fMRI Evidence for Default Mode Network Deactivation Associated with Rapid Eye Movements in Sleep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Acquisition
2.3. Data Analysis
3. Results
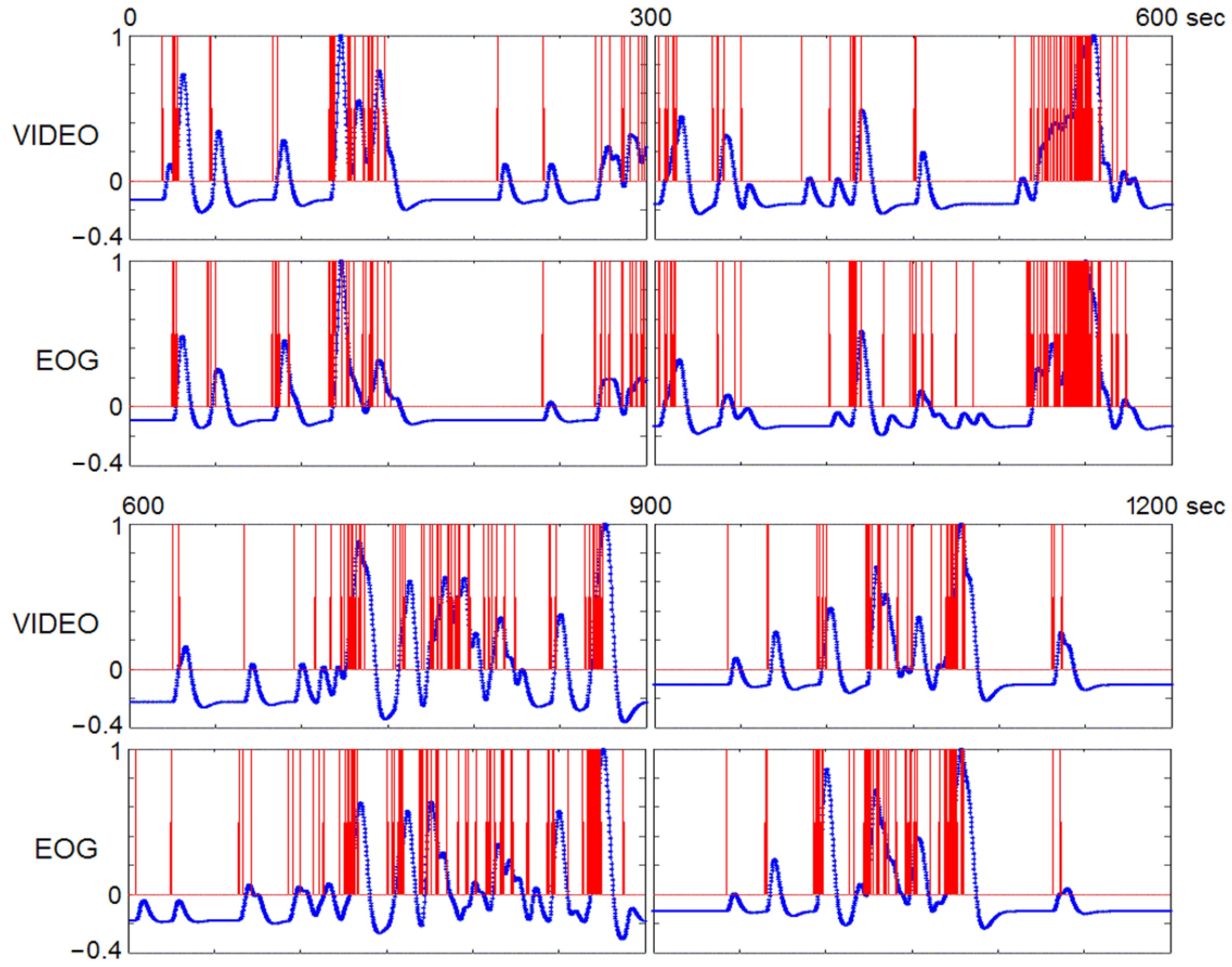
3.1. REM Characteristics
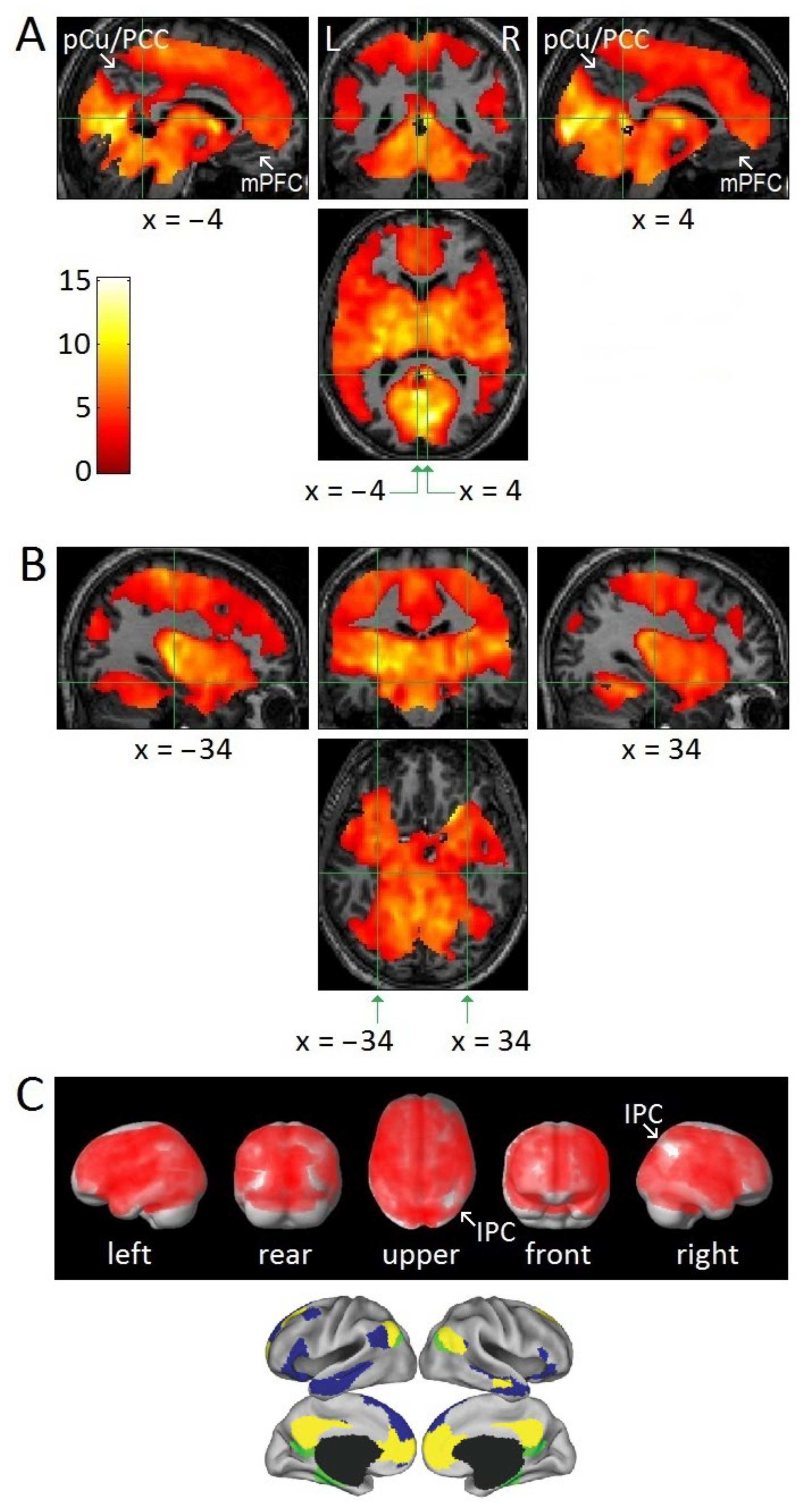
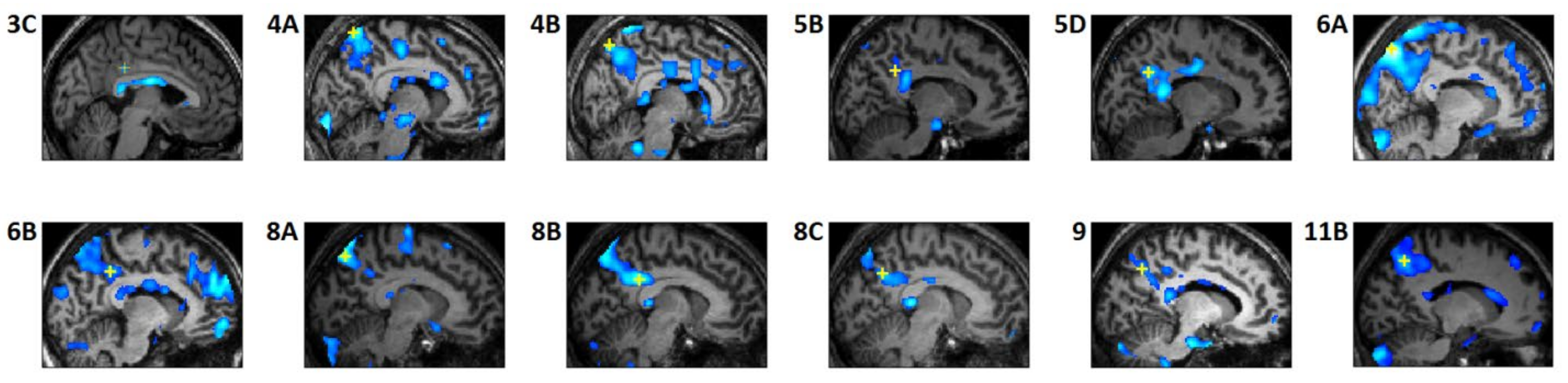
3.2. Attenuation of DMN Activity Associated with REMs
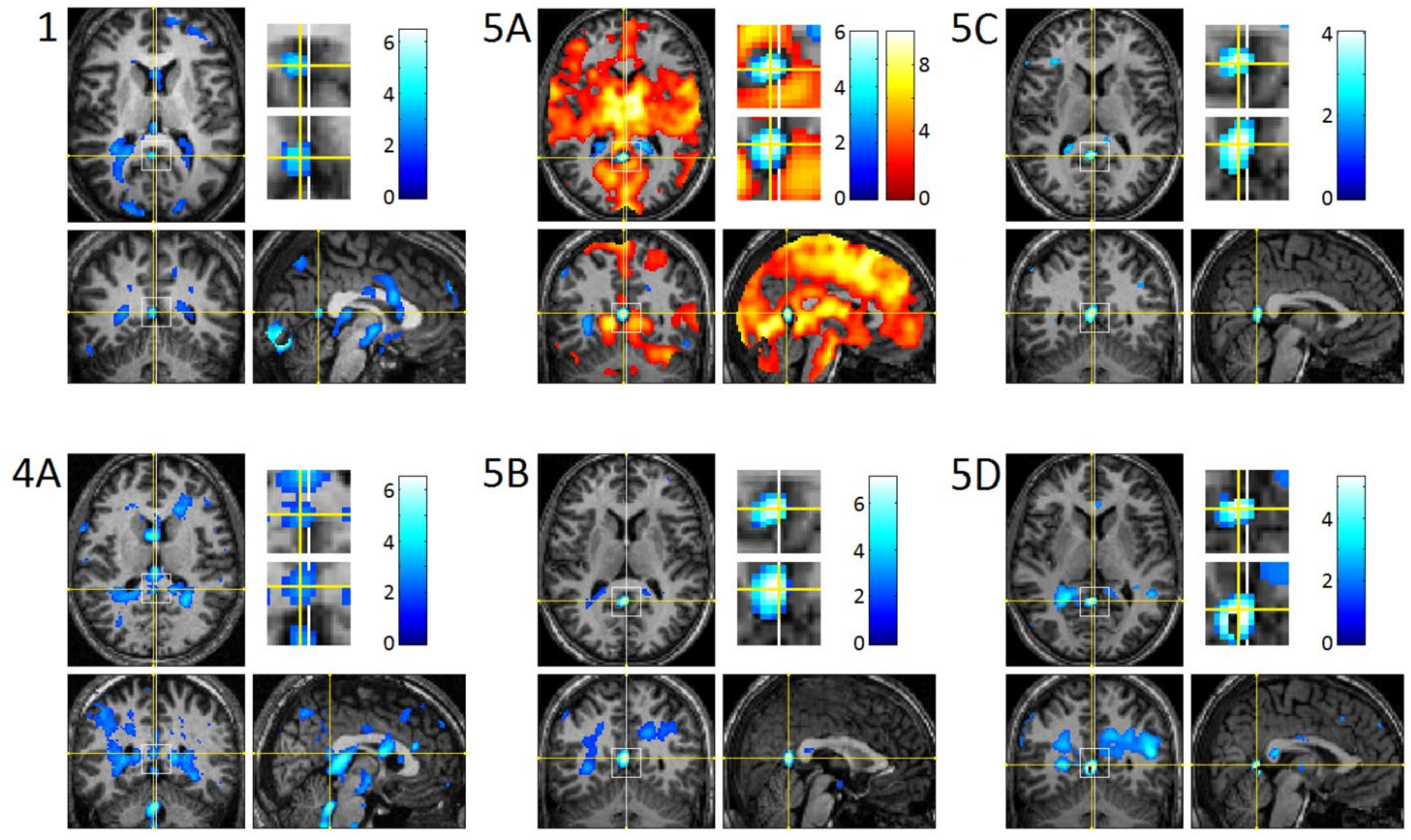
3.3. REM-Locked fMRI Calculations in DMN Nodes
3.4. REM-Locked Periventricular Signal Decrease (PVSD)
4. Discussion
4.1. Relatively Small Islands of Attenuated REM-Locked Cortical Activation
4.2. REMs Are Saccades
4.3. Parallels with Hallucinogen-Induced Changes
4.4. Permissive Deactivation of DMN
4.5. Temporally-Precise REM-Locked Signal Decrease in DMN Nodes May Reflect Upward Inhibitory Messages
4.6. DMN Plays a Role in Aberrant Generation of Perceptual Content
4.7. REM-Locked PVSD Indicates REM-Locked Cerebral Vasoconstriction
4.8. Vascular vs. Neuronal Change in Other Structures
4.9. Timing REMs by Visual Inspection of Video-Recording
4.10. Superb Localization Capacity of fMRI of Video-Timed REMs (vtREM-fMRI)
4.11. Attenuation of REM-Locked Activation in the Hippocampal Formation May Explain Forgetting of Dreaming and Waking Experience
4.12. Limitations of the Present Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Yarkoni, T.; Poldrack, R.A.; Nichols, T.E.; Van Essen, D.C.; Wager, T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 2011, 8, 665–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, B.T.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [Green Version]
- Greicius, M.D.; Kiviniemi, V.; Tervonen, O.; Vainionpää, V.; Alahuhta, S.; Reiss, A.L.; Menon, V. Persistent default-mode network connectivity during light sedation. Hum. Brain Mapp. 2008, 29, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L.; Patel, G.H.; Fox, M.D.; Snyder, A.Z.; Baker, J.T.; Van Essen, D.C.; Zempel, J.M.; Snyder, L.H.; Corbetta, M.; Raichle, M.E. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 2007, 447, 83–86. [Google Scholar] [CrossRef]
- Horovitz, S.G.; Fukunaga, M.; de Zwart, J.A.; van Gelderen, P.; Fulton, S.C.; Balkin, T.J.; Duyn, J.H. Low frequency BOLD fluctuations during resting wakefulness and light sleep: A simultaneous EEG-fMRI study. Hum. Brain Mapp. 2008, 29, 671–682. [Google Scholar] [CrossRef]
- Larson-Prior, L.J.; Zempel, J.M.; Nolan, T.S.; Prior, F.W.; Snyder, A.Z.; Raichle, M.E. Cortical network functional connectivity in the descent to sleep. Proc. Natl. Acad. Sci. USA 2009, 106, 4489–4494. [Google Scholar] [CrossRef] [Green Version]
- Koike, T.; Kan, S.; Misaki, M.; Miyauchi, S. Connectivity pattern changes in default-mode network with deep non-REM and REM sleep. Neurosci. Res. 2011, 69, 322–330. [Google Scholar] [CrossRef]
- Chow, H.M.; Horovitz, S.G.; Carr, W.S.; Picchioni, D.; Coddington, N.; Fukunaga, M.; Xu, Y.; Balkin, T.J.; Duyn, J.H.; Braun, A.R. Rhythmic alternating patterns of brain activity distinguish rapid eye movement sleep from other states of consciousness. Proc. Natl. Acad. Sci. USA 2013, 110, 10300–10305. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.C.-H.; Harris, J.C.; Pearlson, G.D.; Kim, J.-S.; Calhoun, V.D.; Fallon, J.H.; Golay, X.; Gillen, J.S.; Simmonds, D.J.; van Zijl, P.C.M.; et al. fMRI evidence for multisensory recruitment associated with rapid eye movements during sleep. Hum. Brain Mapp. 2009, 30, 1705–1722. [Google Scholar] [CrossRef] [Green Version]
