Predicting the Extent of Resection of Motor-Eloquent Gliomas Based on TMS-Guided Fiber Tracking
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patients and Study Design
2.3. Image Acquisition
2.4. Navigated TMS
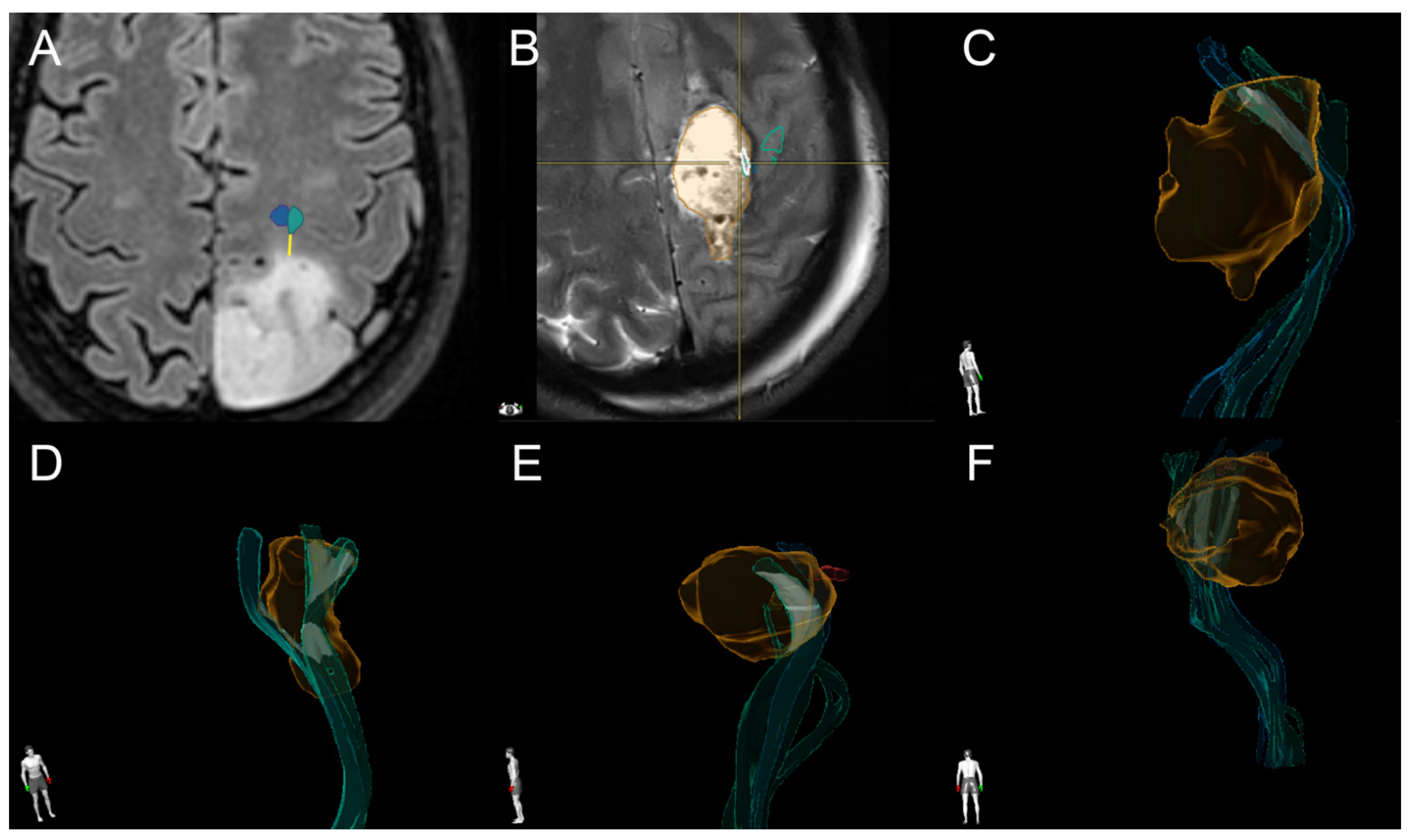
2.5. Tractography
2.6. IOM
