In Time with the Beat: Entrainment in Patients with Phonological Impairment, Apraxia of Speech, and Parkinson’s Disease
Abstract
:1. Introduction
1.1. The Role of Rhythm in Speech Production
1.2. The Influence of Rhythm on Speech Production in Patients with Neurogenic Speech Sound Impairments
1.3. The Role of Rhythm in Speech Perception: Theories of Rhythmic Entrainment
1.4. Rhythmic Auditory Cueing in Patients with Speech Sound Impairments
1.5. Aims
2. Materials and Methods
2.1. Participants
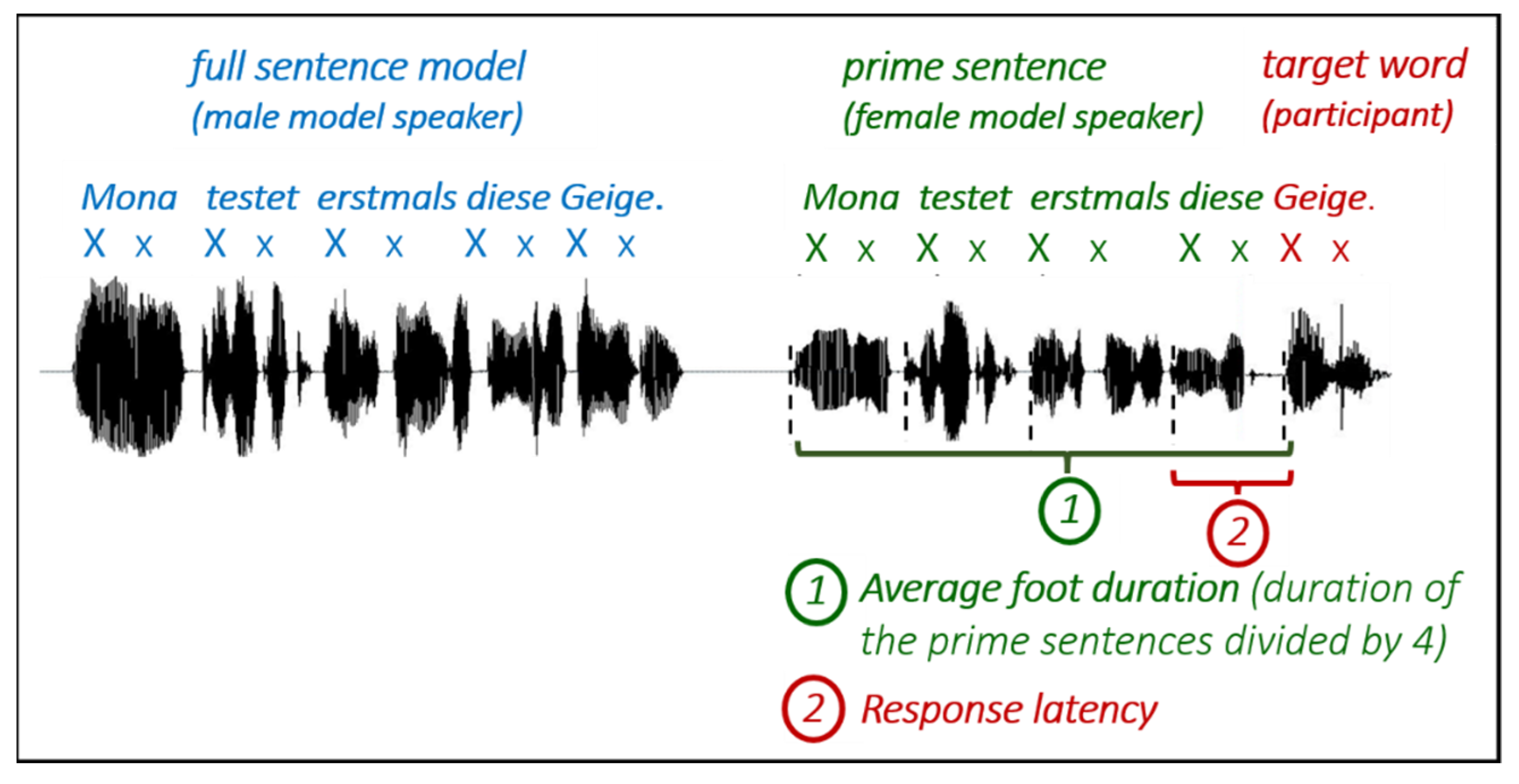
2.2. Materials and Procedure
- (1)
