Flexible Use of Spatial Frames of Reference for Object–Location Memory in Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure and Questionnaires
2.3. Virtual Environment Design
2.4. Spatial Memory Task
2.4.1. Learning Phase
2.4.2. Testing Phase
2.5. Statistical Analyses
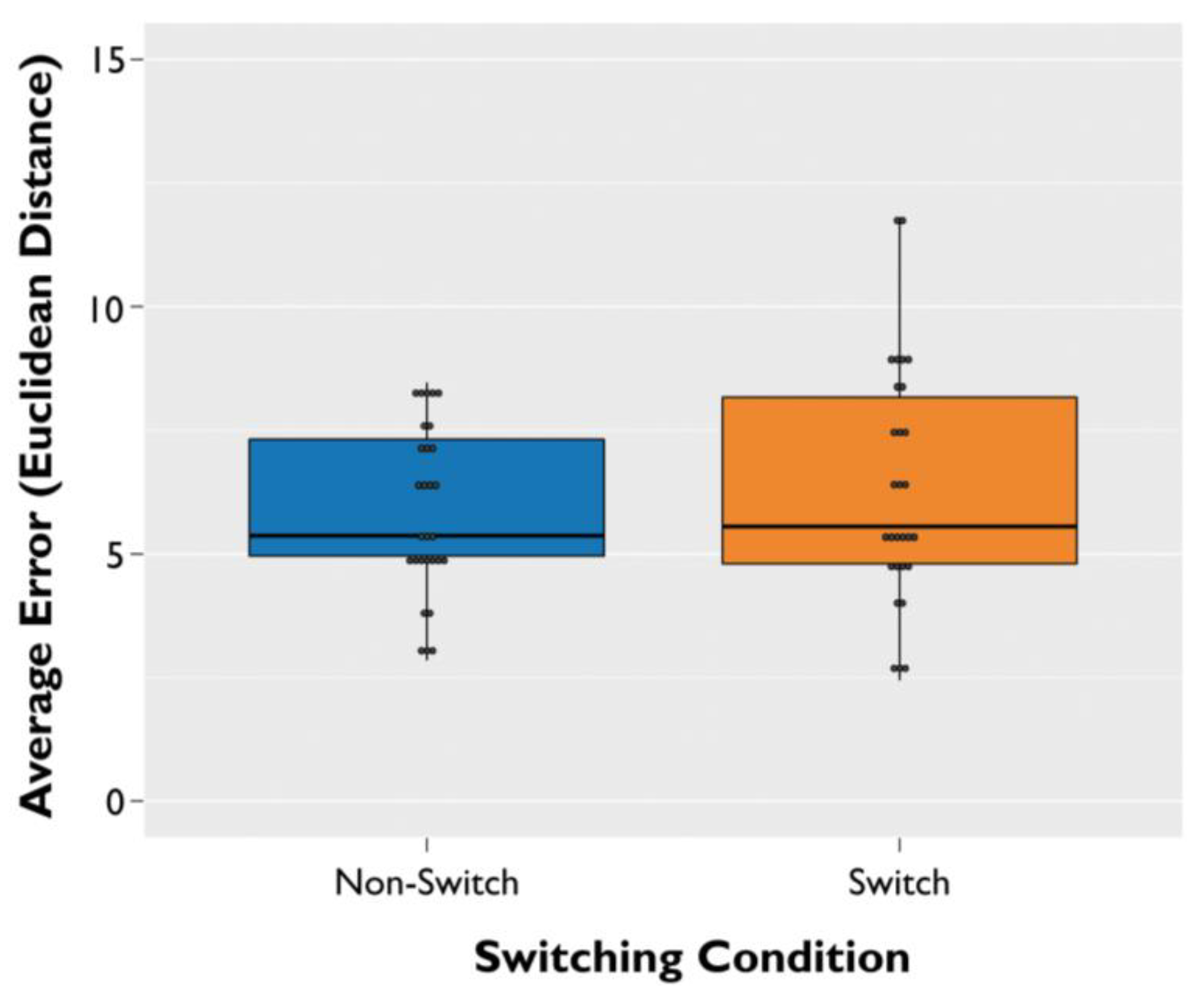
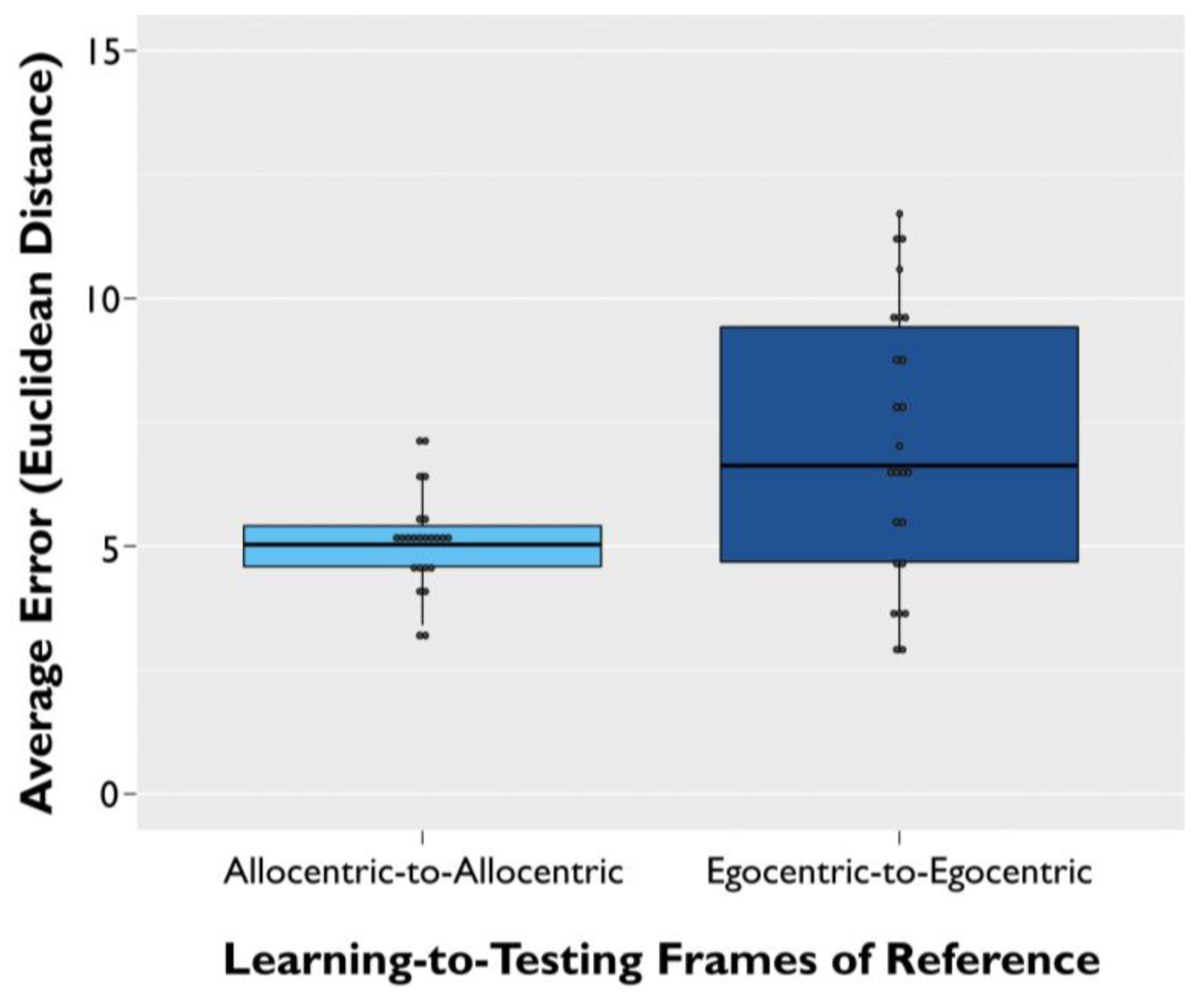
3. Results
3.1. Spatial Memory Error
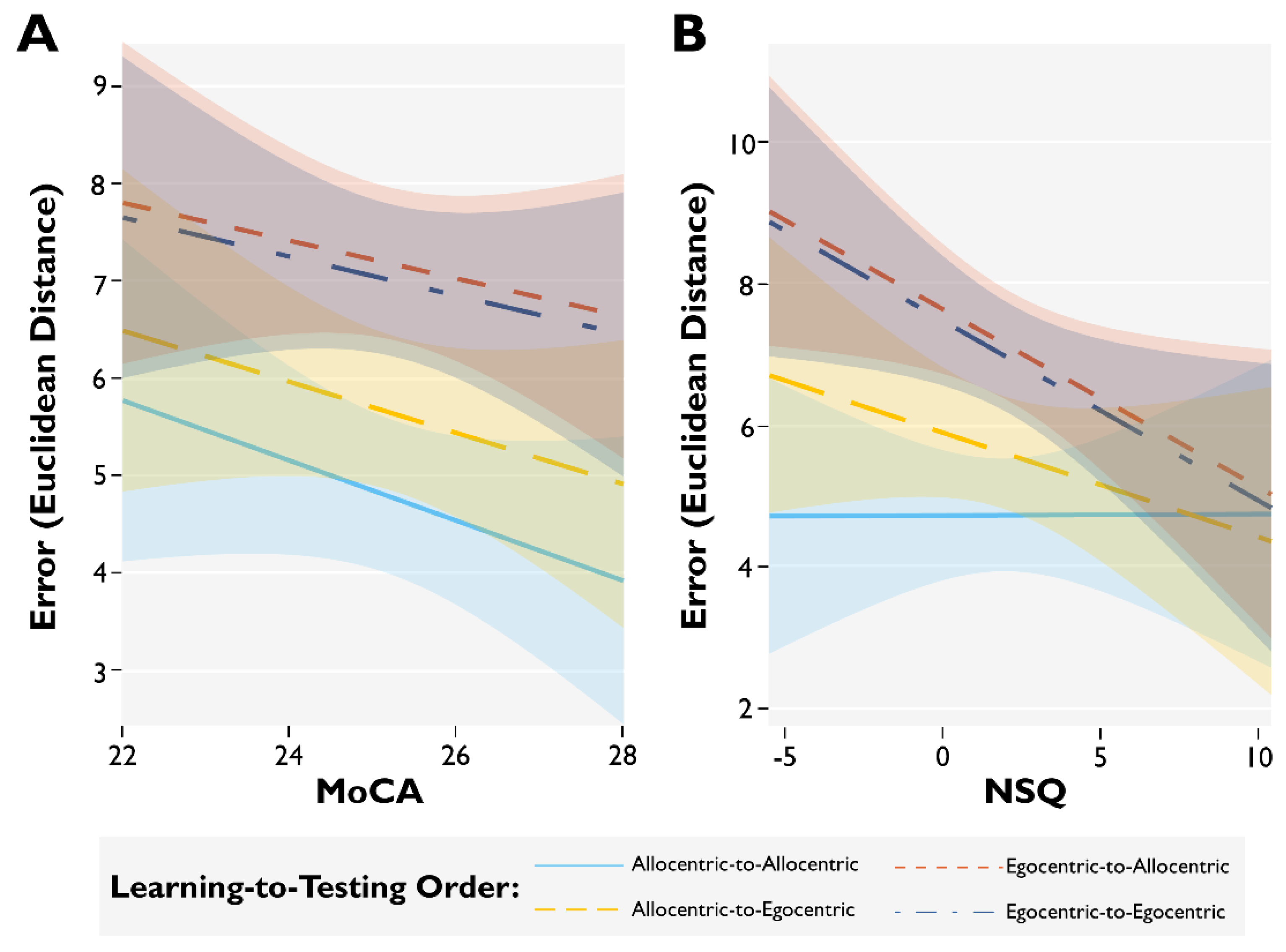
3.2. Modulating Factors of Spatial Memory Accuracy
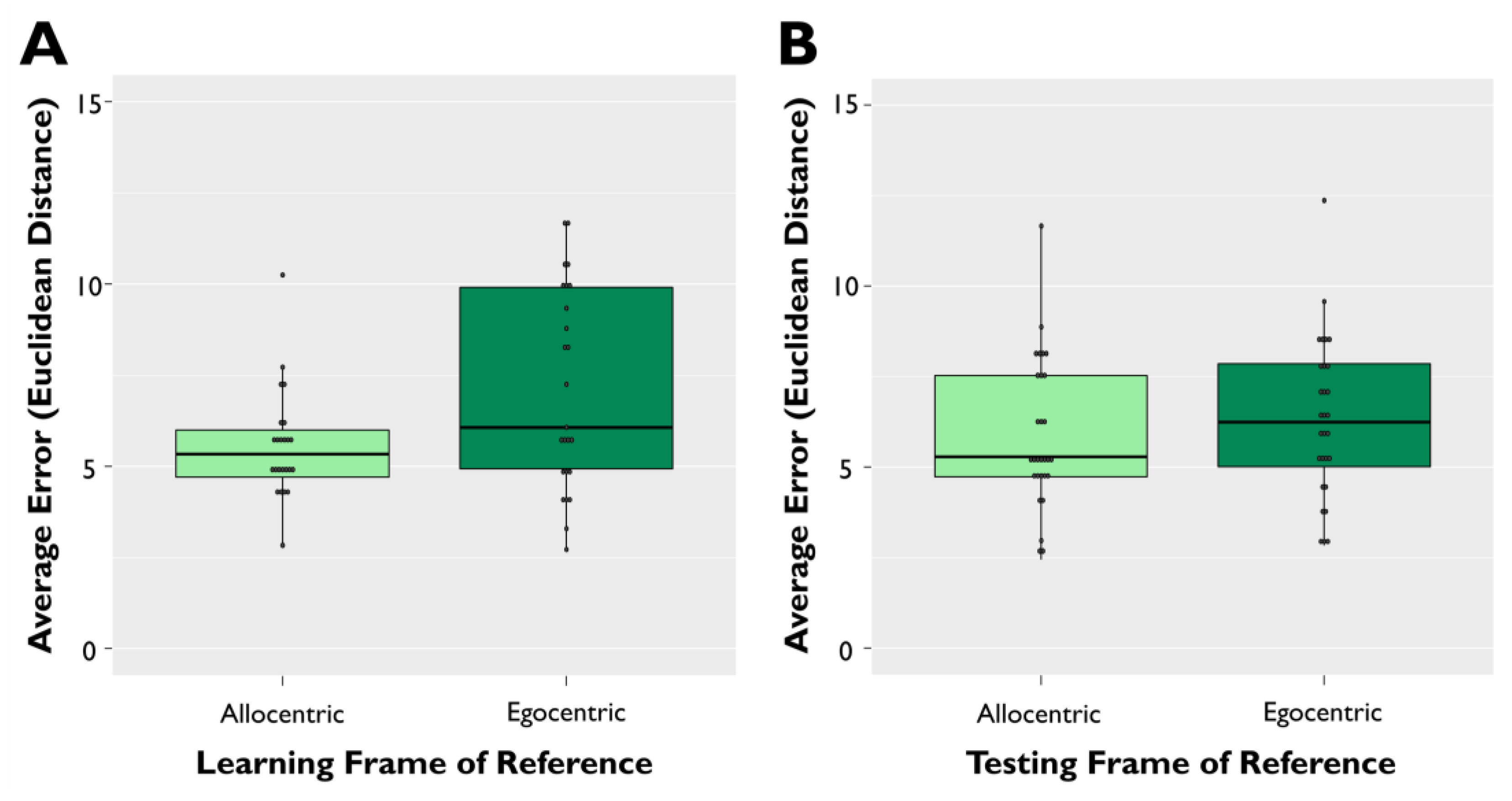
3.3. Exploratory Analysis of Learning Frame of Reference
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chersi, F.; Burgess, N. The Cognitive Architecture of Spatial Navigation: Hippocampal and Striatal Contributions. Neuron 2015, 88, 64–77. [Google Scholar] [CrossRef] [Green Version]
- Ekstrom, A.D.; Isham, E.A. Human Spatial Navigation: Representations across Dimensions and Scales. Curr. Opin. Behav. Sci. 2017, 17, 84–89. [Google Scholar] [CrossRef]
- Meneghetti, C.; Pazzaglia, F.; De Beni, R. Mental Representations Derived from Spatial Descriptions: The Influence of Orientation Specificity and Visuospatial Abilities. Psychol. Res. 2015, 79, 289–307. [Google Scholar] [CrossRef]
