Cognitive and Neural Mechanisms of Social Communication Dysfunction in Primary Progressive Aphasia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cognitive Screening
2.3. Assessment of Social Communication Profiles
2.4. Statistical Analyses
2.5. Image Acquisition
2.6. Voxel-Based Morphometry
2.7. Profiles of Grey Matter Atrophy
2.8. Neural Substrates of Social Communication Changes
3. Results
3.1. Demographic and Neuropsychological Data
3.2. LCQ Performance
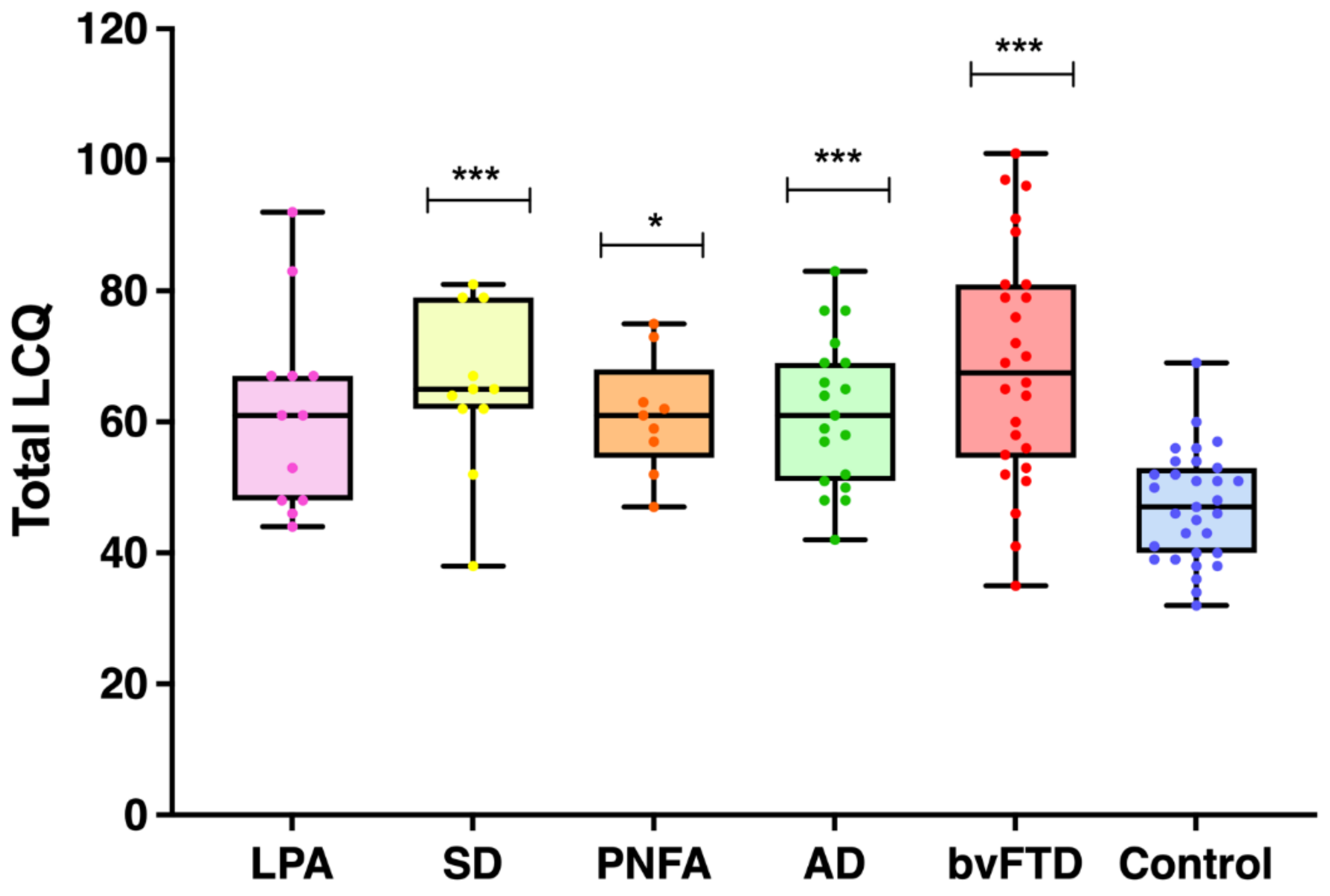
3.2.1. Overall Social Communication Deficits
3.2.2. Social Communication Profiles across Patient Groups
3.2.3. Correlations with Cognitive Function
3.2.4. Relationship between Social Communication Dysfunction and Carer Burden
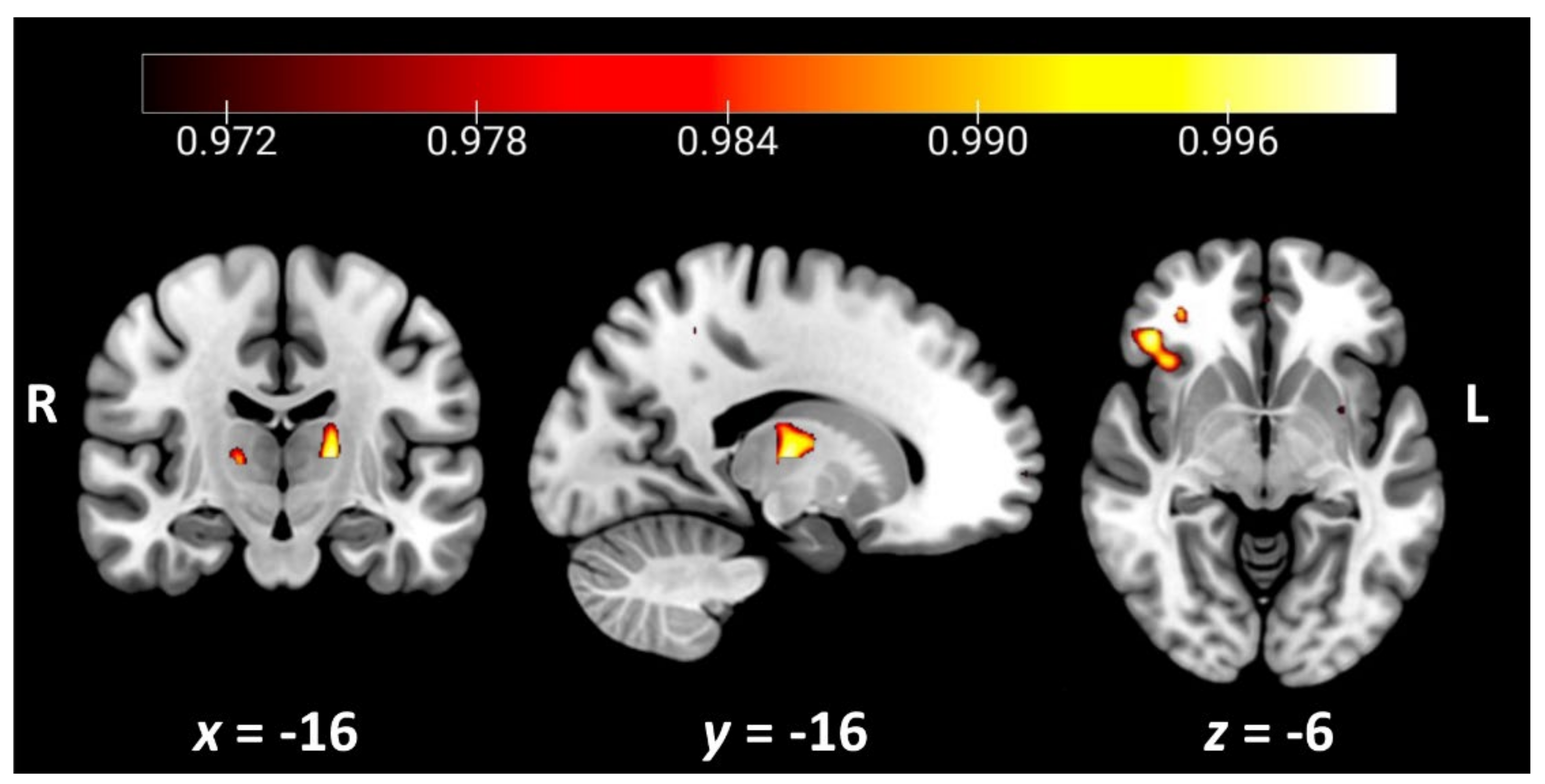
3.3. Neuroimaging Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of primary progressive aphasia and its variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Marshall, C.R.; Hardy, C.J.D.; Volkmer, A.; Russell, L.L.; Bond, R.L.; Fletcher, P.D.; Clark, C.N.; Mummery, C.J.; Schott, J.M.; Rossor, M.N.; et al. Primary progressive aphasia: A clinical approach. J. Neurol. 2018, 265, 1474–1490. [Google Scholar] [CrossRef] [Green Version]
- Tippett, D.C. Classification of primary progressive aphasia: Challenges and complexities. F1000Research 2020, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sajjadi, S.A.; Patterson, K.; Arnold, R.J.; Watson, P.C.; Nestor, P.J. Primary progressive aphasia: A tale of two syndromes and the rest. Neurology 2012, 78, 1670–1677. [Google Scholar] [CrossRef] [Green Version]
- Croot, K.; Ballard, K.; Leyton, C.E.; Hodges, J.R. Apraxia of speech and phonological errors in the diagnosis of nonfluent/agrammatic and logopenic variants of primary progressive aphasia. J. Speech Lang. Hear. Res. 2012, 55, S1562–S1572. [Google Scholar] [CrossRef]
- Foxe, D.; Cheung, S.C.; Cordato, N.J.; Burrell, J.R.; Ahmed, R.M.; Taylor-Rubin, C.; Irish, M.; Piguet, O. Verbal short-term memory disturbance in the primary progressive aphasias: Challenges and distinctions in a clinical setting. Brain Sci. 2021, 11, 1060. [Google Scholar] [CrossRef]
- Foxe, D.; Irish, M.; Hu, A.; Carrick, J.; Hodges, J.R.; Ahmed, R.M.; Burrell, J.R.; Piguet, O. Longitudinal cognitive and functional changes in primary progressive aphasia. J. Neurol. 2021, 268, 1951–1961. [Google Scholar] [CrossRef]