- Dehaene, S.; Changeux, J.P. Experimental and Theoretical Approaches to Conscious Processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef] [Green Version]
- Hohwy, J. The Predictive Mind; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Hong, C.C.-H.; Fallon, J.H.; Friston, K.J.; Harris, J.C. Rapid Eye Movements in Sleep Furnish a Unique Probe Into Consciousness. Front. Psychol. 2018, 9, 2087. [Google Scholar] [CrossRef] [Green Version]
- Andrillon, T.; Nir, Y.; Cirelli, C.; Tononi, G.; Fried, I. Single-neuron activity and eye movements during human REM sleep and awake vision. Nat. Commun. 2015, 6, 7884. [Google Scholar] [CrossRef] [Green Version]
- Hobson, J.A.; Hong, C.C.-H.; Friston, K. Virtual reality and consciousness inference in dreaming. Front. Psychol. 2014, 5, 1133. [Google Scholar] [CrossRef]
- Leclair-Visonneau, L.; Oudiette, D.; Gaymard, B.; Leu-Semenescu, S.; Arnulf, I. Do the eyes scan dream images during rapid eye movement sleep? Evidence from the rapid eye movement sleep behaviour disorder model. Brain 2010, 133, 1737–1746. [Google Scholar] [CrossRef] [Green Version]
- Arnulf, I. The ‘scanning hypothesis’ of rapid eye movements during REM sleep: A review of the evidence. Arch. Ital. Biol. 2011, 149, 367–382. [Google Scholar] [CrossRef]
- Siclari, F.; Baird, B.; Perogamvros, L.; Bernardi, G.; LaRocque, J.J.; Riedner, B.; Boly, M.; Postle, B.R.; Tononi, G. The neural correlates of dreaming. Nat. Neurosci. 2017, 20, 872. [Google Scholar] [CrossRef] [Green Version]
- Ehrsson, H.H. The concept of body ownership and its relation to multisensory integration. In The New Handbook of Multisensory Processes; Stein, B.E., Ed.; MIT Press: Cambridge, MA, USA, 2012; pp. 775–792. [Google Scholar]
- Metzinger, T. The Ego Tunnel: The Science of the Mind and the Myth of the Self; Basic Books: New York, NY, USA, 2009. [Google Scholar]
- Blanke, O. Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci. 2012, 13, 556–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, B.E.; Stanford, T.R. Multisensory integration: Current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 2008, 9, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Metzinger, T. Being No One. The Self-Model Theory of Subjectivity; MIT Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Koroma, M.; Lacaux, C.; Andrillon, T.; Legendre, G.; Léger, D.; Kouider, S. Sleepers Selectively Suppress Informative Inputs during Rapid Eye Movements. Curr. Biol. 2020, 30, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Simor, P.; van der Wijk, G.; Nobili, L.; Peigneux, P. The microstructure of REM sleep: Why phasic and tonic? Sleep Med. Rev. 2020, 52, 101305. [Google Scholar] [CrossRef] [Green Version]
- Funk, C.M.; Honjoh, S.; Rodriguez, A.V.; Cirelli, C.; Tononi, G. Local Slow Waves in Superficial Layers of Primary Cortical Areas during REM Sleep. Curr. Biol. 2016, 26, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Clark, A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 2013, 36, 181–204. [Google Scholar] [CrossRef]