- Siegel, A.W.; White, S.H. The development of spatial representations of large-scale environments. Adv. Child Dev. Behav. 1975, 10, 9–55. [Google Scholar]
- Holmes, C.A.; Marchette, S.A.; Newcombe, N.S. Multiple Views of Space: Continuous Visual Flow Enhances Small-Scale Spatial Learning. J. Exp. Psychol. Learn. Mem. Cogn. 2017, 43, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Spelke, E.S. Updating Egocentric Representations in Human Navigation. Cognition 2000, 77, 215–250. [Google Scholar] [CrossRef]
- Mou, W.; Zhao, M.; McNamara, T.P. Layout Geometry in the Selection of Intrinsic Frames of Reference from Multiple Viewpoints. J. Exp. Psychol. Learn. Mem. Cogn. 2007, 33, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolman, E.C. Cognitive Maps in Rats and Men. Psychol. Rev. 1948, 55, 189–208. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.K.; Bonura, B.M.; Taylor, H.A. The Influence of Semantic Relationships on Older Adult Map Memory. Psychol. Aging 2012, 27, 657–665. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, N.; DeGirolamo, G.J. Differential Effects of Aging on Spatial Learning through Exploratory Navigation and Map Reading. Front. Aging Neurosci. 2012, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutrot, A.; Silva, R.; Manley, E.; de Cothi, W.; Sami, S.; Bohbot, V.D.; Wiener, J.M.; Hölscher, C.; Dalton, R.C.; Hornberger, M.; et al. Global Determinants of Navigation Ability. Curr. Biol. 2018, 28, 2861–2866.e4. [Google Scholar] [CrossRef] [Green Version]