- Butts, A.M.; Machulda, M.M.; Duffy, J.R.; Strand, E.A.; Whitwell, J.L.; Josephs, K.A. Neuropsychological profiles differ among the three variants of primary progressive aphasia. J. Int. Neuropsychol. Soc. 2015, 21, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, S.; Roquet, D.; Goldberg, Z.L.; Hodges, J.R.; Piguet, O.; Irish, M.; Lambon Ralph, M.A. Establishing two principal dimensions of cognitive variation in logopenic progressive aphasia. Brain Commun. 2020, 2, fcaa125. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, S.; Ibanez, A.; Baez, S.; Manes, F.; Sedeno, L.; Garcia, A.M. More than words: Social cognition across variants of primary progressive aphasia. Neurosci. Biobehav. Rev. 2019, 100, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Kumfor, F.; Irish, M.; Hodges, J.R.; Piguet, O. Discrete neural correlates for the recognition of negative emotions: Insights from frontotemporal dementia. PLoS ONE 2013, 8, e67457. [Google Scholar]
- Rohrer, J.D.; Sauter, D.; Scott, S.; Rossor, M.N.; Warren, J.D. Receptive prosody in nonfluent primary progressive aphasias. Cortex 2012, 48, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Hazelton, J.L.; Irish, M.; Hodges, J.R.; Piguet, O.; Kumfor, F. Cognitive and affective empathy disruption in non-fluent primary progressive aphasia syndromes. Brain Impair. 2016, 18, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Kamminga, J.; Kumfor, F.; Burrell, J.R.; Piguet, O.; Hodges, J.R.; Irish, M. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: An examination of clinical characteristics and emotion processing. J. Neurol Neurosurg. Psychiatry 2015, 86, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Piguet, O.; Leyton, C.E.; Gleeson, L.D.; Hoon, C.; Hodges, J.R. Memory and emotion processing performance contributes to the diagnosis of non-semantic primary progressive aphasia syndromes. J. Alzheimers Dis. 2015, 44, 541–547. [Google Scholar] [CrossRef]
- Irish, M.; Hodges, J.R.; Piguet, O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain 2014, 137, 1241–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, C.; Bejanin, A.; Piolino, P.; Laisney, M.; de La Sayette, V.; Belliard, S.; Eustache, F.; Desgranges, B. Theory of mind impairments in patients with semantic dementia. Brain 2012, 135 Pt 1, 228–241. [Google Scholar] [CrossRef] [Green Version]
- Couto, B.; Manes, F.; Montanes, P.; Matallana, D.; Reyes, P.; Velasquez, M.; Yoris, A.; Baez, S.; Ibanez, A. Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front. Hum. Neurosci. 2013, 7, 467. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, S.; Irish, M.; Daveson, N.; Hodges, J.R.; Piguet, O. When one loses empathy: Its effect on carers of patients with dementia. J. Geriatr. Psychiatry Neurol. 2013, 26, 174–184. [Google Scholar] [CrossRef]
- Irish, M.; Kumfor, F.; Hodges, J.R.; Piguet, O. A tale of two hemispheres: Contrasting socioemotional dysfunction in right-versus left-lateralised semantic dementia. Dement. Neuropsychol. 2013, 7, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rankin, K.P.; Gorno-Tempini, M.L.; Allison, S.C.; Stanley, C.M.; Glenn, S.; Weiner, M.W.; Miller, B.L. Structural anatomy of empathy in neurodegenerative disease. Brain 2006, 129 Pt 11, 2945–2956. [Google Scholar] [CrossRef] [Green Version]