- Regular prime sentence—trochaic target word (Xx Xx Xx Xx-Xx)Example: ’Lena ’pflanzte ’damals ’diese-’Tulpe(engl. Lena planted then this-tulip)
- (2)
- Regular prime sentence—iambic target word (Xx Xx Xx Xx-xX)Example: ’Friedrich ’neckte ’häufig ’diesen-’Te’nor(engl. Friedrich teased often this-tenor)
- (3)
- Irregular prime sentence—trochaic target word (Xx xX xX Xx-Xx)Example: ’Jule ver’schenkt je’doch ’diese-’Tulpe(engl. Jule gives away though this-tulip)
- (4)
- Irregular prime sentence—iambic target word (Xx xX xX Xx-xX)Example: ’Mira be’trog zu’nächst ’diesen-Te’nor(engl. Mira cheated at first this-tenor)
2.3. Data Analyses
2.4. Rhythm Discrimination Task
2.5. Statistical Analyses
3. Results
3.1. Rhythm Discrimination Task
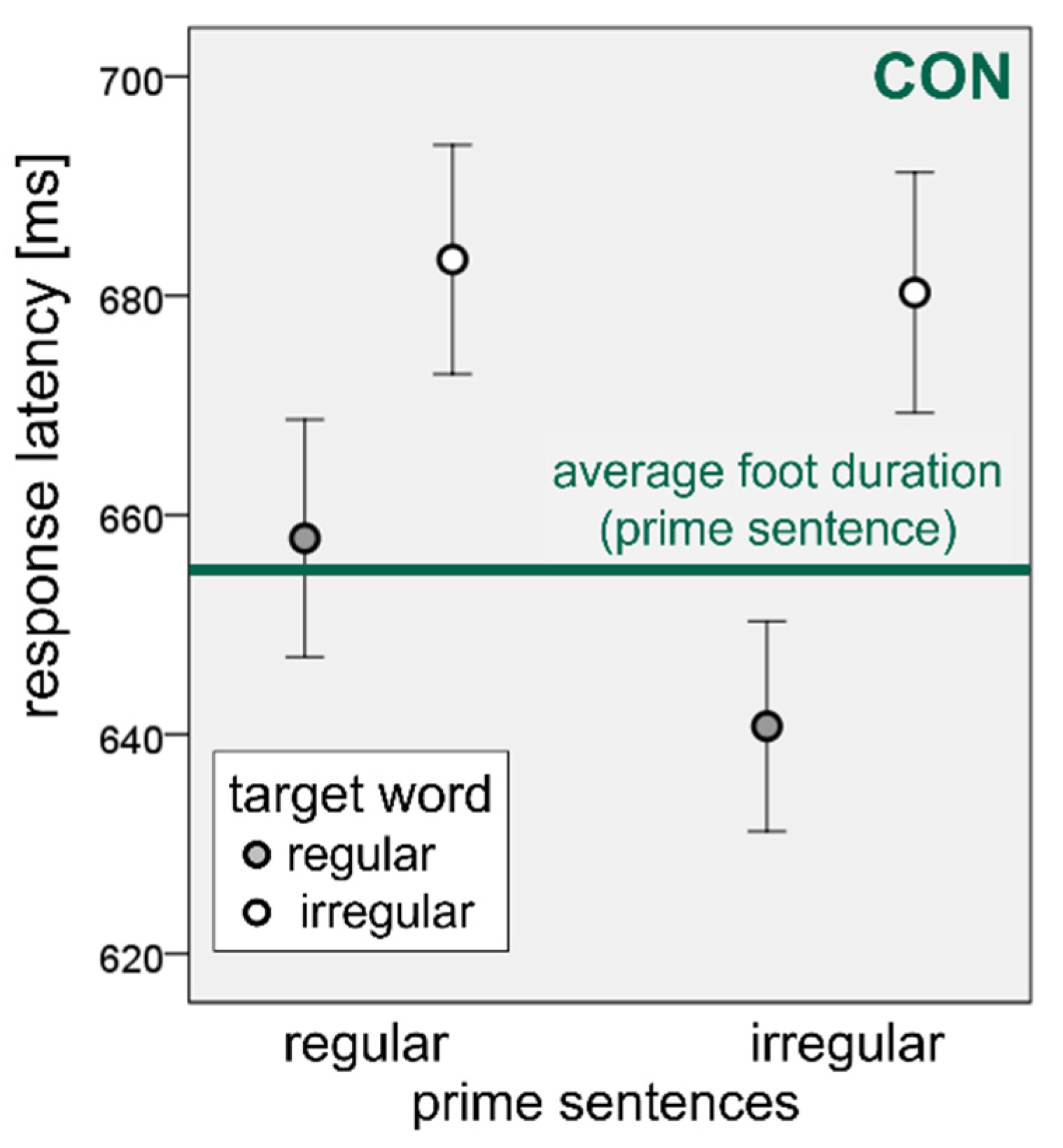
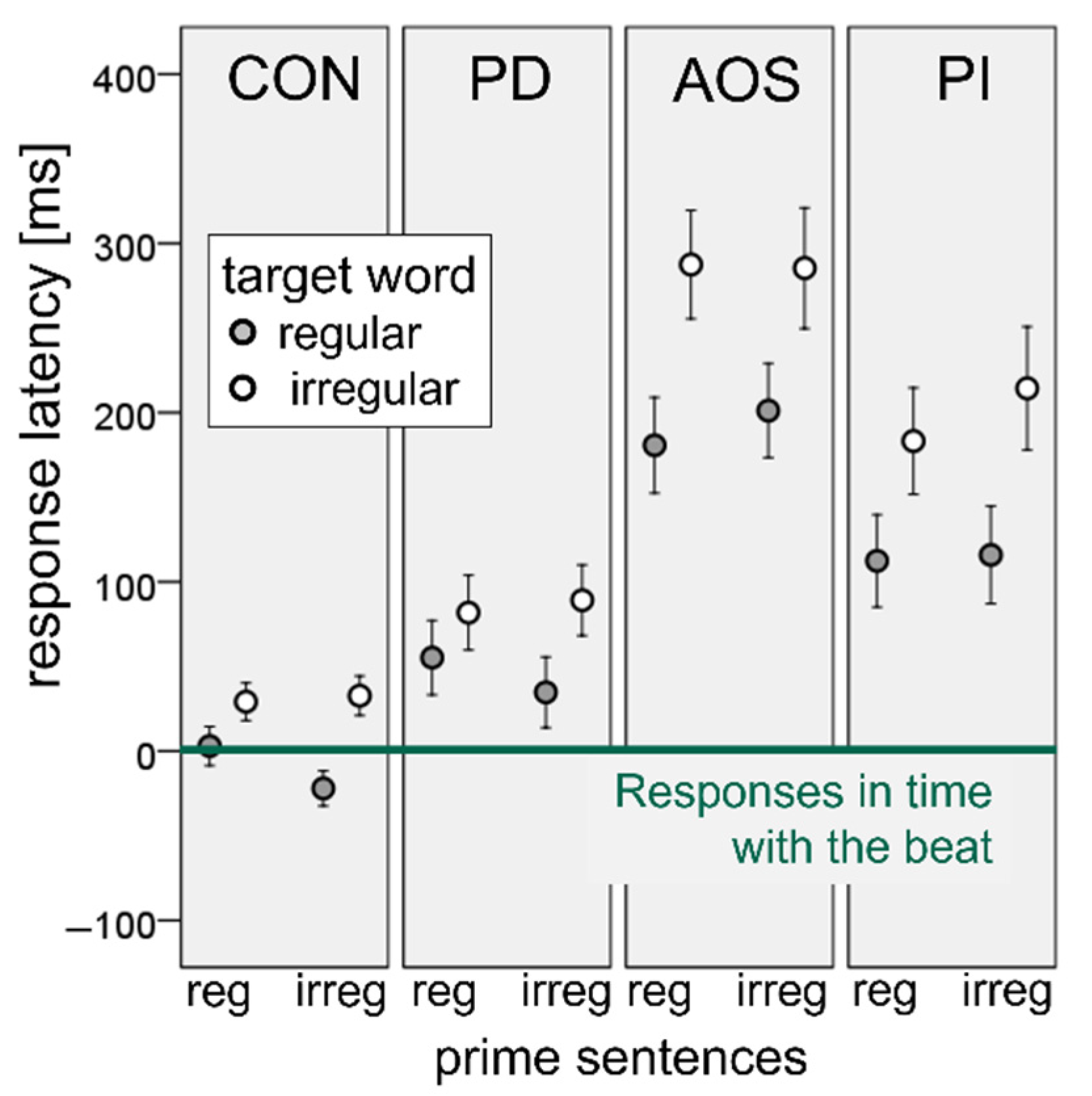
3.2. Sequential Synchronization Paradigm
4. Discussion
4.1. Rhythm Discrimination Task: Were Participants Able to Perceive the Metrical Differences between the Two Priming Conditions?
4.2. Sequential Synchronization Paradigm-“Prime Effect”: Do Participants with Neurogenic Speech Sound Impairments Accommodate Their Speech to the Natural Speech Rhythm of an Auditory Model?