- Lester, A.W.; Moffat, S.D.; Wiener, J.M.; Barnes, C.A.; Wolbers, T. The Aging Navigational System. Neuron 2017, 95, 1019–1035. [Google Scholar] [CrossRef]
- Weisberg, S.M.; Newcombe, N.S. Cognitive Maps: Some People Make Them, Some People Struggle. Curr. Dir. Psychol. Sci. 2018, 27, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Boccia, M.; Di Vita, A.; Diana, S.; Margiotta, R.; Imbriano, L.; Rendace, L.; Campanelli, A.; D’Antonio, F.; Trebbastoni, A.; de Lena, C.; et al. Is Losing One’s Way a Sign of Cognitive Decay? Topographical Memory Deficit as an Early Marker of Pathological Aging. J. Alzheimer’s Dis. 2019, 68, 679–693. [Google Scholar] [CrossRef] [Green Version]
- Colombo, D.; Serino, S.; Tuena, C.; Pedroli, E.; Dakanalis, A.; Cipresso, P.; Riva, G. Egocentric and Allocentric Spatial Reference Frames in Aging: A Systematic Review. Neurosci. Biobehav. Rev. 2017, 80, 605–621. [Google Scholar] [CrossRef]
- Fernandez-Baizan, C.; Diaz-Caceres, E.; Arias, J.L.; Mendez, M.; Fernandez-Baizan, C.; Diaz-Caceres, E.; Arias, J.L.; Mendez, M. Egocentric and Allocentric Spatial Memory in Healthy Aging: Performance on Real-World Tasks. Braz. J. Med. Biol. Res. 2019, 52, e8041. [Google Scholar] [CrossRef] [PubMed]