2.7. Postoperative Image Analysis
2.8. Statistics
3. Results
3.1. Characteristics of Patients and Tumors
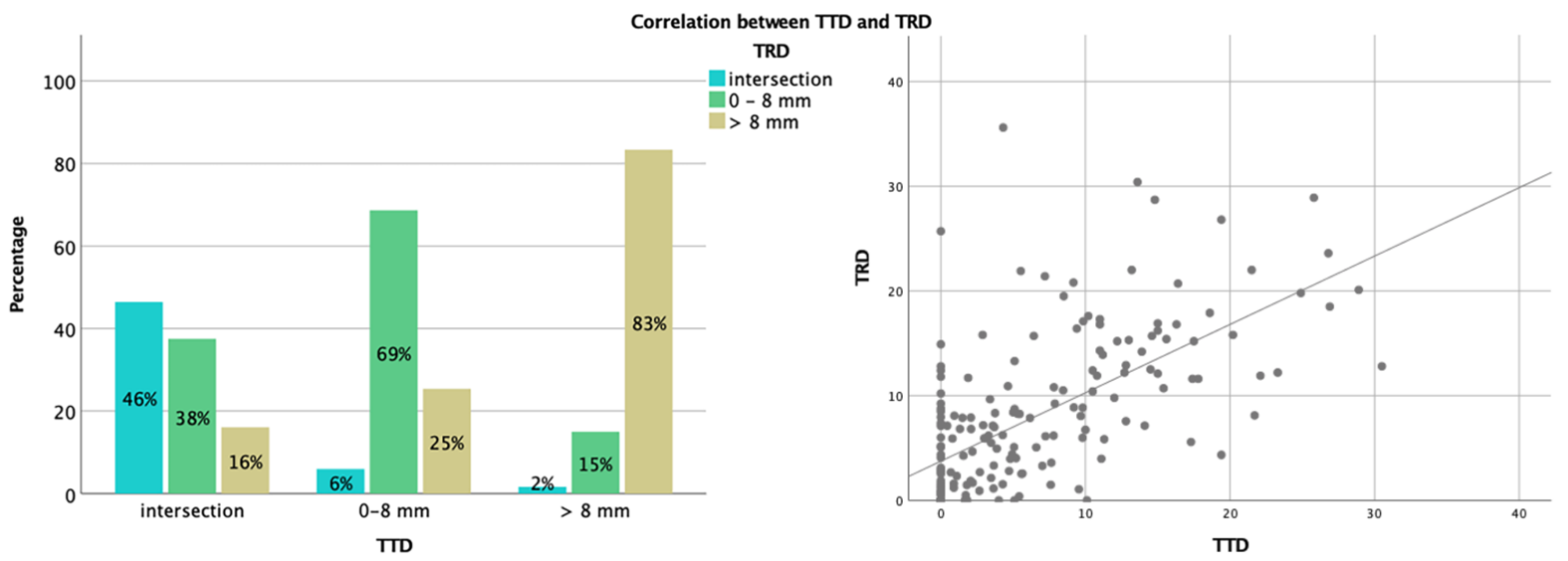
3.2. Analysis of TTD and TRD
3.3. Correlation between TTD, TRD, and Extent of Resection (or Residual Volume)
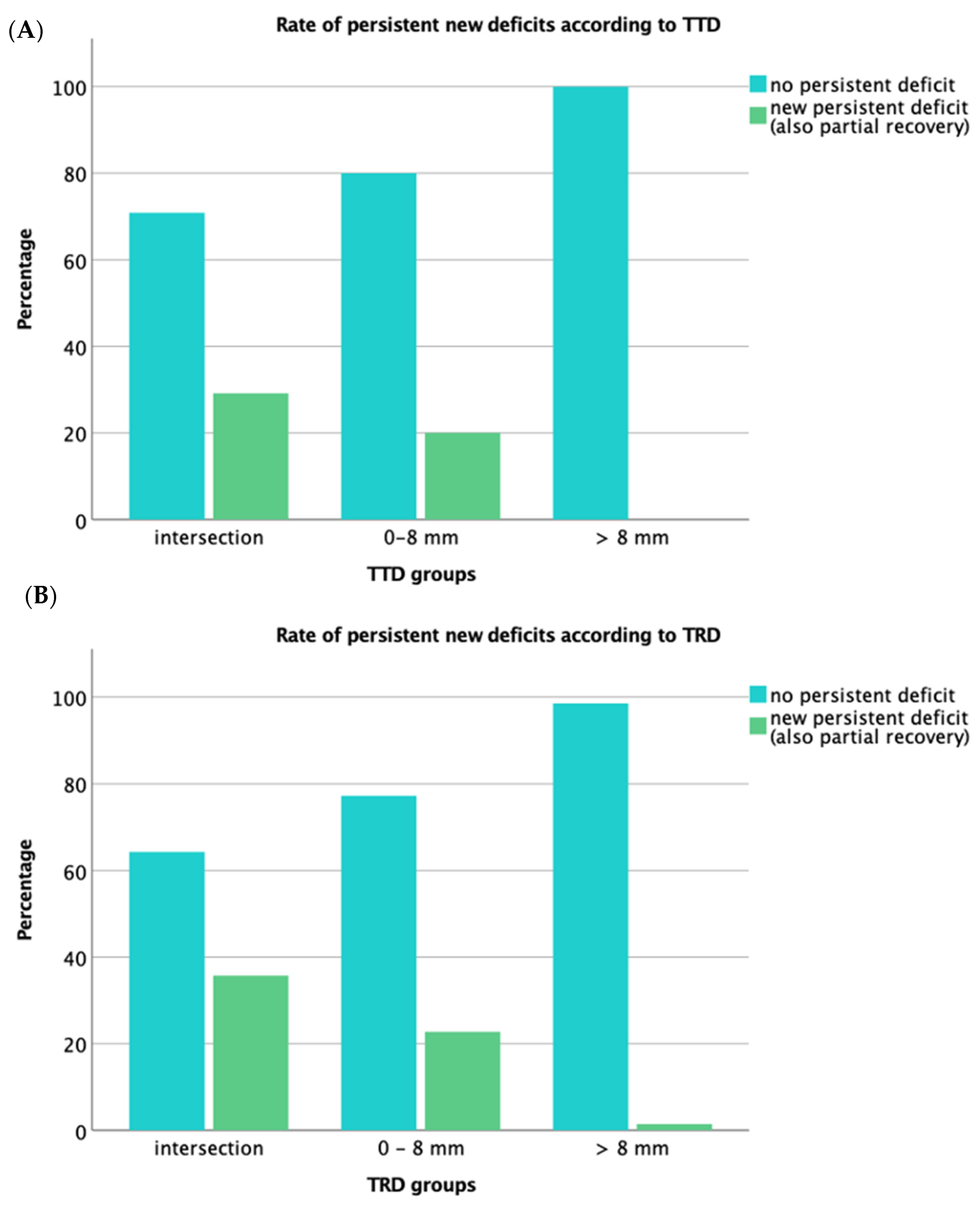