- Friston, K.; Adams, R.A.; Perrinet, L.; Breakspear, M. Perceptions as Hypotheses: Saccades as Experiments. Front. Psychol. 2012, 3, 151. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.C.-H.; Gillin, J.C.; Dow, B.M.; Wu, J.; Buchsbaum, M.S. Localized and lateralized cerebral glucose metabolism associated with eye movements during REM sleep and wakefulness: A positron emission tomography (PET) study. Sleep 1995, 18, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Feldman, H.; Friston, K.J. Attention, uncertainty, and free-energy. Front. Hum. Neurosci. 2010, 4, 215. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.; Adams, R.A.; Parees, I.; Edwards, M.; Friston, K. Active inference, sensory attenuation and illusions. Cogn. Process. 2013, 14, 411–427. [Google Scholar] [CrossRef] [Green Version]
- Bauer, M.; Stenner, M.P.; Friston, K.J.; Dolan, R.J. Attentional modulation of alpha/beta and gamma oscillations reflect functionally distinct processes. J. Neurosci. 2014, 34, 16117–16125. [Google Scholar] [CrossRef] [Green Version]
- Pinotsis, D.A.; Brunet, N.; Bastos, A.; Bosman, C.A.; Litvak, V.; Fries, P.; Friston, K.J. Contrast gain control and horizontal interactions in V1: A DCM study. Neuroimage 2014, 92, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Auksztulewicz, R.; Friston, K. Attentional Enhancement of Auditory Mismatch Responses: A DCM/MEG Study. Cereb. Cortex 2015, 25, 4273–4283. [Google Scholar] [CrossRef] [Green Version]
- Kanai, R.; Komura, Y.; Shipp, S.; Friston, K. Cerebral hierarchies: Predictive processing, precision and the pulvinar. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2015, 370, 20140169. [Google Scholar] [CrossRef] [Green Version]
- Limanowski, J.; Friston, K. ‘Seeing the Dark’: Grounding Phenomenal Transparency and Opacity in Precision Estimation for Active Inference. Front. Psychol. 2018, 9, 643. [Google Scholar] [CrossRef] [Green Version]
- Parr, T.; Friston, K.J. Attention or salience? Curr. Opin. Psychol. 2019, 29, 1–5. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Friston, K.J. The default-mode, ego-functions and free-energy: A neurobiological account of Freudian ideas. Brain 2010, 133, 1265–1283. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Friston, K.J. REBUS and the Anarchic Brain: Toward a Unified Model of the Brain Action of Psychedelics. Pharmacol. Rev. 2019, 71, 316–344. [Google Scholar] [CrossRef]
- de Araujo, D.B.; Ribeiro, S.; Cecchi, G.A.; Carvalho, F.M.; Sanchez, T.A.; Pinto, J.P.; de Martinis, B.S.; Crippa, J.A.; Hallak, J.E.; Santos, A.C. Seeing with the eyes shut: Neural basis of enhanced imagery following Ayahuasca ingestion. Hum. Brain Mapp. 2012, 33, 2550–2560. [Google Scholar] [CrossRef]
- Palhano-Fontes, F.; Andrade, K.C.; Tofoli, L.F.; Santos, A.C.; Crippa, J.A.S.; Hallak, J.E.C.; Ribeiro, S.; de Araujo, D.B. The Psychedelic State Induced by Ayahuasca Modulates the Activity and Connectivity of the Default Mode Network. PLoS ONE 2015, 10, e0118143. [Google Scholar] [CrossRef] [Green Version]
- Kraehenmann, R. Dreams and Psychedelics: Neurophenomenological Comparison and Therapeutic Implications. Curr. Neuropharmacol. 2017, 15, 1032–1042. [Google Scholar] [CrossRef] [Green Version]