- Antonova, E.; Parslow, D.; Brammer, M.; Dawson, G.R.; Jackson, S.H.D.; Morris, R.G. Age-Related Neural Activity during Allocentric Spatial Memory. Memory 2009, 17, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Moffat, S.D.; Kennedy, K.M.; Rodrigue, K.M.; Raz, N. Extrahippocampal Contributions to Age Differences in Human Spatial Navigation. Cereb. Cortex 2007, 17, 1274–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moffat, S.D.; Elkins, W.; Resnick, S.M. Age Differences in the Neural Systems Supporting Human Allocentric Spatial Navigation. Neurobiol. Aging 2006, 27, 965–972. [Google Scholar] [CrossRef]
- Fricke, M.; Bock, O. Egocentric Navigation Is Age-Resistant: First Direct Behavioral Evidence. Curr. Neurobiol. 2018, 9, 69–75. [Google Scholar]
- Gazova, I.; Laczó, J.; Rubinova, E.; Mokrisova, I.; Hyncicova, E.; Andel, R.; Vyhnalek, M.; Sheardova, K.; Coulson, E.J.; Hort, J. Spatial Navigation in Young versus Older Adults. Front. Aging Neurosci. 2013, 5, 94. [Google Scholar] [CrossRef] [Green Version]
- Guderian, S.; Dzieciol, A.M.; Gadian, D.G.; Jentschke, S.; Doeller, C.F.; Burgess, N.; Mishkin, M.; Vargha-Khadem, F. Hippocampal Volume Reduction in Humans Predicts Impaired Allocentric Spatial Memory in Virtual-Reality Navigation. J. Neurosci. 2015, 35, 14123–14131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lithfous, S.; Dufour, A.; Blanc, F.; Després, O. Allocentric but Not Egocentric Orientation Is Impaired during Normal Aging: An ERP Study. Neuropsychology 2014, 28, 761–771. [Google Scholar] [CrossRef]
- Hort, J.; Laczó, J.; Vyhnálek, M.; Bojar, M.; Bureš, J.; Vlček, K. Spatial Navigation Deficit in Amnestic Mild Cognitive Impairment. Proc. Natl. Acad. Sci. USA 2007, 104, 4042–4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunec, I.K.; Robin, J.; Patai, E.Z.; Ozubko, J.D.; Javadi, A.-H.; Barense, M.D.; Spiers, H.J.; Moscovitch, M. Cognitive Mapping Style Relates to Posterior-Anterior Hippocampal Volume Ratio. Hippocampus 2019, 29, 748–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlan, G.; Laczó, J.; Hort, J.; Minihane, A.-M.; Hornberger, M. Spatial Navigation Deficits—Overlooked Cognitive Marker for Preclinical Alzheimer Disease? Nat. Rev. Neurol. 2018, 14, 496–506. [Google Scholar] [CrossRef]
- Ruggiero, G.; Iachini, T. Editorial: Spatial Cognition in Normal Aging, MCI and AD. Available online: https://www.ingentaconnect.com/content/ben/car/2018/00000015/00000003/art00002 (accessed on 26 June 2019).