3.4. Functional Outcome
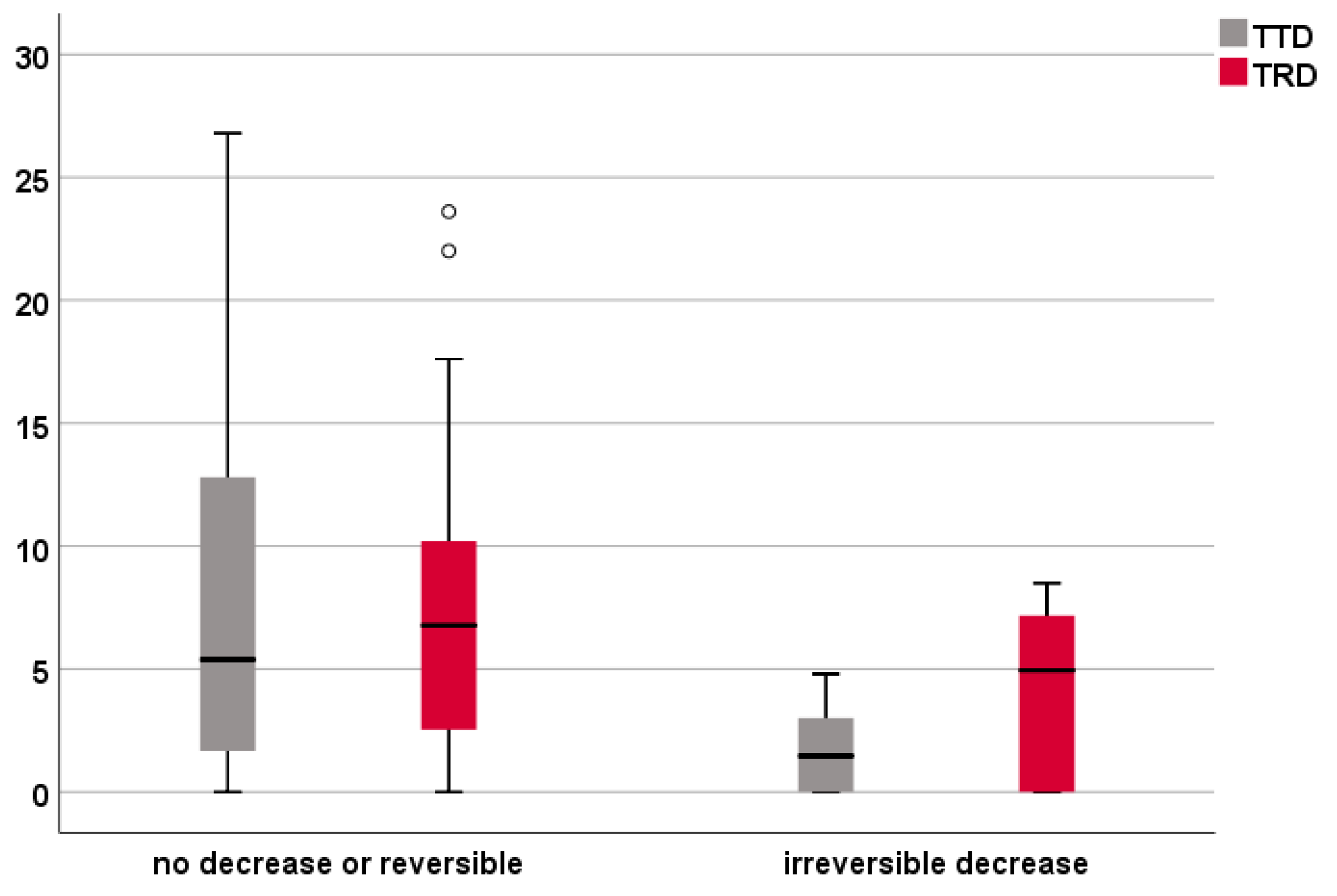
3.5. Analysis of the IOM Subgroup
4. Discussion
- Resection stopped according to IOM to avoid postoperative deficits, leading to an EOR lower than planned (unexpected MEP signal);
- MEP decrease at a certain distance from the CST, as planned, but reaching that distance in only one trajectory (expected MEP response as stop signal, but overestimating the surrounding resection);
- Resection stopped for other anatomical reasons;
- Premature termination of resection due to unclear reasons.