- Sanz, C.; Zamberlan, F.; Erowid, E.; Erowid, F.; Tagliazucchi, E. The Experience Elicited by Hallucinogens Presents the Highest Similarity to Dreaming within a Large Database of Psychoactive Substance Reports. Front. Neurosci. 2018, 12, 7. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef] [Green Version]
- Barrett, F.S.; Griffiths, R.R. Classic Hallucinogens and Mystical Experiences: Phenomenology and Neural Correlates. In Behavioral Neurobiology of Psychedelic Drugs; Halberstadt, A.L., Vollenweider, F.X., Nichols, D.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 36, pp. 393–430. [Google Scholar]
- dos Santos, R.G.; Balthazar, F.M.; Bouso, J.C.; Hallak, J.E. The current state of research on ayahuasca: A systematic review of human studies assessing psychiatric symptoms, neuropsychological functioning, and neuroimaging. J. Psychopharmacol. 2016, 30, 1230–1247. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Osório, F.L.; Crippa, J.A.S.; Hallak, J.E.C. Classical hallucinogens and neuroimaging: A systematic review of human studies: Hallucinogens and neuroimaging. Neurosci. Biobehav. Rev. 2016, 71, 715–728. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Muthukumaraswamy, S.; Roseman, L.; Kaelen, M.; Droog, W.; Murphy, K.; Tagliazucchi, E.; Schenberg, E.E.; Nest, T.; Orban, C.; et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging (early edition). Proc. Natl. Acad. Sci. USA 2016, 7, 4853–4858. [Google Scholar] [CrossRef] [Green Version]
- Müller, F.; Lenz, C.; Dolder, P.; Lang, U.; Schmidt, A.; Liechti, M.; Borgwardt, S. Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr. Scand. 2017, 136, 648–657. [Google Scholar] [CrossRef] [Green Version]
- Müller, F.; Dolder, P.C.; Schmidt, A.; Liechti, M.E.; Borgwardt, S. Altered network hub connectivity after acute LSD administration. Neuroimage Clin. 2018, 18, 694–701. [Google Scholar] [CrossRef]
- Hebbard, F.W.; Fischer, R. Effect of Psilocybin, LSD, and mescaline on small, involuntary eye movements. Psychopharmacologia 1966, 9, 146–156. [Google Scholar] [CrossRef]
- Raichle, M.E. Two views of brain function. Trends Cogn. Sci. 2010, 14, 180–190. [Google Scholar] [CrossRef]
- Margulies, D.S.; Ghosh, S.S.; Goulas, A.; Falkiewicz, M.; Huntenburg, J.M.; Langs, G.; Bezgin, G.; Eickhoff, S.B.; Castellanos, F.X.; Petrides, M.; et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. USA 2016, 113, 12574–12579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastos-Leite, A.J.; Ridgway, G.R.; Silveira, C.; Norton, A.; Reis, S.; Friston, K.J. Dysconnectivity Within the Default Mode in First-Episode Schizophrenia: A Stochastic Dynamic Causal Modeling Study with Functional Magnetic Resonance Imaging. Schizophr. Bull. 2015, 41, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L. The brain’s default network: Origins and implications for the study of psychosis. Dialogues Clin. Neurosci. 2013, 15, 351–358. [Google Scholar] [PubMed]
- Khadka, S.; Meda, S.A.; Stevens, M.C.; Glahn, D.C.; Calhoun, V.D.; Sweeney, J.A.; Tamminga, C.A.; Keshavan, M.S.; O’Neil, K.; Schretlen, D.; et al. Is Aberrant Functional Connectivity A Psychosis Endophenotype? A Resting State Functional Magnetic Resonance Imaging Study. Biol. Psychiatry 2013, 74, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Parr, T.; Benrimoh, D.; Vincent, P.; Friston, K. Precision and false perceptual inference. Front. Integr. Neurosci. 2018, 74, 458–466. [Google Scholar] [CrossRef]