- Rusconi, M.L.; Suardi, A.; Zanetti, M.; Rozzini, L. Spatial Navigation in Elderly Healthy Subjects, Amnestic and Non Amnestic MCI Patients. J. Neurol. Sci. 2015, 359, 430–437. [Google Scholar] [CrossRef]
- Bierbrauer, A.; Kunz, L.; Gomes, C.A.; Luhmann, M.; Deuker, L.; Getzmann, S.; Wascher, E.; Gajewski, P.D.; Hengstler, J.G.; Fernandez-Alvarez, M.; et al. Unmasking Selective Path Integration Deficits in Alzheimer’s Disease Risk Carriers. Sci. Adv. 2020, 6, eaba1394. [Google Scholar] [CrossRef]
- Coughlan, G.; Coutrot, A.; Khondoker, M.; Minihane, A.-M.; Spiers, H.; Hornberger, M. Toward Personalized Cognitive Diagnostics of At-Genetic-Risk Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2019, 116, 9285–9292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verghese, J.; Lipton, R.; Ayers, E. Spatial Navigation and Risk of Cognitive Impairment: A Prospective Cohort Study. Alzheimers Dement. 2017, 13, 985–992. [Google Scholar] [CrossRef]
- Harris, M.A.; Wiener, J.M.; Wolbers, T. Aging Specifically Impairs Switching to an Allocentric Navigational Strategy. Front. Aging Neurosci. 2012, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morganti, F.; Riva, G. Virtual Reality as Allocentric/Egocentric Technology for the Assessment of Cognitive Decline in the Elderly. Stud. Health Technol. Inform. 2014, 196, 278–284. [Google Scholar] [PubMed]
- Ruggiero, G.; Iavarone, A.; Iachini, T. Allocentric to Egocentric Spatial Switching: Impairment in AMCI and Alzheimer’s Disease Patients? Curr. Alzheimer Res. 2018, 15, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sele, S.; Liem, F.; Mérillat, S.; Jäncke, L. Age-Related Decline in the Brain: A Longitudinal Study on Inter-Individual Variability of Cortical Thickness, Area, Volume, and Cognition. NeuroImage 2021, 240, 118370. [Google Scholar] [CrossRef]
- Morse, C.K. Does Variability Increase with Age? An Archival Study of Cognitive Measures. Psychol. Aging 1993, 8, 156–164. [Google Scholar] [CrossRef]
- Hultsch, D.F.; MacDonald, S.W.S.; Hunter, M.A.; Levy-Bencheton, J.; Strauss, E. Intraindividual Variability in Cognitive Performance in Older Adults: Comparison of Adults with Mild Dementia, Adults with Arthritis, and Healthy Adults. Neuropsychology 2000, 14, 588–598. [Google Scholar] [CrossRef]
- Konishi, K.; Bohbot, V. Spatial Navigational Strategies Correlate with Gray Matter in the Hippocampus of Healthy Older Adults Tested in a Virtual Maze. Front. Aging Neurosci. 2013, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Cherry, K.E.; Park, D.C. Individual Difference and Contextual Variables Influence Spatial Memory in Younger and Older Adults. Psychol. Aging 1993, 8, 517–526. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Dong, Y.; Lee, W.Y.; Basri, N.A.; Collinson, S.L.; Merchant, R.A.; Venketasubramanian, N.; Chen, C.L.-H. The Montreal Cognitive Assessment Is Superior to the Mini–Mental State Examination in Detecting Patients at Higher Risk of Dementia. Int. Psychogeriatr. 2012, 24, 1749–1755. [Google Scholar] [CrossRef] [Green Version]
- Roalf, D.R.; Moberg, P.J.; Xie, S.X.; Wolk, D.A.; Moelter, S.T.; Arnold, S.E. Comparative Accuracies of Two Common Screening Instruments for Classification of Alzheimer’s Disease, Mild Cognitive Impairment, and Healthy Aging. Alzheimer’s Dement. 2013, 9, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Reitan, R.M.; Wolfson, D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation; Reitan Neuropsychology: Tucson, AZ, USA, 1985; Volume 4. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale, 4th ed.; Pearson Assessment: San Antonio, TX, USA, 2008. [Google Scholar]
