Surgical Aspects of Corpus Callosotomy
Abstract
:1. Introduction
2. Indications for CC
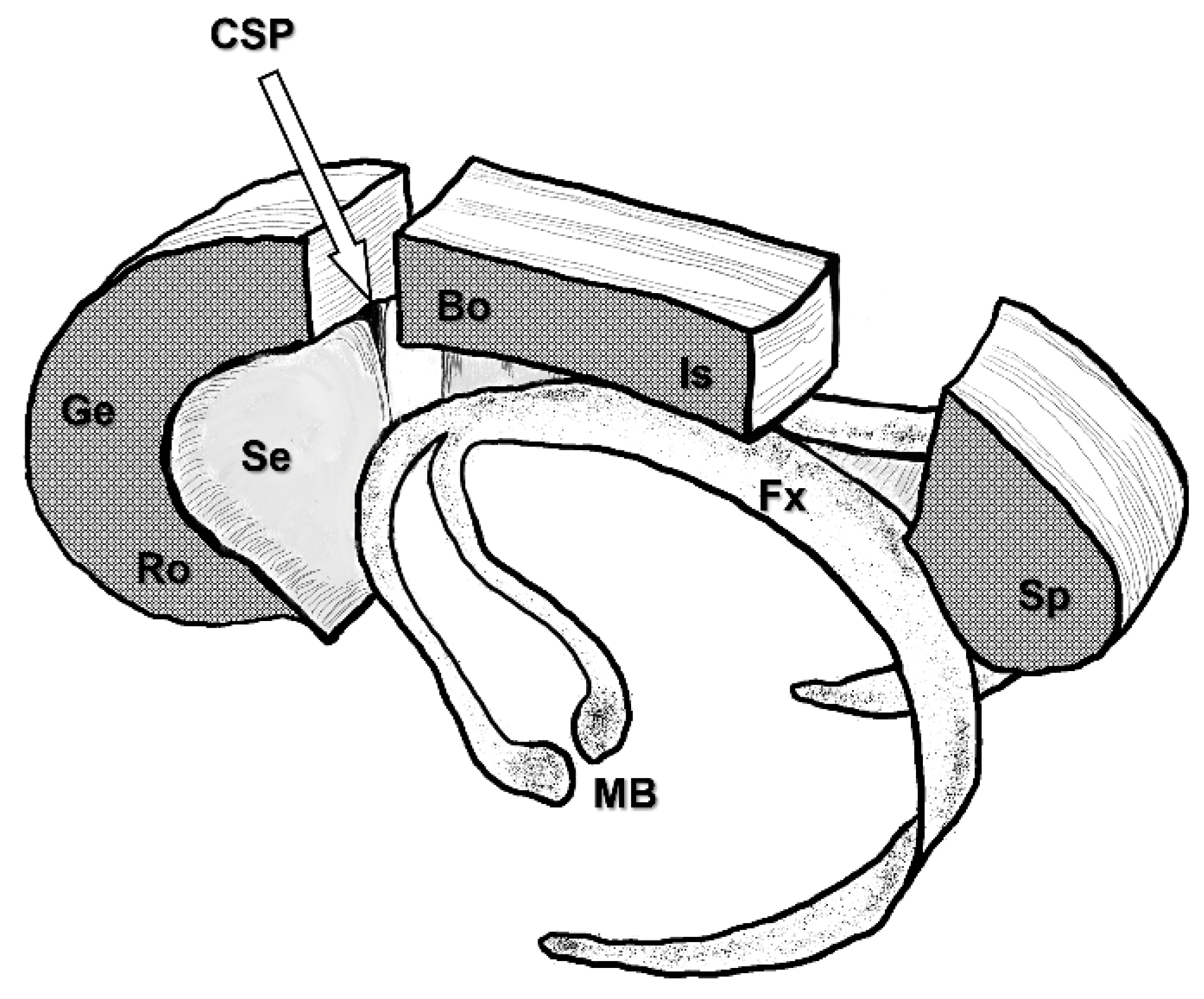
3. Disconnection Syndrome and Extent of Corpus Callosum Disconnection
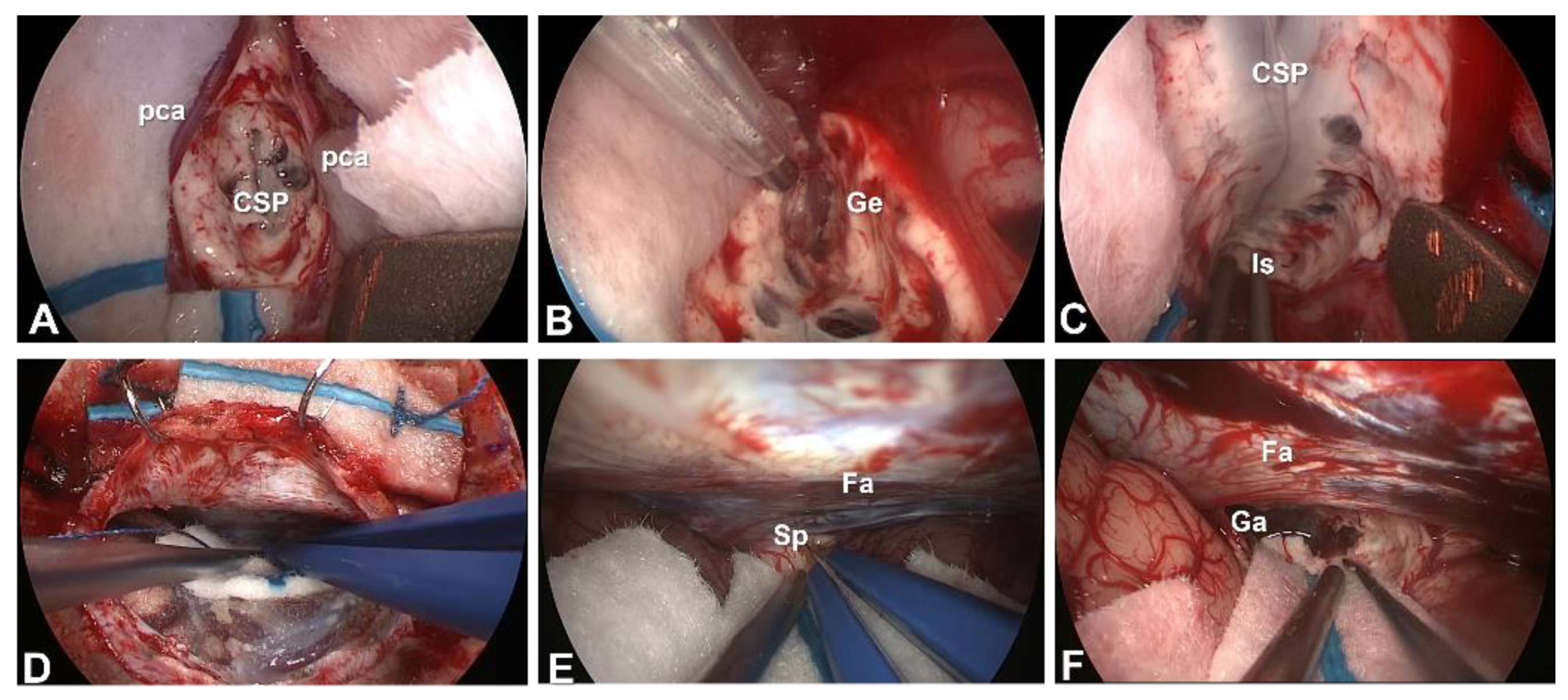
4. Surgery
4.1. Microsurgical tCC and aCC
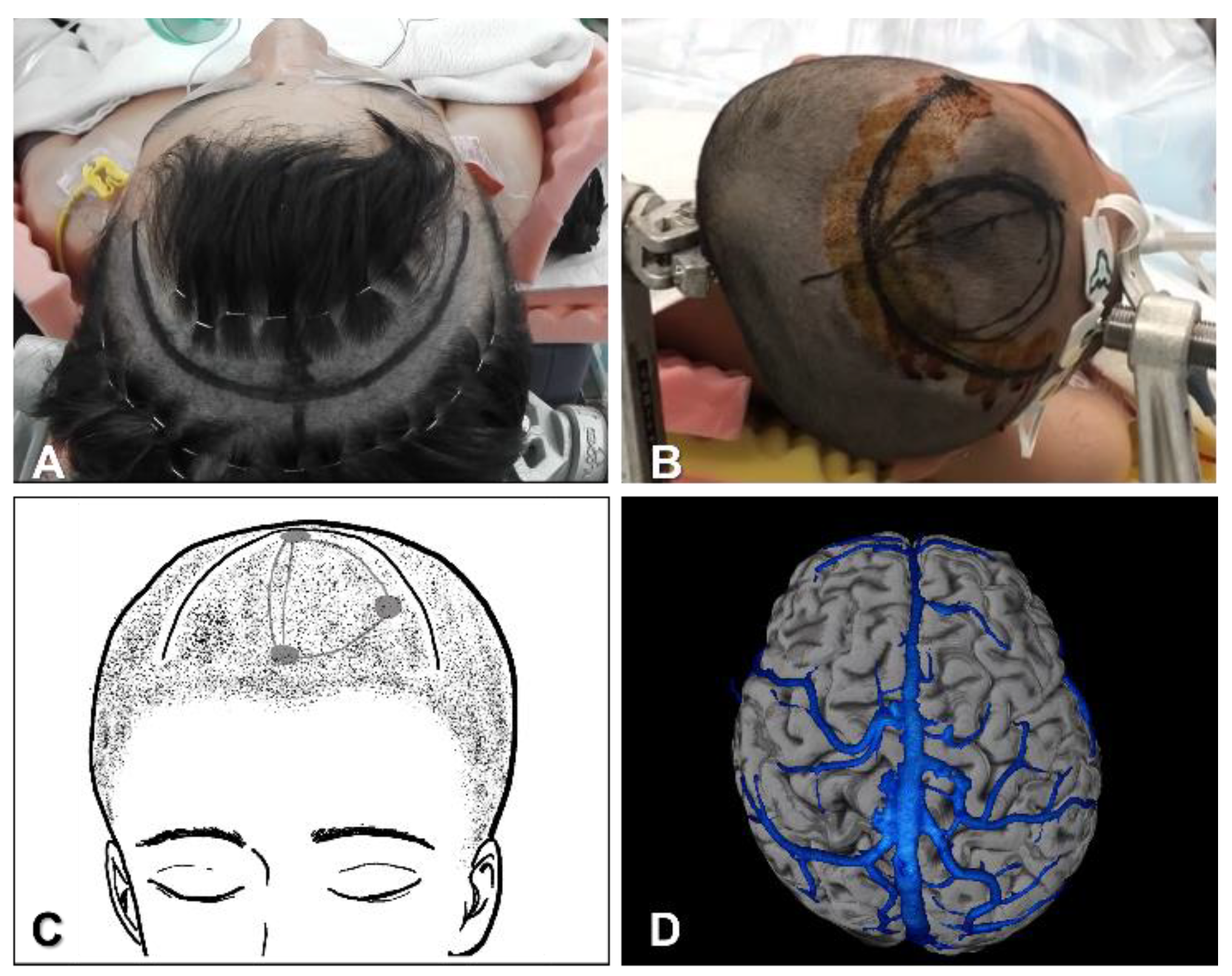
4.1.1. Position, Skin Incision and Craniotomy
4.1.2. Interhemispheric Fissure Dissection
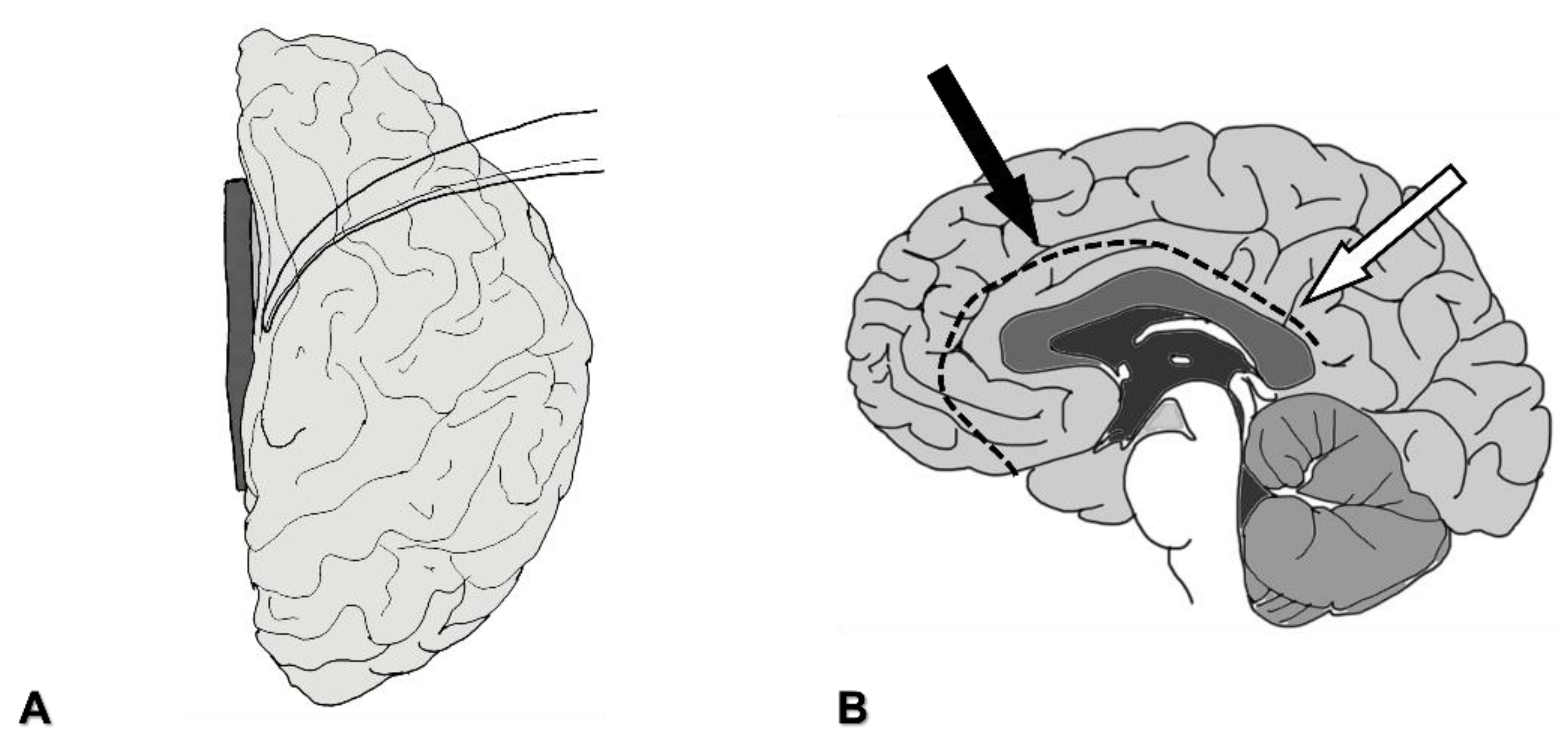
4.1.3. Severing the Corpus Callosum
4.2. Microsurgical pCC
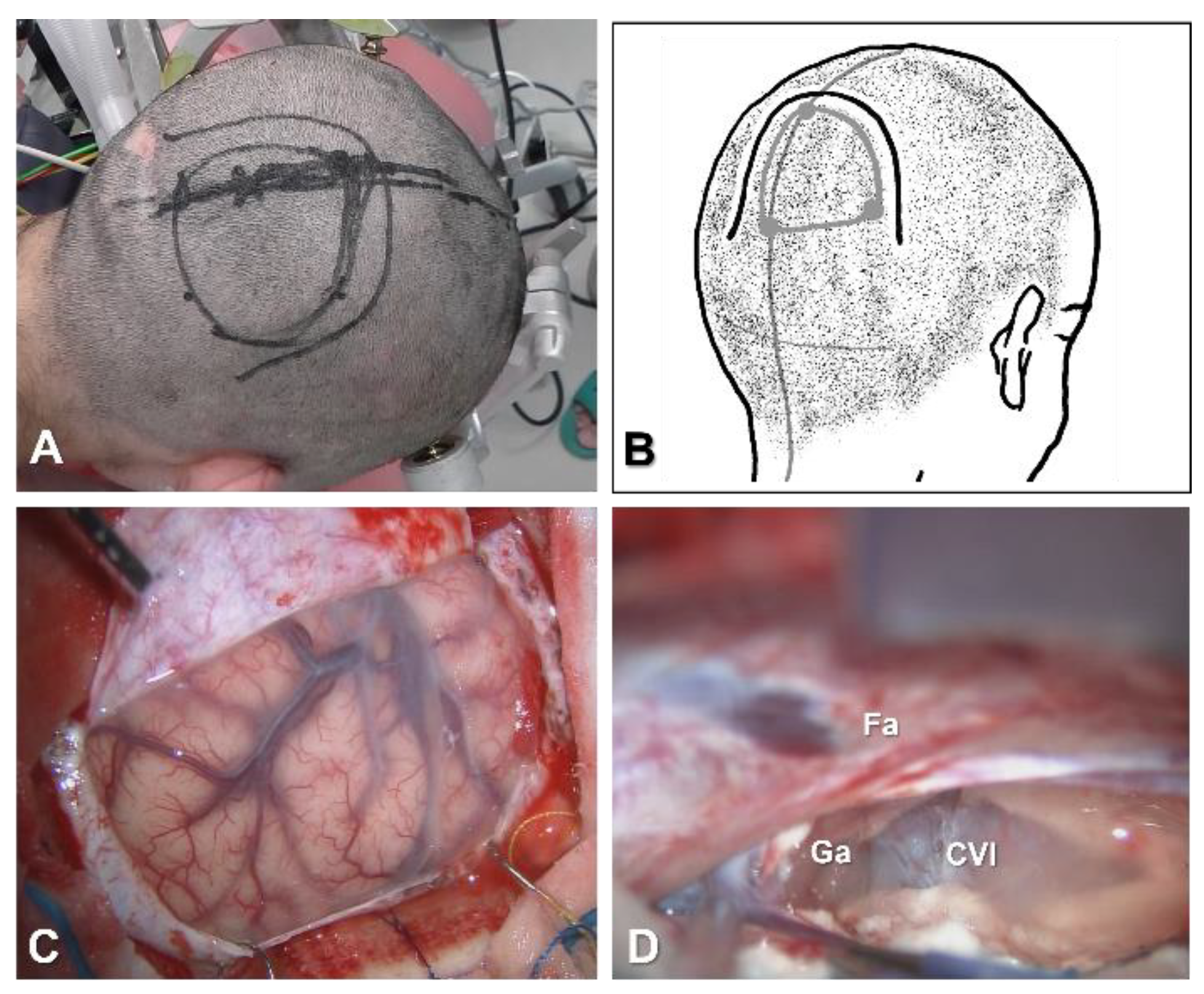
4.2.1. Position, Skin Incision and Craniotomy
4.2.2. Interhemispheric Fissure Dissection
4.2.3. Severing the Corpus Callosum
4.3. Endoscopic Corpus Callosotomy
4.4. CC without Craniotomy
5. Surgical Complications
5.1. Hydrocephalus and Subdural Fluid Collection
5.2. Hemorrhage and Infarction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, A.Y.; Rolston, J.D.; Lee, B.; Vadera, S.; Englot, D.J. Rates and predictors of seizure outcome after corpus callosotomy for drug-resistant epilepsy: A meta-analysis. J. Neurosurg. 