- Henry, J.D.; von Hippel, W.; Molenberghs, P.; Lee, T.; Sachdev, P.S. Clinical assessment of social cognitive function in neurological disorders. Nat. Rev. Neurol. 2016, 12, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Grice, H.P. Studies in the Way of Words; Harvard University Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Searle, J.R. What is an intentional state? Mind 1979, 88, 74–92. [Google Scholar] [CrossRef]
- Hyter, Y.D. Pragmatic assessment and intervention in children. In Research in Clinical Pragmatics; Cummings, L., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 493–526. [Google Scholar]
- Wilson, D. New directions for research on pragmatics and modularity. Lingua 2005, 115, 1129–1146. [Google Scholar] [CrossRef]
- Catani, M.; Bambini, V. A model for social communication and language evolution and development (SCALED). Curr. Opin. Neurobiol. 2014, 28, 165–171. [Google Scholar] [CrossRef]
- Bambini, V.; Tonini, E.; Ceccato, I.; Lecce, S.; Marocchini, E.; Cavallini, E. How to improve social communication in aging: Pragmatic and cognitive interventions. Brain Lang. 2020, 211, 104864. [Google Scholar] [CrossRef] [PubMed]
- Sobral, A.; de Araujo, C.M.T.; Sobral, M.F.F. Mild cognitive impairment in the elderly relationship between communication and functional capacity. Dement. Neuropsychol. 2018, 12, 165–172. [Google Scholar] [CrossRef]
- Douglas, J.M.; O’Flaherty, C.A.; Snow, P.C. Measuring perception of communicative ability: The development and evaluation of the La Trobe communication questionnaire. Aphasiology 2000, 14, 251–268. [Google Scholar] [CrossRef]
- Blair, M.; Marczinski, C.A.; Davis-Faroque, N.; Kertesz, A. A longitudinal study of language decline in Alzheimer’s disease and frontotemporal dementia. J. Int. Neuropsychol. Soc. 2007, 13, 237–245. [Google Scholar] [CrossRef]
- Hardy, C.J.; Buckley, A.H.; Downey, L.E.; Lehmann, M.; Zimmerer, V.C.; Varley, R.A.; Crutch, S.J.; Rohrer, J.D.; Warrington, E.K.; Warren, J.D. The language profile of behavioral variant frontotemporal dementia. J. Alzheimers Dis. 2016, 50, 359–371. [Google Scholar] [CrossRef]
- Dermody, N.; Wong, S.; Ahmed, R.; Piguet, O.; Hodges, J.R.; Irish, M. Uncovering the neural bases of cognitive and affective empathy deficits in alzheimer’s disease and the behavioral-variant of frontotemporal dementia. J. Alzheimers Dis. 2016, 53, 801–816. [Google Scholar] [CrossRef]
- Bambini, V.; Arcara, G.; Bechi, M.; Buonocore, M.; Cavallaro, R.; Bosia, M. The communicative impairment as a core feature of schizophrenia: Frequency of pragmatic deficit, cognitive substrates, and relation with quality of life. Compr. Psychiatry 2016, 71, 106–120. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134 Pt 9, 2456–2477. [Google Scholar] [CrossRef]