4.3. Sequential Synchronization Paradigm-“Target Effect”: Is There a Facilitation Effect of Regular (Trochaic) Word Stress on Speech Production in Patients with Neurogenic Speech Sound Impairments?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cason, N.; Astésano, C.; Schön, D. Bridging music and speech rhythm: Rhythmic priming and audio–motor training affect speech perception. Acta Psychol. 2015, 155, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Thaut, M.H.; McIntosh, G.C.; Hoemberg, V. Neurobiological foundations of neurologic music therapy: Rhythmic entrainment and the motor system. Front. Psychol. 2015, 5, 1185. [Google Scholar] [CrossRef] [Green Version]
- Falk, S.; Dalla Bella, S. It is better when expected: Aligning speech and motor rhythms enhances verbal processing. Lang. Cogn. Neurosci. 2016, 31, 699–708. [Google Scholar] [CrossRef]
- Lagrois, M.; Palmer, C.; Peretz, I. Poor Synchronization to Musical Beat Generalizes to Speech. Brain Sci. 2019, 9, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repp, B.H. Sensorimotor synchronization: A review of the tapping literature. Psychon. Bull. Rev. 2005, 12, 969–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalla Bella, S.; Białuńska, A.; Sowiński, J. Why movement is captured by music, but less by speech: Role of temporal regularity. PLoS ONE 2013, 8, e71945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.D. Music, Language, and the Brain; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Drake, C.; Jones, M.R.; Baruch, C. The development of rhythmic attending in auditory sequences: Attunement, referent period, focal attending. Cognition 2000, 77, 251–288. [Google Scholar] [CrossRef]
- Post, B.; Payne, E. Speech rhythm in development: What is the child acquiring? In Prosodic Development in First Language Acquisition; Esteve-Gibert, N., Prieto, P., Eds.; John Benjamins: Amsterdam, The Netherlands, 2018; pp. 125–144. [Google Scholar]
- Chen-Hafteck, L. Music and Language Development in Early Childhood: Integrating Past Research in the Two Domains. Early Child Dev. Care 1997, 130, 85–97. [Google Scholar] [CrossRef]
- Fletcher, J. The prosody of speech: Timing and rhythm. In The Handbook of Phonetic Sciences, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 521–602. [Google Scholar]
- Kager, R. Feet and metrical stress. In The Cambridge Handbook of Phonology; Lacy, P., Ed.; Cambridge University Press: Cambridge, UK, 2007; pp. 195–228. [Google Scholar]
- Domahs, U.; Wiese, R.; Bornkessel-Schlesewsky, I.; Schlesewsky, M. The processing of German word stress: Evidence for the prosodic hierarchy. Phonology 2008, 25, 1–36. [Google Scholar] [CrossRef]
- Fèry, C. German word stress in optimality theory. J. Comp. Ger. Linguist. 1998, 2, 101–142. [Google Scholar] [CrossRef]
- Levelt, W.J.M.; Roelofs, A.; Meyer, A.S. A theory of lexical access in speech production. Behav. Brain Sci. 1999, 22, 1–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levelt, W.J.M. Speaking. From Intention to Articulation; MIT Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Roelofs, A.; Meyer, A.S. Metrical structure in planning the production of spoken words. J. Exp. Psychol. Learn. Mem. Cogn. 1998, 24, 922–939. [Google Scholar] [CrossRef]
- Schiller, N.O.; Fikkert, P.; Levelt, C.C. Stress priming in picture naming: An SOA study. Brain Lang. 2004, 90, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Laganaro, M. Phonetic encoding in utterance production: A review of open issues from 1989 to 2018. Lang. Cogn. Neurosci. 2019, 34, 1193–1201. [Google Scholar] [CrossRef]
- Levelt, W.J.M.; Wheeldon, L.R. Do speakers have access to a mental syllabary? Cognition 1994, 50, 239–269. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, L.; Fowler, C.A. Articulatory Phonology: A phonology for public language use. In Phonetics and Phonology in Language Comprehension and Production: Differences and Similarities; Schiller, N.O., Meyer, A.S., Eds.; Mouton de Gruyter: Berlin, Germany, 2003; pp. 159–207. [Google Scholar]
- Ziegler, W. A nonlinear model of word length effects in apraxia of speech. Cogn. Neuropsychol. 2005, 22, 603–623. [Google Scholar] [CrossRef]