- Kok, P.; Rahnev, D.; Jehee, J.F.M.; Lau, H.C.; de Lange, F.P. Attention Reverses the Effect of Prediction in Silencing Sensory Signals. Cereb. Cortex 2012, 22, 2197–2206. [Google Scholar] [CrossRef] [Green Version]
- Hobson, J.A.; Friston, K.J. Waking and dreaming consciousness: Neurobiological and functional considerations. Prog. Neurobiol. 2012, 98, 82–98. [Google Scholar] [CrossRef] [Green Version]
- Clark, A. The many faces of precision (Replies to commentaries on “Whatever next? Neural prediction, situated agents, and the future of cognitive science”). Front. Psychol. 2013, 4, 270. [Google Scholar] [CrossRef] [Green Version]
- Lisman, J. Excitation, inhibition, local oscillations, or large-scale loops: What causes the symptoms of schizophrenia? Curr. Opin. Neurobiol. 2012, 22, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Corlett, P.R.; Honey, G.D.; Krystal, J.H.; Fletcher, P.C. Glutamatergic model psychoses: Prediction error, learning, and inference. Neuropsychopharmacology 2011, 36, 294–315. [Google Scholar] [CrossRef]
- Powers, A.R.; Mathys, C.; Corlett, P.R. Pavlovian conditioning–induced hallucinations result from overweighting of perceptual priors. Science 2017, 357, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Powers, A.R., 3rd; Gancsos, M.G.; Finn, E.S.; Morgan, P.T.; Corlett, P.R. Ketamine-Induced Hallucinations. Psychopathology 2015, 48, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Leigh, R.J.; Zee, D.S. The saccadic system. In The Neurology of Eye Movements, 5th ed.; Oxford University Press: New York, NY, USA, 2015; pp. 169–288. [Google Scholar]
- Pace-Schott, E.F. Neurobiology of dreaming. In Principles and Practice of Sleep Medicine, 5th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; Saunders: Philadelphia, PA, USA, 2011; pp. 563–575. [Google Scholar]
- Domhoff, G.W. The neural substrate for dreaming: Is it a subsystem of the default network? Conscious. Cogn. 2011, 20, 1163–1174. [Google Scholar] [CrossRef]
- Domhoff, G.W.; Fox, K.C.R. Dreaming and the default network: A review, synthesis, and counterintuitive research proposal. Conscious. Cogn. 2015, 33, 342–353. [Google Scholar] [CrossRef]
- Christoff, K.; Irving, Z.C.; Fox, K.C.R.F.; Spreng, R.N.; Andrews-Hanna, J.R. Mind-wandering as spontaneous thought: A dynamic framework. Nat. Rev. Neurosci. 2016, 17, 718–731. [Google Scholar] [CrossRef]
- Hobson, J.A.; Pace-Schott, E.F.; Stickgold, R. Dreaming and the brain: Toward a cognitive neuroscience of conscious states. Behav. Brain Sci. 2000, 23, 793–842. [Google Scholar] [CrossRef]
- Fox, K.; Nijeboer, S.; Solomonova, E.; Domhoff, G.W.; Christoff, K. Dreaming as mind wandering: Evidence from functional neuroimaging and first-person content reports. Front. Hum. Neurosci. 2013, 7, 412. [Google Scholar] [CrossRef] [Green Version]
- Wehrle, R.; Czisch, M.; Kaufmann, C.; Wetter, T.C.; Holsboer, F.; Auer, D.P.; Pollmächer, T. Rapid eye movement-related brain activation in human sleep: A functional magnetic resonance imaging study. NeuroReport 2005, 16, 853–857. [Google Scholar] [CrossRef]