- Mioshi, E.; Hsieh, S.; Savage, S.; Hornberger, M.; Hodges, J.R. Clinical staging and disease progression in frontotemporal dementia. Neurology 2010, 74, 1591–1597. [Google Scholar] [CrossRef]
- Bédard, M.; Molloy, D.W.; Squire, L.; Dubois, S.; Lever, J.A.; O’Donnell, M. The Zarit Burden interview a new short version and screening version. Gerontologist 2001, 41, 652–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, S.; Schubert, S.; Hoon, C.; Mioshi, E.; Hodges, J.R. Validation of the addenbrooke’s cognitive examination III in frontotemporal dementia and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2013, 36, 242–250. [Google Scholar] [CrossRef]
- So, M.; Foxe, D.; Kumfor, F.; Murray, C.; Hsieh, S.; Savage, G.; Ahmed, R.M.; Burrell, J.R.; Hodges, J.R.; Irish, M.; et al. Addenbrooke’s cognitive examination III: Psychometric characteristics and relations to functional ability in dementia. J. Int. Neuropsychol. Soc. 2018, 24, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Memory Scale Administration and Scoring Manual, 3rd ed.; Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Reitan, R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]
- Rey, A. L’examen psychologique dans les cas d’encéphalopathie traumatique. Arch. Psychol. 1941, 28, 215–285. [Google Scholar]
- Irish, M.; Piguet, O.; Hodges, J.R.; Hornberger, M. Common and unique grey matter correlates of episodic memory dysfunction in frontotemporal dementia and Alzheimer’s disease. Hum. Brain Mapp. 2014, 35, 1422–1435. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.A.; Hsieh, S.; Leslie, F.; Foxe, D.; Piguet, O.; Hodges, J.R. Distinguishing subtypes in primary progressive aphasia: Application of the Sydney language battery. Dement. Geriatr. Cogn. Disord. 2013, 35, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Burgess, P.; Shallice, T. (Eds.) The Hayling and Brixton Tests; Thames Valley Test Company: Bury St Edmonds, UK, 1997. [Google Scholar]
- Struchen, M.A.; Pappadis, M.R.; Mazzei, D.K.; Clark, A.N.; Davis, L.C.; Sander, A.M. Perceptions of communication abilities for persons with traumatic brain injury: Validity of the La trobe communication questionnaire. Brain Inj. 2008, 22, 940–951. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11 Pt 1, 805–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23 (Suppl. 1), S208–S219. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imag. 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Jenkinson, M.; Smith, S. Non-linear optimisation. In FMRIB Technical Report TR07JA1; University of Oxford FMRIB Centre: Oxford, UK, 2007. [Google Scholar]
- Andersson, J.L.R.; Jenkinson, M.; Smith, S. Non-linear registration, aka spatial normalisation. In FMRIB Technical Report TR07JA2; University of Oxford FMRIB Centre: Oxford, UK, 2007. [Google Scholar]