- Benedict, R. Brief Visuospatial Memory Test–Revised; Psychological Assessment Resources: Lutz, FL, USA, 1997. [Google Scholar]
- Schmidt, M. Rey Auditory Verbal Learning Test; Western Psychological Services: Torrance, CA, USA, 1996. [Google Scholar]
- Marchette, S.A.; Bakker, A.; Shelton, A.L. Cognitive Mappers to Creatures of Habit: Differential Engagement of Place and Response Learning Mechanisms Predicts Human Navigational Behavior. J. Neurosci. 2011, 31, 15264–15268. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative Data Stratified by Age and Education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Brody, D.J.; Kramarow, E.A.; Taylor, C.A.; McGuire, L.C. Cognitive Performance in Adults Aged 60 and Over: National Health and Nutrition Examination Survey, 2011–2014; National Center for Health Statistics: Hyattsville, MD, USA, 2019; pp. 1–23.
- Tombaugh, T.N.; Kozak, J.; Rees, L. Normative Data Stratified by Age and Education for Two Measures of Verbal Fluency: FAS and Animal Naming. Arch. Clin. Neuropsychol. 1999, 14, 167–177. [Google Scholar] [CrossRef]
- Alsbury-Nealy, K.; Wang, H.; Howarth, C.; Gordienko, A.; Schlichting, M.; Duncan, K. OpenMaze: An Open-Source Toolbox for Creating Virtual Environment Experiments. Behav. Res. Methods 2021, 1–14. [Google Scholar]
- Brodeur, M.B.; Guérard, K.; Bouras, M. Bank of Standardized Stimuli (BOSS) Phase II: 930 New Normative Photos. PLoS ONE 2014, 9, e106953. [Google Scholar] [CrossRef]
- Brodeur, M.B.; Dionne-Dostie, E.; Montreuil, T.; Lepage, M. The Bank of Standardized Stimuli (BOSS), a New Set of 480 Normative Photos of Objects to Be Used as Visual Stimuli in Cognitive Research. PLoS ONE 2010, 5, e10773. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bozdogan, H. Model Selection and Akaike’s Information Criterion (AIC): The General Theory and Its Analytical Extensions. Psychometrika 1987, 52, 345–370. [Google Scholar] [CrossRef]
- Olsen, R.K.; Lee, Y.; Kube, J.; Rosenbaum, R.S.; Grady, C.L.; Moscovitch, M.; Ryan, J.D. The Role of Relational Binding in Item Memory: Evidence from Face Recognition in a Case of Developmental Amnesia. J. Neurosci. 2015, 35, 5342–5350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, R.K.; Moses, S.N.; Riggs, L.; Ryan, J.D. The Hippocampus Supports Multiple Cognitive Processes through Relational Binding and Comparison. Front. Hum. Neurosci. 2012, 6. [Google Scholar] [CrossRef] [Green Version]
- Moffat, S.D.; Resnick, S.M. Effects of Age on Virtual Environment Place Navigation and Allocentric Cognitive Mapping. Behav. Neurosci. 2002, 116, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Montefinese, M.; Sulpizio, V.; Galati, G.; Committeri, G. Age-Related Effects on Spatial Memory across Viewpoint Changes Relative to Different Reference Frames. Psychol. Res. 2015, 79, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.H.; Buckley, M.J.; Pegman, S.J.; Spiers, H.; Scahill, V.L.; Gaffan, D.; Bussey, T.J.; Davies, R.R.; Kapur, N.; Hodges, J.R.; et al. Specialization in the Medial Temporal Lobe for Processing of Objects and Scenes. Hippocampus 2005, 15, 782–797. [Google Scholar] [CrossRef]