- Miyauchi, S.; Misaki, M.; Kan, S.; Fukunaga, T.; Koike, T. Human brain activity time-locked to rapid eye movements during REM sleep. Exp. Brain Res. 2009, 192, 657–667. [Google Scholar] [CrossRef] [Green Version]
- Vatansever, D.; Menon, D.K.; Manktelow, A.E.; Sahakian, B.J.; Stamatakis, E.A. Default mode network connectivity during task execution. NeuroImage 2015, 122, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Sormaz, M.; Murphy, C.; Wang, H.-T.; Hymers, M.; Karapanagiotidis, T.; Poerio, G.; Margulies, D.S.; Jefferies, E.; Smallwood, J. Default mode network can support the level of detail in experience during active task states. Proc. Natl. Acad. Sci. USA 2018, 115, 9318–9323. [Google Scholar] [CrossRef] [Green Version]
- Braga, R.M.; Sharp, D.J.; Leeson, C.; Wise, R.J.S.; Leech, R. Echoes of the brain within default mode, association, and heteromodal cortices. J. Neurosci. 2013, 33, 14031–14039. [Google Scholar] [CrossRef] [Green Version]
- Pearson, J.M.; Hayden, B.Y.; Raghavachari, S.; Platt, M.L. Neurons in Posterior Cingulate Cortex Signal Exploratory Decisions in a Dynamic Multioption Choice Task. Curr. Biol. 2009, 19, 1532–1537. [Google Scholar] [CrossRef] [Green Version]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2013, 137, 12–32. [Google Scholar] [CrossRef] [Green Version]
- Ioannides, A.A.; Corsi-Cabrera, M.; Fenwick, P.B.C.; Rio Portilla, Y.; Laskaris, N.A.; Khurshudyan, A.; Theofilou, D.; Shibata, T.; Uchida, S.; Nakabayashi, T.; et al. MEG tomography of human cortex and brainstem activity in waking and REM sleep saccades. Cereb. Cortex 2004, 14, 56–72. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Zhu, H.; Giovanello, K.S.; Smith, J.K.; Shen, D.; Gilmore, J.H.; Lin, W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. USA 2009, 106, 6790–6795. [Google Scholar] [CrossRef] [Green Version]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Broyd, S.J.; Demanuele, C.; Debener, S.; Helps, S.K.; James, C.J.; Sonuga-Barke, E.J.S. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci. Biobehav. Rev. 2009, 33, 279–296. [Google Scholar] [CrossRef]
- Calhoun, V.; Eichele, T.; Pearlson, G. Functional brain networks in schizophrenia: A review. Front. Hum. Neurosci. 2009, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.-L.; Zong, X.-F.; Mann, J.J.; Zheng, J.-J.; Liao, Y.-H.; Li, Z.-C.; He, Y.; Chen, X.-G.; Tang, J.-S. A review of the functional and anatomical default mode network in schizophrenia. Neurosci. Bull. 2017, 33, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Padmanabhan, A.; Lynch, C.J.; Schaer, M.; Menon, V. The Default Mode Network in Autism. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.C.-H.; Potkin, S.G.; Antrobus, J.S.; Dow, B.M.; Callaghan, G.M.; Gillin, J.C. REM sleep eye movement counts correlate with visual imagery in dreaming: A pilot study. Psychophysiology 1997, 34, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Bianciardi, M.; Fukunaga, M.; Van Gelderen, P.; De Zwart, J.A.; Duyn, J.H. Negative BOLD-fMRI signals in large cerebral veins. J. Cereb. Blood Flow Metab. 