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almenawer, S.A.; Badhiwala, J.H.; Alhazzani, W.; Greenspoon, J.; Farrokhyar, F.; Yarascavitch, B.; Algird, A.; Kachur, E.; Cenic, A.; Sharieff, W.; et al. Biopsy versus Partial versus Gross Total Resection in Older Patients with High-Grade Glioma: A Systematic Review and Meta-Analysis. Neuro Oncol. 2015, 17, 868–881. [Google Scholar] [CrossRef] [Green Version]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgård, G.; Solheim, O. Comparison of a Strategy Favoring Early Surgical Resection vs a Strategy Favoring Watchful Waiting in Low-Grade Gliomas. JAMA 2012, 308, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Kim, D.G.; Kim, J.W.; Han, J.H.; Kim, Y.H.; Park, C.K.; Kim, C.Y.; Paek, S.H.; Jung, H.W. The Role of Surgical Resection in the Management of Brain Metastasis: A 17-Year Longitudinal Study. Acta Neurochir. (Wien) 2013, 155, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of Resection and Survival in Glioblastoma Multiforme: Identification of and Adjustment for Bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [PubMed]
- De Witt Hamer, P.C.; Robles, S.G.; Zwinderman, A.H.; Duffau, H.; Berger, M.S. Impact of Intraoperative Stimulation Brain Mapping on Glioma Surgery Outcome: A Meta-Analysis. J. Clin. Oncol. 2012, 30, 2559–2565. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Berger, M.S. Glioma Extent of Resection and Its Impact on Patient Outcome. Neurosurgery 2008, 62, 753–764, discussion 264–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quiñones-Hinojosa, A.R. Independent Association of Extent of Resection with Survival in Patients with Malignant Brain Astrocytoma. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Meyer, E.J.; Gaggl, W.; Gilloon, B.; Swan, B.; Greenstein, M.; Voss, J.; Hussain, N.; Holdsworth, R.L.; Nair, V.A.; Meyerand, M.E.; et al. The Impact of Intracranial Tumor Proximity to White Matter Tracts on Morbidity and Mortality: A Retrospective Diffusion Tensor Imaging Study. Neurosurgery 2017, 80, 193–200. [Google Scholar] [CrossRef]
- Costabile, J.D.; Alaswad, E.; D’Souza, S.; Thompson, J.A.; Ormond, D.R. Current Applications of Diffusion Tensor Imaging and Tractography in Intracranial Tumor Resection. Front. Oncol. 2019, 9, 426. [Google Scholar] [CrossRef]
- Duffau, H.; Lopes, M.; Arthuis, F.; Bitar, A.; Sichez, J.P.; Van Effenterre, R.; Capelle, L. Contribution of Intraoperative Electrical Stimulations in Surgery of Low Grade Gliomas: A Comparative Study between Two Series without (1985–96) and with (1996–2003) Functional Mapping in the Same Institution. J. Neurol. Neurosurg. Psychiatry 2005, 76, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Kombos, T.; Picht, T.; Derdilopoulos, A.; Suess, O. Impact of Intraoperative Neurophysiological Monitoring on Surgery of High-Grade Gliomas. J. Clin. Neurophysiol. 2009, 26, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Raffa, G.; Granata, F.; Rizzo, V.; Germanò, A.; Tomasello, F. Navigated Transcranial Magnetic Stimulation for “Somatotopic” Tractography of the Corticospinal Tract. Neurosurgery 2014, 10 (Suppl. 4), 542–554, discussion 554. [Google Scholar] [CrossRef] [PubMed]