- Ziegler, W.; Lehner, K.; Pfab, J.; Aichert, I. The nonlinear gestural model of speech apraxia: Clinical implications and applications. Aphasiology 2021, 35, 462–484. [Google Scholar] [CrossRef]
- Ziegler, W. Modelling the architecture of phonetic plans: Evidence from apraxia of speech. Lang. Cogn. Process. 2009, 24, 631–661. [Google Scholar] [CrossRef]
- Keating, P.A.; Shattuck-Hufnagel, S. A Prosodic View of Word Form Encoding for Speech Production; UCLA: Los Angeles, CA, USA, 2002; pp. 112–156. [Google Scholar]
- Aichert, I.; Ziegler, W. Segmental and metrical encoding in aphasia: Two case reports. Aphsiology 2004, 18, 1201–1211. [Google Scholar] [CrossRef]
- Laganaro, M.; Vacheresse, F.; Frauenfelder, U.H. Selective impairment of lexical stress assignment in an Italian-speaking aphasic patient. Brain Lang. 2002, 81, 601–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickels, L.; Howard, D. Effects of lexical stress on aphasic word production. Clin. Linguist. Phon. 1999, 13, 269–294. [Google Scholar] [CrossRef]
- Janßen, U.; Domahs, F. Going on with optimised feet: Evidence for the interaction between segmental and metrical structure in phonological encoding from a case of primary progressive aphasia. Aphasiology 2008, 22, 1157–1175. [Google Scholar] [CrossRef]
- Ballard, K.J.; Granier, J.P.; Robin, D.A. Understanding the nature of apraxia of speech: Theory, analysis, and treatment. Aphasiology 2000, 14, 969–995. [Google Scholar] [CrossRef]
- Ziegler, W. Phonology vs. phonetics in speech sound disorders. In Speech Motor Control in Normal and Disordered Speech. Future Developments in Theory and Methodology; van Lieshout, P., Maassen, B., Terband, H., Eds.; ASHA Press: Rockville, MD, USA, 2016; pp. 223–255. [Google Scholar]
- McNeil, M.R.; Ballard, K.J.; Duffy, J.R.; Wambaugh, J.L. Apraxia of speech. Theory, assessment, differential diagnosis and treatment: Past, present and future. In Speech Motor Control in Normal and Disordered Speech: Future Developments in Theory and Methodology; Van Lieshout, P., Maassen, B., Terband, H., Eds.; ASHA Press: Rockville, MD, USA, 2016; pp. 195–221. [Google Scholar]
- Croot, K. Diagnosis of AOS: Definition and criteria. Semin. Speech Lang. 2002, 23, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Wambaugh, J.L.; Duffy, J.R.; McNeil, M.R.; Robin, D.A.; Rogers, M.A. Treatment Guidelines for Acquired Apraxia of Speech: A Synthesis and Evaluation of the Evidence. J. Med. Speech Lang. Pathol. 2006, 14, xv. [Google Scholar]
- Aichert, I.; Späth, M.; Ziegler, W. The role of metrical information in apraxia of speech. Perceptual and acoustic analysis of word stress. Neuropsychologia 2016, 82, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.J.; Bunker, L.; Mauszycki, S.; Wambaugh, J.L. Reliability and stability of the metrical stress effect on segmental production accuracy in persons with apraxia of speech. Int. J. Lang. Commun. Disord. 2019, 54, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, W.; Aichert, I. How much is a word? Predicting ease of articulation planning from apraxic speech error patterns. Cortex 2015, 69, 24–39. [Google Scholar] [CrossRef]
- Duffy, J.R. Motor Speech Disorders. Substrates, Differential Diagnosis, and Management, 4th ed.; Elsevier: St. Louis, MO, USA, 2020. [Google Scholar]
- Skodda, S.; Schlegel, U. Speech rate and rhythm in Parkinson’s disease. Mov. Disord. 2008, 23, 985–992. [Google Scholar] [CrossRef]
- Lowit, A.; Marchetti, A.; Corson, S.; Kuschmann, A. Rhythmic performance in hypokinetic dysarthria: Relationship between reading, spontaneous speech and diadochokinetic tasks. J. Commun. Disord. 2018, 72, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.L.; McHugh, A.; Martin, J.G. Reaction time to phoneme targets as a function of rhythmic cues in continuous speech. J. Exp. Psychol. 1974, 102, 250–255. [Google Scholar] [CrossRef]
- Meltzer, R.H.; Martin, J.G.; Mills, C.B.; Imhoff, D.L.; Zohar, D. Reaction time to temporally-displaced phoneme targets in continuous speech. J. Exp. Psychol. Hum. Percept. Perform. 1976, 2, 277–290. [Google Scholar] [CrossRef]
- Pitt, M.A.; Samuel, A.G. The use of rhythm in attending to speech. J. Exp. Psychol. Hum. Percept. Perform. 1990, 16, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Cason, N.; Schon, D. Rhythmic priming enhances the phonological processing of speech. Neuropsychologia 2012, 50, 2652–2658. [Google Scholar] [CrossRef]