2018, 130, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Sunaga, S.; Shimizu, H.; Sugano, H. Long-term follow-up of seizure outcomes after corpus callosotomy. Seizure 2009, 18, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Graham, D.; Tisdall, M.M.; Gill, D. Corpus callosotomy outcomes in pediatric patients: A systematic review. Epilepsia 2016, 57, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Jea, A.; Vachhrajani, S.; Widjaja, E.; Nilsson, D.; Raybaud, C.; Shroff, M.; Rutka, J.T. Corpus callosotomy in children and the disconnection syndromes: A review. Child’s Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2008, 24, 685–692. [Google Scholar] [CrossRef]
- Pristas, N.; Rosenberg, N.; Pindrik, J.; Ostendorf, A.P.; Lundine, J.P. An observational report of swallowing outcomes following corpus callosotomy. Epilepsy Behav. 2021, 123, 108271. [Google Scholar] [CrossRef]
- Jalilian, L.; Limbrick, D.D.; Steger-May, K.; Johnston, J.; Powers, A.K.; Smyth, M.D. Complete versus anterior two-thirds corpus callosotomy in children: Analysis of outcome. J. Neurosurg. Pediatr. 2010, 6, 257–266. [Google Scholar] [CrossRef]
- Thirunavu, V.; Du, R.; Wu, J.Y.; Berg, A.T.; Lam, S.K. The role of surgery in the management of Lennox-Gastaut syndrome: A systematic review and meta-analysis of the clinical evidence. Epilepsia 2021, 62, 888–907. [Google Scholar] [CrossRef] [PubMed]
- Paglioli, E.; Martins, W.A.; Azambuja, N.; Portuguez, M.; Frigeri, T.M.; Pinos, L.; Saute, R.; Salles, C.; Hoefel, J.R.; Soder, R.B.; et al. Selective posterior callosotomy for drop attacks: A new approach sparing prefrontal connectivity. Neurology 2016, 87, 1968–1974. [Google Scholar] [CrossRef]