- Frey, D.; Schilt, S.; Strack, V.; Zdunczyk, A.; Rösler, J.; Niraula, B.; Vajkoczy, P.; Picht, T. Navigated Transcranial Magnetic Stimulation Improves the Treatment Outcome in Patients with Brain Tumors in Motor Eloquent Locations. Neuro Oncol. 2014, 16, 1365–1372. [Google Scholar] [CrossRef] [Green Version]
- Raffa, G.; Conti, A.; Scibilia, A.; Sindorio, C.; Quattropani, M.C.; Visocchi, M.; Germanò, A.; Tomasello, F. Functional Reconstruction of Motor and Language Pathways Based on Navigated Transcranial Magnetic Stimulation and DTI Fiber Tracking for the Preoperative Planning of Low Grade Glioma Surgery: A New Tool for Preservation and Restoration of Eloquent Networks. Acta Neurochir. Suppl. 2017, 124, 251–261. [Google Scholar] [PubMed]
- Picht, T.; Mularski, S.; Kuehn, B.; Vajkoczy, P.; Kombos, T.; Suess, O. Navigated Transcranial Magnetic Stimulation for Preoperative Functional Diagnostics in Brain Tumor Surgery. Neurosurgery 2009, 65, 93–98, discussion 98–99. [Google Scholar] [CrossRef]
- Picht, T.; Schmidt, S.; Brandt, S.; Frey, D.; Hannula, H.; Neuvonen, T.; Karhu, J.; Vajkoczy, P.; Suess, O. Preoperative Functional Mapping for Rolandic Brain Tumor Surgery: Comparison of Navigated Transcranial Magnetic Stimulation to Direct Cortical Stimulation. Neurosurgery 2011, 69, 581–588, discussion 588. [Google Scholar] [CrossRef]
- Tarapore, P.E.; Tate, M.C.; Findlay, A.M.; Honma, S.M.; Mizuiri, D.; Berger, M.S.; Nagarajan, S.S. Preoperative Multimodal Motor Mapping: A Comparison of Magnetoencephalography Imaging, Navigated Transcranial Magnetic Stimulation, and Direct Cortical Stimulation. J. Neurosurg. 2012, 117, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Krieg, S.M.; Shiban, E.; Buchmann, N.; Gempt, J.; Foerschler, A.; Meyer, B.; Ringel, F. Utility of Presurgical Navigated Transcranial Magnetic Brain Stimulation for the Resection of Tumors in Eloquent Motor Areas. J. Neurosurg. 2012, 116, 994–1001. [Google Scholar] [CrossRef]
- Picht, T.; Schulz, J.; Hanna, M.; Schmidt, S.; Suess, O.; Vajkoczy, P. Assessment of the Influence of Navigated Transcranial Magnetic Stimulation on Surgical Planning for Tumors in or near the Motor Cortex. Neurosurgery 2012, 70, 1248–1256, discussion 1256–1257. [Google Scholar] [CrossRef] [Green Version]
- Krieg, S.M.; Sabih, J.; Bulubasova, L.; Obermueller, T.; Negwer, C.; Janssen, I.; Shiban, E.; Meyer, B.; Ringel, F. Preoperative Motor Mapping by Navigated Transcranial Magnetic Brain Stimulation Improves Outcome for Motor Eloquent Lesions. Neuro Oncol. 2014, 16, 1274–1282. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, T.; Grittner, U.; Acker, G.; Schwarzer, V.; Kulchytska, N.; Vajkoczy, P.; Picht, T. Risk Stratification in Motor Area-Related Glioma Surgery Based on Navigated Transcranial Magnetic Stimulation Data. J. Neurosurg. 2017, 126, 1227–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenstock, T.; Giampiccolo, D.; Schneider, H.; Runge, S.J.; Bährend, I.; Vajkoczy, P.; Picht, T. Specific DTI Seeding and Diffusivity-Analysis Improve the Quality and Prognostic Value of TMS-Based Deterministic DTI of the Pyramidal Tract. Neuroimage Clin. 2017, 16, 276–285. [Google Scholar] [CrossRef]