- Schmidt-Kassow, M.; Kotz, S.A. Event-related brain potentials suggest a late interaction of meter and syntax in the P600. J. Cogn. Neurosci. 2009, 21, 1693–1708. [Google Scholar] [CrossRef] [PubMed]
- Rothermich, K.; Schmidt-Kassow, M.; Schwartze, M.; Kotz, S.A. Event-related potential responses to metric violations: Rules versus meaning. NeuroReport 2010, 21, 580–584. [Google Scholar] [CrossRef]
- Rothermich, K.; Schmidt-Kassow, M.; Kotz, S.A. Rhythm's gonna get you: Regular meter facilitates semantic sentence processing. Neuropsychologia 2012, 50, 232–244. [Google Scholar] [CrossRef]
- Falk, S.; Lanzilotti, C.; Schön, D. Tuning Neural Phase Entrainment to Speech. J. Cogn. Neurosci. 2017, 29, 1378–1389. [Google Scholar] [CrossRef]
- Roncaglia-Denissen, M.P.; Schmidt-Kassow, M.; Kotz, S.A. Speech rhythm facilitates syntactic ambiguity resolution: ERP evidence. PLoS ONE 2013, 8, e56000. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Kassow, M.; Kotz, S.A. Entrainment of syntactic processing? ERP-responses to predictable time intervals during syntactic reanalysis. Brain Res. 2008, 1226, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Giraud, A.; Poeppel, D. Cortical oscillations and speech processing: Emerging computational principles and operations. Nat. Neurosci. 2012, 15, 511–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donhauser, P.W.; Baillet, S. Two Distinct Neural Timescales for Predictive Speech Processing. Neuron 2020, 105, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.; Gross, J.; Thut, G. A New Unifying Account of the Roles of Neuronal Entrainment. Curr. Biol. 2019, 29, R890–R905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etard, O.; Reichenbach, T. Neural Speech Tracking in the Theta and in the Delta Frequency Band Differentially Encode Clarity and Comprehension of Speech in Noise. J. Neurosci. 2019, 39, 5750–5759. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.K.; McGettigan, C.; Eisner, F. A little more conversation, a little less action—candidate roles for the motor cortex in speech perception. Nat. Rev. Neurosci. 2009, 10, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummins, F. Rhythm as an affordance for the entrainment of movement. Phonetica 2009, 66, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.P. Studies on the metronome effect on stuttering. Behav. Res. Ther. 1969, 7, 197–204. [Google Scholar] [CrossRef]
- Ingham, R.J.; Bothe, A.K.; Jang, E.; Yates, L.; Cotton, J.; Seybold, I. Measurement of speech effort during fluency-inducing conditions in adults who do and do not stutter. J. Speech Lang. Hear. Res. 2009, 52, 1286–1301. [Google Scholar] [CrossRef] [Green Version]
- Zumbansen, A.; Tremblay, P. Music-based interventions for aphasia could act through a motor-speech mechanism: A systematic review and case–control analysis of published individual participant data. Aphasiology 2019, 33, 466–497. [Google Scholar] [CrossRef]
- Square, P.; Martin, R.E.; Bose, A. Nature and treatment of neuromotor speech disorders in aphasia. In Language Intervention Strategies in Aphasia and Related Neurogenic Communication Disorders, 4th ed.; Chapey, R., Ed.; Lippincott Williams & Williams: Philadelphia, PA, USA, 2001; pp. 847–884. [Google Scholar]
- Wertz, R.T.; Lapointe, L.L.; Rosenbek, J.C. Apraxia of Speech in Adults: The Disorder and Its Management; Grune & Stratton: Orlando, FL, USA, 1984. [Google Scholar]
- Rubow, R.T.; Rosenbek, J.; Collins, M.; Longstreth, D. Vibrotactile stimulation for intersystemic reorganization in the treatment of apraxia of speech. Arch. Phys. Med. Rehabil. 1982, 63, 150–153. [Google Scholar] [PubMed]
- Wambaugh, J.L.; Martinez, A.L. Effects of rate and rhythm control treatment on consonant production accuracy in apraxia of speech. Aphasiology 2000, 14, 851–871. [Google Scholar] [CrossRef]
- Shane, H.C.; Darley, F.L. The effect of auditory rhythmic stimulation on articulatory accuracy in apraxia of speech. Cortex 1978, 14, 444–450. [Google Scholar] [CrossRef]
- Brendel, B.; Ziegler, W. Effectiveness of metrical pacing in the treatment of apraxia of speech. Aphasiology 2008, 22, 77–102. [Google Scholar] [CrossRef]