2011, 31, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.P.; Liu, P.; Aslan, S.; King, K.S.; van Osch, M.J.P.; Lu, H. Physiologic underpinnings of negative BOLD cerebrovascular reactivity in brain ventricles. NeuroImage 2013, 83, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Bright, M.G.; Bianciardi, M.; de Zwart, J.A.; Murphy, K.; Duyn, J.H. Early anti-correlated BOLD signal changes of physiologic origin. NeuroImage 2014, 87, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Fultz, N.E.; Bonmassar, G.; Setsompop, K.; Stickgold, R.A.; Rosen, B.R.; Polimeni, J.R.; Lewis, L.D. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019, 366, 628–631. [Google Scholar] [CrossRef]
- Mandell, D.M.; Han, J.S.; Poublanc, J.; Crawley, A.P.; Kassner, A.; Fisher, J.A.; Mikulis, D.J. Selective Reduction of Blood Flow to White Matter during Hypercapnia Corresponds With Leukoaraiosis. Stroke 2008, 39, 1993–1998. [Google Scholar] [CrossRef] [Green Version]
- Shmuel, A.; Augath, M.; Oeltermann, A.; Logothetis, N.K. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 2006, 9, 569–577. [Google Scholar] [CrossRef]
- Maguire, E.A. The retrosplenial contribution to human navigation: A review of lesion and neuroimaging findings. Scand. J. Psychol. 2001, 42, 225–238. [Google Scholar] [CrossRef]
- Vann, S.D.; Aggleton, J.P.; Maguire, E.A. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 2009, 10, 792–802. [Google Scholar] [CrossRef]
- Kimmig, H.; Greenlee, M.; Gondan, M.; Schira, M.; Kassubek, J.; Mergner, T. Relationship between saccadic eye movements and cortical activity as measured by fMRI: Quantitative and qualitative aspects. Exp. Brain Res. 2001, 141, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Shein-Idelson, M.; Ondracek, J.M.; Liaw, H.-P.; Reiter, S.; Laurent, G. Slow waves, sharp waves, ripples, and REM in sleeping dragons. Science 2016, 352, 590–595. [Google Scholar] [CrossRef]
- Stark, C.E.L.; Squire, L.R. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proc. Natl. Acad. Sci. USA 2001, 98, 12760–12766. [Google Scholar] [CrossRef] [Green Version]
- Stickgold, R. Sleep-dependent memory consolidation. Nature 2005, 437, 1272–1278. [Google Scholar] [CrossRef]
- Buzsáki, G. The Hippocampo-Neocortical Dialogue. Cereb. Cortex 1996, 6, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Freud, S. Die Traumdeutung (Tranlated to Korean by Byung-Gil Chang); Eulyoo Publishing Co.: Seoul, Korea, 1963. [Google Scholar]
- McCarley, R.W.; Hobson, J.A. The neurobiological origins of psychoanalytic dream theory. Am. J. Psychiatry 1977, 134, 1211–1221. [Google Scholar]
- Koukkou, M.; Lehmann, D. Dreaming: The functional state-shift hypothesis. A neuropsychophysiological model. Br. J. Psychiatry 1983, 142, 221–231. [Google Scholar] [CrossRef]
- Hobson, J.A. REM sleep and dreaming: Towards a theory of protoconsciousness. Nat. Rev. Neurosci. 2009, 10, 803–813. [Google Scholar] [CrossRef]
- Buzsáki, G. Two-stage model of memory trace formation: A role for “noisy” brain states. Neuroscience 1989, 31, 551–570. [Google Scholar] [CrossRef]
- Boyce, R.; Glasgow, S.D.; Williams, S.; Adamantidis, A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 2016, 352, 812–816. [Google Scholar] [CrossRef]