- Frey, D.; Strack, V.; Wiener, E.; Jussen, D.; Vajkoczy, P.; Picht, T. A New Approach for Corticospinal Tract Reconstruction Based on Navigated Transcranial Stimulation and Standardized Fractional Anisotropy Values. Neuroimage 2012, 62, 1600–1609. [Google Scholar] [CrossRef]
- Gerhardt, J.; Sollmann, N.; Hiepe, P.; Kirschke, J.S.; Meyer, B.; Krieg, S.M.; Ringel, F. Retrospective Distortion Correction of Diffusion Tensor Imaging Data by Semi-Elastic Image Fusion-Evaluation by Means of Anatomical Landmarks. Clin. Neurol. Neurosurg. 2019, 183, 105387. [Google Scholar] [CrossRef]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-Invasive Magnetic Stimulation of Human Motor Cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Rossini, P.M.; Burke, D.; Chen, R.; Cohen, L.G.; Daskalakis, Z.; Di Iorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald, P.B.; George, M.S.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord, Roots and Peripheral Nerves: Basic Principles and Procedures for Routine Clinical and Research Application. An Updated Report from an I.F.C.N. Committee. Clin. Neurophysiol. 2015, 126, 1071–1107. [Google Scholar] [CrossRef]
- Mori, S.; Crain, B.J.; Chacko, V.P.; van Zijl, P.C. Three-Dimensional Tracking of Axonal Projections in the Brain by Magnetic Resonance Imaging. Ann. Neurol. 1999, 45, 265–269. [Google Scholar] [CrossRef]
- Zolal, A.; Hejčl, A.; Vachata, P.; Bartoš, R.; Humhej, I.; Malucelli, A.; Nováková, M.; Hrach, K.; Derner, M.; Sameš, M. The Use of Diffusion Tensor Images of the Corticospinal Tract in Intrinsic Brain Tumor Surgery. Neurosurgery 2012, 71, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Tuncer, M.S.; Salvati, L.F.; Grittner, U.; Hardt, J.; Schilling, R.; Bährend, I.; Silva, L.L.; Fekonja, L.S.; Faust, K.; Vajkoczy, P.; et al. Towards a Tractography-Based Risk Stratification Model for Language Area Associated Gliomas. NeuroImage Clin. 2021, 29, 102541. [Google Scholar] [CrossRef]
- Schilling, K.G.; Nath, V.; Hansen, C.; Parvathaneni, P.; Blaber, J.; Gao, Y.; Neher, P.; Aydogan, D.B.; Shi, Y.; Ocampo-Pineda, M.; et al. Limits to Anatomical Accuracy of Diffusion Tractography Using Modern Approaches. Neuroimage 2019, 185, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydogan, D.B.; Jacobs, R.; Dulawa, S.; Thompson, S.L.; Francois, M.C.; Toga, A.W.; Dong, H.; Knowles, J.A.; Shi, Y. When Tractography Meets Tracer Injections: A Systematic Study of Trends and Variation Sources of Diffusion-Based Connectivity. Brain Struct. Funct. 2018, 223, 2841–2858. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hein, K.H.; Neher, P.F.; Houde, J.C.; Côté, M.A.; Garyfallidis, E.; Zhong, J.; Chamberland, M.; Yeh, F.C.; Lin, Y.C.; Ji, Q.; et al. The Challenge of Mapping the Human Connectome Based on Diffusion Tractography. Nat. Commun. 2017, 8, 1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tournier, J.D. Diffusion MRI in the Brain-Theory and Concepts. Prog. Nucl. Magn. Reson. Spectrosc. 2019, 112–113, 1–16. [Google Scholar] [CrossRef] [PubMed]