- Frigeri, T.; Paglioli, E.; Soder, R.B.; Martins, W.A.; Paglioli, R.; Mattiello, R.; Paganin, R.; Palmini, A. Control of drop attacks with selective posterior callosotomy: Anatomical and prognostic data. Epilepsy Res. 2021, 171, 106544. [Google Scholar] [CrossRef]
- Ito, H.; Morino, M.; Niimura, M.; Takamizawa, S.; Shimizu, Y. Posterior callosotomy using a parietooccipital interhemispheric approach in the semi-prone park-bench position. J. Neurosurg. 2015, 123, 1322–1325. [Google Scholar] [CrossRef]
- Tubbs, R.S.; Smyth, M.D.; Salter, G.; Doughty, K.; Blount, J.P. Eyebrow incision with supraorbital trephination for endoscopic corpus callosotomy: A feasibility study. Child’s Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2004, 20, 188–191. [Google Scholar] [CrossRef]
- Bahuleyan, B.; Vogel, T.W.; Robinson, S.; Cohen, A.R. Endoscopic total corpus callosotomy: Cadaveric demonstration of a new approach. Pediatr. Neurosurg. 2011, 47, 455–460. [Google Scholar] [CrossRef]
- Sood, S.; Asano, E.; Altinok, D.; Luat, A. Endoscopic posterior interhemispheric complete corpus callosotomy. J. Neurosurg. Pediatr. 2016, 25, 689–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sood, S.; Marupudi, N.I.; Asano, E.; Haridas, A.; Ham, S.D. Endoscopic corpus callosotomy and hemispherotomy. J. Neurosurg. Pediatr. 2015, 16, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Rich, C.W.; Fasano, R.E.; Isbaine, F.; Saindane, A.M.; Qiu, D.; Curry, D.J.; Gross, R.E.; Willie, J.T. MRI-guided stereotactic laser corpus callosotomy for epilepsy: Distinct methods and outcomes. J. Neurosurg. 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Tao, J.X.; Satzer, D.; Issa, N.P.; Collins, J.; Wu, S.; Rose, S.; Henry, J.; Santos de Lima, F.; Nordli, D.; Warnke, P.C. Stereotactic laser anterior corpus callosotomy for Lennox-Gastaut syndrome. Epilepsia 2020, 61, 1190–1200. [Google Scholar] [CrossRef]
- Tripathi, M.; Maskara, P.; Rangan, V.S.; Mohindra, S.; De Salles, A.A.F.; Kumar, N. Radiosurgical corpus callosotomy: A review of literature. World Neurosurg. 