- Rueckert, D.; Sonoda, L.I.; Hayes, C.; Hill, D.L.G.; Leach, M.O.; Hawkes, D.J. Nonrigid registration using free-form deformations: Application to breast MR images. IEEE Trans. Med. Imag. 1999, 18, 712–721. [Google Scholar] [CrossRef]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Bennett, C.M.; Wolford, G.L.; Miller, M.B. The principled control of false positives in neuroimaging. Soc. Cogn. Affect. Neurosci. 2009, 4, 417–422. [Google Scholar] [CrossRef]
- Davies, K.; Howe, T. Experiences of living with primary progressive aphasia: A scoping review of qualitative studies. Am. J. Alzheimers Dis. Other Demen. 2020, 35, 1533317519886218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strikwerda-Brown, C.; Grilli, M.D.; Andrews-Hanna, J.R.; Irish, M. “All is not lost”—Rethinking the nature of memory and the self in dementia. Ageing Res. Rev. 2019, 54, 100932. [Google Scholar] [CrossRef] [Green Version]
- Taylor-Rubin, C.; Croot, K.; Power, E.; Savage, S.A.; Hodges, J.R.; Togher, L. Communication behaviors associated with successful conversation in semantic variant primary progressive aphasia. Int. Psychogeriatr. 2017, 29, 1619–1632. [Google Scholar] [CrossRef]
- Meteyard, L.; Patterson, K. The relation between content and structure in language production: An analysis of speech errors in semantic dementia. Brain Lang. 2009, 110, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Irish, M.; Kamminga, J.; Addis, D.R.; Crain, S.; Thornton, R.; Hodges, J.R.; Piguet, O. “Language of the past”—Exploring past tense disruption during autobiographical narration in neurodegenerative disorders. J. Neuropsychol. 2016, 10, 295–316. [Google Scholar] [CrossRef]
- Bozeat, S.; Gregory, C.A.; Ralph, M.A.; Hodges, J.R. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J. Neurol. Neurosurg. Psychiatry 2000, 69, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulin, T.; Roquet, D.; Kenett, Y.N.; Savage, G.; Irish, M. The effect of semantic memory degeneration on creative thinking: A voxel-based morphometry analysis. Neuroimage 2020, 220, 117073. [Google Scholar] [CrossRef]
- Modirrousta, M.; Price, B.H.; Dickerson, B.C. Neuropsychiatric symptoms in primary progressive aphasia: Phenomenology, pathophysiology, and approach to assessment and treatment. Neurodegener. Dis. Manag. 2013, 3, 133–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irish, M.; Addis, D.R.; Hodges, J.R.; Piguet, O. Considering the role of semantic memory in episodic future thinking: Evidence from semantic dementia. Brain 2012, 135 Pt 7, 2178–2191. [Google Scholar] [CrossRef] [Green Version]
- Irish, M.; Addis, D.R.; Hodges, J.R.; Piguet, O. Exploring the content and quality of episodic future simulations in semantic dementia. Neuropsychologia 2012, 50, 3488–3495. [Google Scholar] [CrossRef]