- Sparks, R.W.; Holland, A.L. Method: Melodic Intonation Therapy for aphasia. J. Speech Hear. Disord. 1976, 41, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.L.; Sparks, R.W.; Helm, N.A. Melodic intonation therapy for aphasia. Arch. Neurol. 1973, 29, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Zumbansen, A.; Peretz, I.; Hébert, S. Melodic Intonation Therapy: Back to Basics for Future Research. Front. Neurol. 2014, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, B.; Kotz, S.A.; Henseler, I.; Turner, R.; Geyer, S. Rhythm in disguise: Why singing may not hold the key to recovery from aphasia. Brain 2011, 134, 3083–3093. [Google Scholar] [CrossRef] [Green Version]
- Mainka, S.; Mallien, G. Rhythmic speech cueing (RSC). In Handbook of Neurologic Music Therapy; Oxford University Press: New York, NY, USA, 2014; pp. 150–160. [Google Scholar]
- Pilon, M.A.; Mcintosh, K.W.; Thaut, M.H. Auditory vs visual speech timing cues as external rate control to enhance verbal intelligibility in mixed spastic-ataxic dysarthric speakers: A pilot study. Brain Inj. 1998, 12, 793–803. [Google Scholar] [CrossRef]
- Thaut, M.H.; Mcintosh, K.W.; McIntosh, G.C.; Hoemberg, V. Auditory rhythmicity enhances movement and speech motor control in patients with Parkinson's disease. Funct. Neurololy 2001, 16, 163–172. [Google Scholar]
- Skodda, S.; Flasskamp, A.; Schlegel, U. Instability of syllable repetition as a model for impaired motor processing: Is Parkinson’s disease a “rhythm disorder”? J. Neural. Transm. 2010, 117, 605–612. [Google Scholar] [CrossRef]
- Spaulding, S.J.; Barber, B.; Colby, M.; Cormack, B.; Mick, T.; Jenkins, M.E. Cueing and Gait Improvement Among People with Parkinson's Disease: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2013, 94, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Späth, M.; Aichert, I.; Ceballos-Baumann, A.; Wagner-Sonntag, E.; Miller, N.; Ziegler, W. Entraining with another person’s speech rhythm: Evidence from healthy speakers and individuals with Parkinson’s disease. Clin. Linguist. Phon. 2016, 30, 68–85. [Google Scholar] [CrossRef]
- Aichert, I.; Lehner, K.; Falk, S.; Späth, M.; Ziegler, W. Do Patients With Neurogenic Speech Sound Impairments Benefit From Auditory Priming With a Regular Metrical Pattern? J. Speech Lang. Hear. Res. 2019, 62, 3104–3118. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Poeck, K.; Weniger, D.; Willmes, K. Aachener Apasie Test (AAT); Hogrefe: Göttingen, Germany, 1983. [Google Scholar]
- Stadie, N.; Cholewa, J.; De Bleser, R. LEMO 2.0. Lexikon Modellorientiert—Diagnostik Für Aphasie, Dyslexie Und Dysgraphie; NAT-Verlag: Hofheim, Germany, 2013. [Google Scholar]
- Ziegler, W.; Aichert, I.; Staiger, A.; Schimeczek, M. HWL-Kompakt. Available online: https://neurophonetik.de/sprechapraxie-wortlisten (accessed on 30 July 2021).
- Baayen, R.H.; Piepenbrock, R.; Gulikers, L. The CELEX Lexical Database (CD-ROM). Linguistic Data Consortium; University of Pennsylvania: Philadelphia, PA, USA, 1995. [Google Scholar]
- Peirce, J.W. PsychoPy—Psychophysics Software in Python. J. Neurosci. Methods 2007, 162, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boersma, P.; Weenink, D. PRAAT: Doing Phonetics by Computer [Computer Program], Version 5.3.42; 2013. Available online: https://www.fon.hum.uva.nl/praat/ (accessed on 8 February 2021).
- Team, R.C. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 30 July 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Large, E.; Jones, M. The dynamics of attending: How people track time-varying events. Psychol. Rev. 1999, 106, 119–159. [Google Scholar] [CrossRef]
- Jones, M.; Moynihan, H.; MacKenzie, N.; Puente, J. Temporal Aspects of Stimulus-Driven Attending in Dynamic Arrays. Psychol. Sci. 2002, 13, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J. Grouping and short-term memory: Different means and patterns of grouping. Q. J. Exp. Psychol. 1969, 21, 137–147. [Google Scholar] [CrossRef]
- Hartley, T.; Hurlstone, M.J.; Hitch, G.J. Effects of rhythm on memory for spoken sequences: A model and tests of its stimulus-driven mechanism. Cogn. Psychol. 2016, 87, 135–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grahn, J.A.; Brett, M. Impairment of beat-based rhythm discrimination in Parkinson’s disease. Cortex 2009, 45, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.R.; Jahanshahi, M. Motor and perceptual timing in Parkinson’s disease. Adv. Exp. Med. Biol. 2014, 829, 265–290. [Google Scholar] [CrossRef]
