%) | 1091 (21.1) | 262 (9.9) |
| No (%) | 4081 (78.9) | 2376 (90.1) |
| Apathy | ||
| Yes (%) | 1447 (28.0) | 358 (13.6) |
| No (%) | 3724 (72.0) | 2280 (86.4) |
| Obsessive behavior | ||
| Yes (%) | 1302 (25.2) | 354 (13.4) |
| No (%) | 3869 (74.8) | 2283 (86.6) |
| Psychosis | ||
| Yes (%) | 181 (3.5) | 61 (2.3) |
| No (%) | 4990 (96.5) | 2576 (97.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achenbach, J.; Saft, C. Another Perspective on Huntington’s Disease: Disease Burden in Family Members and Pre-Manifest HD When Compared to Genotype-Negative Participants from ENROLL-HD. Brain Sci. 2021, 11, 1621. https://doi.org/10.3390/brainsci11121621
Achenbach J, Saft C. Another Perspective on Huntington’s Disease: Disease Burden in Family Members and Pre-Manifest HD When Compared to Genotype-Negative Participants from ENROLL-HD. Brain Sciences. 2021; 11(12):1621. https://doi.org/10.3390/brainsci11121621
Chicago/Turabian StyleAchenbach, Jannis, and Carsten Saft. 2021. "Another Perspective on Huntington’s Disease: Disease Burden in Family Members and Pre-Manifest HD When Compared to Genotype-Negative Participants from ENROLL-HD" Brain Sciences 11, no. 12: 1621. https://doi.org/10.3390/brainsci11121621
APA StyleAchenbach, J., & Saft, C. (2021). Another Perspective on Huntington’s Disease: Disease Burden in Family Members and Pre-Manifest HD When Compared to Genotype-Negative Participants from ENROLL-HD. Brain Sciences, 11(12), 1621. https://doi.org/10.3390/brainsci11121621