- McKinnon, M.; Nica, E.; Sengdy, P.; Kovacevic, N.; Moscovitch, M.; Freedman, M.; Miller, B.; Black, S.; Levine, B. Autobiographical memory and patterns of brain atrophy in fronto-temporal lobar degeneration. J. Cogn. Neurosci. 2008, 20, 1839–1853. [Google Scholar] [CrossRef]
- Ruffman, T.; Murray, J.; Halberstadt, J.; Taumoepeau, M. Verbosity and emotion recognition in older adults. Psychol. Aging 2010, 25, 492–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabria, M.; Cotelli, M.; Adenzato, M.; Zanetti, O.; Miniussi, C. Empathy and emotion recognition in semantic dementia: A case report. Brain Cogn. 2009, 70, 247–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumfor, F.; Irish, M.; Leyton, C.; Miller, L.; Lah, S.; Devenney, E.; Hodges, J.R.; Piguet, O. Tracking the progression of social cognition in neurodegenerative disorders. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1076–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strikwerda-Brown, C.; Ramanan, S.; Irish, M. Neurocognitive mechanisms of theory of mind impairment in neurodegeneration: A transdiagnostic approach. Neuropsychiatr. Dis. Treat. 2019, 15, 557–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irish, M.; Piguet, O.; Hodges, J.R. Self-projection and the default network in frontotemporal dementia. Nat. Rev. Neurol. 2012, 8, 152–161. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Shine, J.M.; Hodges, J.R.; Andrews-Hanna, J.R.; Irish, M. Hippocampal atrophy and intrinsic brain network dysfunction relate to alterations in mind wandering in neurodegeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 3316–3321. [Google Scholar] [CrossRef] [Green Version]
- Grossman, M. Linguistic aspects of primary progressive aphasia. Annu. Rev. Linguist. 2018, 4, 377–403. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Rolls, E.T. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004, 72, 341–372. [Google Scholar] [CrossRef]
- Husain, M.; Roiser, J.P. Neuroscience of apathy and anhedonia: A transdiagnostic approach. Nat. Rev. Neurosci. 2018, 19, 470–484. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.M.; Devenney, E.M.; Irish, M.; Ittner, A.; Naismith, S.; Ittner, L.M.; Rohrer, J.D.; Halliday, G.M.; Eisen, A.; Hodges, J.R.; et al. Neuronal network disintegration: Common pathways linking neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1234–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwigsen, G.; Neef, N.E.; Camilleri, J.A.; Margulies, D.S.; Eickhoff, S.B. Functional segregation of the right inferior frontal gyrus: Evidence from coactivation-based parcellation. Cereb. Cortex 2019, 29, 1532–1546. [Google Scholar] [CrossRef]