- Watkins, K.E.; Strafella, A.P.; Paus, T. Seeing and hearing speech excites the motor system involved in speech production. Neuropsychologia 2003, 41, 989–994. [Google Scholar] [CrossRef]
- Ziegler, W.; Ackermann, H. Subcortical Contributions to Motor Speech: Phylogenetic, Developmental, Clinical. Trends Neurosci. 2017, 40, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A. The Role of the Basal Ganglia in Beat Perception. Ann. N. Y. Acad. Sci. 2009, 1169, 35–45. [Google Scholar] [CrossRef]
- Shi, E.R.; Zhang, Q. A domain-general perspective on the role of the basal ganglia in language and music: Benefits of music therapy for the treatment of aphasia. Brain Lang. 2020, 206, 104811. [Google Scholar] [CrossRef]
- Kotz, S.A.; Schmidt-Kassow, M. Basal ganglia contribution to rule expectancy and temporal predictability in speech. Cortex 2015, 68, 48–60. [Google Scholar] [CrossRef]
- Kotz, S.A.; Gunter, T.C. Can rhythmic auditory cuing remediate language-related deficits in Parkinson's disease? Ann. N. Y. Acad. Sci. 2015, 1337, 62–68. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, G.C.; Brown, S.H.; Rice, R.R.; Thaut, M.H. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 1997, 62, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, D.; Smith, K. The effects of lexical stress in aphasic word production. Aphasiology 2002, 16, 198–237. [Google Scholar] [CrossRef]
| Demographics and Test Scores | Apraxia of Speech (AOS; n = 12) | Phonological Impairment (PI; n = 12) | Hypokinetic Dysarthria (PD; n = 12) |
|---|---|---|---|
| Age in years: median (range) | 55 (30–78) | 60 (41–73) | 75 (54–83) |
| Months post onset: median (range) | 63 (2–191) | 6 (1–97) | 63 (9–213) |
| Word repetition accuracy 1 | |||
| Phonetic | 44 (2–75) | 93 (73–100) | 97 (89–100) |
| Phonemic | 64 (19–94) | 78 (29–96) | 100 (99–100) |
| Fluency | 59 (6–90) | 78 (42–98) | 99 (94–100) |
| Auditory word discrimination (LEMO, test V1 2) | 10 unimpaired, 1 at threshold, 1 impaired | 6 unimpaired, 6 impaired | 9 unimpaired, 3 impaired |
| Auditory word-picture matching (LEMO, test 11 2) | 8 unimpaired, 1 at threshold, 3 impaired | 7 unimpaired, 3 at threshold, 2 impaired | 12 unimpaired |
| (LEMO, test 11 2) | |||
| (LEMO, test 11 2) | |||
| Verbal naming (LEMO, test 13 2,3) | 3 unimpaired, 9 impaired | 1 unimpaired, 2 at threshold, 9 impaired | 10 unimpaired, 2 at threshold |
| Stimulus 1 | Regular | Irregular | ||||
|---|---|---|---|---|---|---|
| Stimulus 2 | Regular | Irregular | Total | Regular | Irregular | Total |
| AOS | 8 (27.8) | 3 (17.5) | 6 (9.7) | 59 (49.4) | 29 (45.7) | 44 (31.5) |
| PI | 5 (22.3) | 10 (30.7) | 8 (13.2 | 43 (49.7) | 42 (49.6) | 42 (25.5) |
| PD | 11 (31.9) | 6 (23.3) | 9 (16.5) | 32 (46.8) | 33 (47.3) | 32 (28.0) |
| CON | 1 (11.7) | 1 (8.3) | 1 (3.5) | 14 (34.3) | 9 (28.7) | 11 (18.4) |
| Total | 5 (21.3) | 4 (18.4) | 4 (10.1) | 29 (45.4) | 22 (41.2) | 25 (27.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aichert, I.; Lehner, K.; Falk, S.; Späth, M.; Franke, M.; Ziegler, W. In Time with the Beat: Entrainment in Patients with Phonological Impairment, Apraxia of Speech, and Parkinson’s Disease. Brain Sci. 2021, 11, 1524. https://doi.org/10.3390/brainsci11111524
Aichert I, Lehner K, Falk S, Späth M, Franke M, Ziegler W. In Time with the Beat: Entrainment in Patients with Phonological Impairment, Apraxia of Speech, and Parkinson’s Disease. Brain Sciences. 2021; 11(11):1524. https://doi.org/10.3390/brainsci11111524
Chicago/Turabian StyleAichert, Ingrid, Katharina Lehner, Simone Falk, Mona Späth, Mona Franke, and Wolfram Ziegler. 2021. "In Time with the Beat: Entrainment in Patients with Phonological Impairment, Apraxia of Speech, and Parkinson’s Disease" Brain Sciences 11, no. 11: 1524. https://doi.org/10.3390/brainsci11111524
APA StyleAichert, I., Lehner, K., Falk, S., Späth, M., Franke, M., & Ziegler, W. (2021). In Time with the Beat: Entrainment in Patients with Phonological Impairment, Apraxia of Speech, and Parkinson’s Disease. Brain Sciences, 11(11), 1524. https://doi.org/10.3390/brainsci11111524