2021, 145, 323–333. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uda, T.; Kunihiro, N.; Umaba, R.; Koh, S.; Kawashima, T.; Ikeda, S.; Ishimoto, K.; Goto, T. Surgical Aspects of Corpus Callosotomy. Brain Sci. 2021, 11, 1608. https://doi.org/10.3390/brainsci11121608
Uda T, Kunihiro N, Umaba R, Koh S, Kawashima T, Ikeda S, Ishimoto K, Goto T. Surgical Aspects of Corpus Callosotomy. Brain Sciences. 2021; 11(12):1608. https://doi.org/10.3390/brainsci11121608
Chicago/Turabian StyleUda, Takehiro, Noritsugu Kunihiro, Ryoko Umaba, Saya Koh, Toshiyuki Kawashima, Shohei Ikeda, Kotaro Ishimoto, and Takeo Goto. 2021. "Surgical Aspects of Corpus Callosotomy" Brain Sciences 11, no. 12: 1608. https://doi.org/10.3390/brainsci11121608
APA StyleUda, T., Kunihiro, N., Umaba, R., Koh, S., Kawashima, T., Ikeda, S., Ishimoto, K., & Goto, T. (2021). Surgical Aspects of Corpus Callosotomy. Brain Sciences, 11(12), 1608. https://doi.org/10.3390/brainsci11121608