- Saalmann, Y.B.; Kastner, S. The cognitive thalamus. Front. Syst. Neurosci. 2015, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Wolff, M.; Vann, S.D. The cognitive thalamus as a gateway to mental representations. J. Neurosci. 2019, 39, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Foxe, D.; Irish, M.; Ramanan, S.; Stark, S.; Cordato, N.J.; Burrell, J.R.; Piguet, O. Longitudinal changes in behaviour, mood, and functional capacity in the primary progressive aphasia variants. Eur. J. Neurosci. 2021. awaiting final decision. [Google Scholar]
- Shaw, S.R.; El-Omar, H.; Roquet, D.; Hodges, J.R.; Piguet, O.; Ahmed, R.M.; Whitton, A.E.; Irish, M. Uncovering the prevalence and neural substrates of anhedonia in frontotemporal dementia. Brain 2021, 5, 1551–1564. [Google Scholar] [CrossRef]
- Shaw, S.R.; El-Omar, H.; Ramanan, S.; Piguet, O.; Ahmed, R.M.; Whitton, A.E.; Irish, M. Anhedonia in semantic dementia-exploring right hemispheric contributions to the loss of pleasure. Brain Sci. 2021, 11, 998. [Google Scholar] [CrossRef]
- Wong, S.; Irish, M.; Husain, M.; Hodges, J.R.; Piguet, O.; Kumfor, F. Apathy and its impact on carer burden and psychological wellbeing in primary progressive aphasia. J. Neurol. Sci. 2020, 416, 117007. [Google Scholar] [CrossRef]
- Merrilees, J.; Dowling, G.A.; Hubbard, E.; Mastick, J.; Ketelle, R.; Miller, B.L. Characterization of apathy in persons with frontotemporal dementia and the impact on family caregivers. Alzheimer Dis. Assoc. Disord. 2013, 27, 62–67. [Google Scholar] [CrossRef]
- Alsawy, S.; Mansell, W.; McEvoy, P.; Tai, S. What is good communication for people living with dementia? A mixed-methods systematic review. Int. Psychogeriatr. 2017, 29, 1785–1800. [Google Scholar] [CrossRef]
- Keegan, L.C.; Murdock, M.; Suger, C.; Togher, L. Improving natural social interaction: Group rehabilitation after traumatic brain injury. Neuropsychol. Rehabil. 2020, 30, 1497–1522. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, A.; Power, E.; O’Halloran, R.; Rietdijk, R. Common and distinct components of communication partner training programmes in stroke, traumatic brain injury and dementia. Int. J. Lang. Commun. Disord. 2018, 53, 1150–1168. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.; Lind, C.; Young, J.A.; Okell, E.; van Steenbrugge, W. Familiar communication partners’ facilitation of topic management in conversations with individuals with dementia. Int. J. Lang. Commun. Disord. 2018, 53, 564–575. [Google Scholar] [CrossRef] [PubMed]

| Demographics | LPA (n = 12) | SD (n = 11) | PNFA (n = 9) | AD (n = 19) | bvFTD (n = 26) | Control (n = 31) | F-Test | Post Hoc |
|---|---|---|---|---|---|---|---|---|
| Age (y) | 69.8 (7.0) | 63.8 (6.2) | 67.1 (6.1) | 66.1 (7.6) | 64.8 (5.8) | 65.4 (6.3) | NS | - |
| Education (y) | 11.9 (3.5) | 13.8 (2.3) | 12.4 (3.2) | 13.2 (3.7) | 12.1 (3.7) | 13.9 (3.7) | NS | - |