- Carelli, L.; Rusconi, M.L.; Scarabelli, C.; Stampatori, C.; Mattioli, F.; Riva, G. The Transfer from Survey (Map-like) to Route Representations into Virtual Reality Mazes: Effect of Age and Cerebral Lesion. J. Neuroeng. Rehabil. 2011, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Merriman, N.A.; Ondřej, J.; Roudaia, E.; O’Sullivan, C.; Newell, F.N. Familiar Environments Enhance Object and Spatial Memory in Both Younger and Older Adults. Exp. Brain Res. 2016, 234, 1555–1574. [Google Scholar] [CrossRef]
- Lopez, A.; Caffò, A.O.; Bosco, A. Topographical Disorientation in Aging. Familiarity with the Environment Does Matter. Neurol. Sci. 2018, 39, 1519–1528. [Google Scholar] [CrossRef]
- Førsund, L.H.; Grov, E.K.; Helvik, A.-S.; Juvet, L.K.; Skovdahl, K.; Eriksen, S. The Experience of Lived Space in Persons with Dementia: A Systematic Meta-Synthesis. BMC Geriatr. 2018, 18, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, K.; Mckenzie, S.; Etchamendy, N.; Roy, S.; Bohbot, V.D. Hippocampus-Dependent Spatial Learning Is Associated with Higher Global Cognition among Healthy Older Adults. Neuropsychologia 2017, 106, 310–321. [Google Scholar] [CrossRef]
- Ladyka-Wojcik, N.; Barense, M.D. Reframing Spatial Frames of Reference: What Can Aging Tell Us about Egocentric and Allocentric Navigation? WIREs Cogn. Sci. 2021, 12, e1549. [Google Scholar] [CrossRef]
- O’Shea, A.; Cohen, R.; Porges, E.; Nissim, N.; Woods, A. Cognitive Aging and the Hippocampus in Older Adults. Front. Aging Neurosci. 2016, 8, 298. [Google Scholar] [CrossRef] [PubMed]



| Neuropsychological Assessment | Mean Score (SD) | Compared Normative Sample |
|---|---|---|
| Trail Making Test | ||
| Trails A | 40.81 (8.75) | >60th %ile (75–79 years old) [48] |
| Trails B | 100.52 (36.13) | >60th %ile (75–79 years old) [48] |
| Digit Symbol Substitution | 44.52 (14.67) | >70th %ile (71–81 years old) [49] |
| Verbal Fluency | ||
| Phonemic (FAS) | 47.96 (12.30) | >80th %ile (60–79 years old) [50] |
| Categorical (Animals) | 19.63 (5.73) | >75th %ile (60–79 years old) [50] |
| BVMT-R | ||
| Learning | 43.96 (12.30) | >80th %ile (70–79 years old) [45] |
| Delayed | 8.85 (4.06) | >75th %ile (70–79 years old) [45] |
| RAVLT | ||
| Total (trials I–IV) Learning | 5.30 (2.09) | >75th %ile (72–79 years old) [46] |
| Delayed | 8.00 (2.39) | >55th %ile (72–79 years old) [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladyka-Wojcik, N.; Olsen, R.K.; Ryan, J.D.; Barense, M.D. Flexible Use of Spatial Frames of Reference for Object–Location Memory in Older Adults. Brain Sci. 2021, 11, 1542. https://doi.org/10.3390/brainsci11111542
Ladyka-Wojcik N, Olsen RK, Ryan JD, Barense MD. Flexible Use of Spatial Frames of Reference for Object–Location Memory in Older Adults. Brain Sciences. 2021; 11(11):1542. https://doi.org/10.3390/brainsci11111542
Chicago/Turabian StyleLadyka-Wojcik, Natalia, Rosanna K. Olsen, Jennifer D. Ryan, and Morgan D. Barense. 2021. "Flexible Use of Spatial Frames of Reference for Object–Location Memory in Older Adults" Brain Sciences 11, no. 11: 1542. https://doi.org/10.3390/brainsci11111542
APA StyleLadyka-Wojcik, N., Olsen, R. K., Ryan, J. D., & Barense, M. D. (2021). Flexible Use of Spatial Frames of Reference for Object–Location Memory in Older Adults. Brain Sciences, 11(11), 1542. https://doi.org/10.3390/brainsci11111542