- Izawa, S.; Chowdhury, S.; Miyazaki, T.; Mukai, Y.; Ono, D.; Inoue, R.; Ohmura, Y.; Mizoguchi, H.; Kimura, K.; Yoshioka, M.; et al. REM sleep–active MCH neurons are involved in forgetting hippocampus-dependent memories. Science 2019, 365, 1308–1313. [Google Scholar] [CrossRef]
- Siclari, F.; LaRocque, J.; Postle, B.; Tononi, G. Assessing sleep consciousness within subjects using a serial awakening paradigm. Front. Psychol. 2013, 4, 542. [Google Scholar] [CrossRef] [Green Version]

| Participant | Study | Age | Sex | Analyzed Sleep Duration a | REM Count b | Inter-Scan Interval c |
|---|---|---|---|---|---|---|
| 1 | 25 | M | 20.0 | 230 | ||
| 2 | 19 | F | 27.3 | 389 | ||
| 3 | a | 18 | F | 14.6 | 174 | |
| b | 12.1 | 108 | 5 months | |||
| c | 6.2 | 43 | 17 months | |||
| d | 13.6 | 189 | 19 months | |||
| 4 | a | 37 | M | 22.1 | 105 | |
| b | 10.8 | 103 | 20.9 h | |||
| 5 | a | 20 | M | 33.3 | 192 | |
| b | 29.1 | 156 | 2.1 h | |||
| c | 17.7 | 283 | 25.5 h | |||
| d | 28.4 | 324 | 27.7 h | |||
| e | 20.5 | 334 | 6 months | |||
| 6 | a | 22 | F | 12.0 | 76 | |
| b | 11.4 | 122 | 23.4 h | |||
| c | 23.2 | 309 | 26.1 h | |||
| 7 | 24 | F | 16.6 | 236 | ||
| 8 | a | 22 | M | 22.7 | 154 | |
| b | 25.7 | 337 | 23.4 h | |||
| c | 7.8 | 70 | 25.3 h | |||
| 9 | 23 | M | 26.2 | 417 | ||
| 10 | 23 | F | 9.0 | 76 | ||
| 11 | a | 25 | M | 13.2 | 170 | |
| b | 16.4 | 223 | 2.1 h |
| Study | Maximum Signal Decrease Voxel | |||
|---|---|---|---|---|
| x | y | z | t | |
| 3C | 4 | −31 | 35 | 2.2 |
| 4A | 8 | −61 | 68 | 4.7 |
| 4B | −6 | −67 | 57 | 4.5 |
| 5B | 12 | −47 | 34 | 2.1 |
| 5D | 14 | −55 | 32 | 3.2 |
| 6A | −10 | −71 | 53 | 7.1 |
| 6B | 10 | −45 | 39 | 3.6 |
| 8A | 10 | −69 | 53 | 4.3 |
| 8B | 8 | −41 | 32 | 5.6 |
| 8C | 8 | −58 | 38 | 3.8 |
| 9 | 12 | −60 | 42 | 2.9 |
| 11B | −14 | −60 | 49 | 5.2 |
| Study | Maximum Signal Decrease Voxel | Cluster Size | |||
|---|---|---|---|---|---|
| x | y | z | t | ||
| 1 | −2 | −52 | 14 | 3.8 | 55 |
| 4A | −2 | −42 | 17 | 3.1 | 9257 |
| 5A | −2 | −54 | 14 | 6.0 | 86 |
| 5B | 0 | −52 | 14 | 7.1 | 139 |
| 5C | −2 | −52 | 14 | 4.0 | 95 |
| 5D | −2 | −52 | 8 | 5.3 | 107 |
| Study | Maximum Signal Decrease Voxel | Cluster Size | |||
|---|---|---|---|---|---|
| x | y | z | t | ||
| LV | −20 | −42 | 13 | 10.5 | 11,468 |
| 3V | 0 | −6 | −8 | 2.5 | 12 |
| 4V | −2 | −44 | −25 | 5.6 | 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, C.C.-H.; Fallon, J.H.; Friston, K.J. fMRI Evidence for Default Mode Network Deactivation Associated with Rapid Eye Movements in Sleep. Brain Sci. 2021, 11, 1528. https://doi.org/10.3390/brainsci11111528
Hong CC-H, Fallon JH, Friston KJ. fMRI Evidence for Default Mode Network Deactivation Associated with Rapid Eye Movements in Sleep. Brain Sciences. 2021; 11(11):1528. https://doi.org/10.3390/brainsci11111528
Chicago/Turabian StyleHong, Charles Chong-Hwa, James H. Fallon, and Karl J. Friston. 2021. "fMRI Evidence for Default Mode Network Deactivation Associated with Rapid Eye Movements in Sleep" Brain Sciences 11, no. 11: 1528. https://doi.org/10.3390/brainsci11111528
APA StyleHong, C. C.-H., Fallon, J. H., & Friston, K. J. (2021). fMRI Evidence for Default Mode Network Deactivation Associated with Rapid Eye Movements in Sleep. Brain Sciences, 11(11), 1528. https://doi.org/10.3390/brainsci11111528