| Sex (M:F) | 7:5 | 7:4 | 2:7 | 10:9 | 19:7 | 16:15 | NS | - |
| Disease Duration (y) | 5.2 (2.5) | 6.7 (2.9) | 6.1 (3.0) | 7.2 (5.1) | 7.5 (4.7) | - | NS | - |
| ACE-III Total (100) | 63.7 (17.1) | 71.1 (21.7) | 80.0 (16.1) | 69.6 (12.5) | 82.8 (9.3) | 94.1 (3.8) | *** | CN > Patients |
| FRS (Rasch score) | 0.85 (1.4) | 0.82 (1.4) | 2.7 (1.8) | 0.42 (1.1) | −0.38 (1.3) | - | *** | PNFA > AD, bvFTD |
| ZBI (48) | 13.2 (6.8) | 18.7 (8.7) | 11.4 (6.5) | 15.2 (8.7) | 21.4 (10.0) | - | * | bvFTD > PNFA |
| LCQ Score | LPA (n = 12) | SD (n = 11) | PNFA (n = 9) | AD (n = 19) | bvFTD (n = 26) | Control (n = 31) | F-Test | Post Hoc |
|---|---|---|---|---|---|---|---|---|
| Total (120) | 61.4 (15.0) | 64.9 (12.5) | 61.0 (9.0) | 61.5 (11.4) | 68.6 (17.8) | 47.1 (8.4) | *** | CN < LPA, SD, AD, bvFTD |
| Initiation/Flow | 22.0 (6.1) | 21.6 (6.0) | 22.8 (4.2) | 19.3 (4.6) | 23.2 (7.6) | 14.5 (3.2) | *** | CN < Patients |
| Disinhibition | 12.3 (3.3) | 13.7 (3.0) | 12.2 (2.4) | 12.5 (3.8) | 15.0 (4.6) | 11.1 (2.3) | ** | CN < bvFTD |
| Conversational Effectiveness | 13.4 (4.3) | 14.7 (4.3) | 13.9 (2.2) | 14.6 (3.2) | 13.7 (4.2) | 10.8 (3.6) | ** | CN < AD |
| Partner Sensitivity | 7.9 (2.9) | 8.6 (2.5) | 7.2 (1.7) | 9.2 (2.7) | 9.9 (3.2) | 6.7 (1.6) | *** | CN < AD, bvFTD |
| Contrast | Regions | Side | Cluster Size | Cluster Peak MNI Coordinates | t-Value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| LCQ Total | Orbitofrontal cortex, insular cortex, inferior frontal gyrus | R | 204 | 44 | 32 | −6 | 3.20 |
| Thalamus | L | 113 | −16 | −16 | 6 | 3.09 | |
| Frontal pole | R | 57 | 32 | 64 | 2 | 2.67 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldberg, Z.-L.; El-Omar, H.; Foxe, D.; Leyton, C.E.; Ahmed, R.M.; Piguet, O.; Irish, M. Cognitive and Neural Mechanisms of Social Communication Dysfunction in Primary Progressive Aphasia. Brain Sci. 2021, 11, 1600. https://doi.org/10.3390/brainsci11121600
Goldberg Z-L, El-Omar H, Foxe D, Leyton CE, Ahmed RM, Piguet O, Irish M. Cognitive and Neural Mechanisms of Social Communication Dysfunction in Primary Progressive Aphasia. Brain Sciences. 2021; 11(12):1600. https://doi.org/10.3390/brainsci11121600
Chicago/Turabian StyleGoldberg, Zoë-Lee, Hashim El-Omar, David Foxe, Cristian E. Leyton, Rebekah M. Ahmed, Olivier Piguet, and Muireann Irish. 2021. "Cognitive and Neural Mechanisms of Social Communication Dysfunction in Primary Progressive Aphasia" Brain Sciences 11, no. 12: 1600. https://doi.org/10.3390/brainsci11121600
APA StyleGoldberg, Z.-L., El-Omar, H., Foxe, D., Leyton, C. E., Ahmed, R. M., Piguet, O., & Irish, M. (2021). Cognitive and Neural Mechanisms of Social Communication Dysfunction in Primary Progressive Aphasia. Brain Sciences, 11(12), 1600. https://doi.org/10.3390/brainsci11121600