| Demographic Characteristics | ||||||
| Age—years | mean (SD) [range] | 50 (16) [21–81] | ||||
| Sex—no. (%) | males 114 (62%) | females 69 (38%) | ||||
| Clinical characteristics | ||||||
| Preoperative motor function (MRC) no. (%) | ||||||
| 0 | 4 (2.2%) | |||||
| 1 | 2 (1.1%) | |||||
| 2 | -- | |||||
| 3 | 14 (7.7%) | |||||
| 4 | 38 (20.7%) | |||||
| 5 | 126 (68.9%) | |||||
| Functional outcome at 7 days after surgery—no. (%) | Functional outcome at 3 months after surgery—no. (%) | |||||
| Improved | 18 (9.8%) | 22 (13.4%) | ||||
| Stable | 121 (66.1%) | 111 (67.7%) | ||||
| Worsened | 44 (24.0%) | 31 (18.9%) | ||||
| Tumor characteristics | ||||||
| Tumor Location—no. (%) | ||||||
| Frontal | Parietal | Temporal | Insular | |||
| total | 68 * (37.2%) | total | 52 (28.4%) | total | 15 (8.2%) | 23 (12.6%) |
| precentral | 17 (9.3%) | postcentral | 23 (12.6%) | temporo-occipital | 1 (0.5%) | |
| fronto-temporal | 4 (2.2%) | parieto-occipital | 6 * (3.3%) | |||
| fronto-parietal (central region) | 27 (14.8%) | parieto-temporal | 2 (1.1%) | |||
| parieto-temporo-occipital | 1 (0.5%) | |||||
| WHO tumor grade—no. (%) | Affected hemisphere—no. (%) | |||||
| I | 3 (1.6%) | right | 98 (53.6%) | |||
| II | 29 (15.8%) | left | 84 (45.9%) | |||
| III | 44 (24.0%) | both | 1 (0.5%) | |||
| IV | 107 (58.5%) | |||||
| Tumor volume and surgical results | ||||||
| Mean (SD) | Median (interquartile range—IQR) | |||||
| Tumor volume—mL | 39 (38) | 26 (13–53) | ||||
| Residual volume—mL | 5 (15) | 0 (0–2) | ||||
| Extent of resection—% | 91.9 (18) | 100 (95–100) | ||||
| CST Infiltration | n | Persistent New Deficit (n=): Infiltration (%) | p-Value | Kendall’s Tau-b |
|---|---|---|---|---|
| TTD | ||||
| intersection | 48 | 14 (29.2%) | <0.001 | 0.30 |
| 0–8 mm | 60 | 12 (20.0%) | ||
| >8 mm | 56 | 0 (0%) | ||
| TRD | ||||
| intersection | 28 | 10 (35.7%) | <0.001 | 0.35 |
| 0–8 mm | 66 | 15 (22.7%) | ||
| >8 mm | 70 | 1 (1.4%) * |
| New Deficit at 7 Days (n = 183) | New Deficit at 3 Months (n = 164) | |
|---|---|---|
| TTD | 0.74 (0.67–0.82) | 0.66 (0.57–0.76) |
| TRD | 0.79 (0.72–0.86) | 0.72 (0.63–0.81) |
| p-value for comparison of ROC curves | 0.241 | 0.137 |
| TTD + TRD | 0.80 (0.67–0.82) | 0.72 (0.63–0.81) |
| Cases with No Decrease or Reversible (n = 42) | Cases with Irreversible Decrease (n = 6) | P (Mann–Whitney U Test) | ||
|---|---|---|---|---|
| TTD | intersection | 9 | 3 | |
| 0–8 mm | 16 | 3 | ||
| >8 mm | 17 | 0 | ||
| median (IQR) | 5.4 (1.6–12.8) mm | 1.5 (0–3.5) mm | 0.033 | |
| TRD | intersection | 5 | 2 | |
| 0–8 mm | 20 | 3 | ||
| >8 mm | 17 | 1 | ||
| median (IQR) | 6.8 (1.4–10.6) mm | 4.9 (0–7.5) mm | 0.249 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belotti, F.; Tuncer, M.S.; Rosenstock, T.; Ivren, M.; Vajkoczy, P.; Picht, T. Predicting the Extent of Resection of Motor-Eloquent Gliomas Based on TMS-Guided Fiber Tracking. Brain Sci. 2021, 11, 1517. https://doi.org/10.3390/brainsci11111517
Belotti F, Tuncer MS, Rosenstock T, Ivren M, Vajkoczy P, Picht T. Predicting the Extent of Resection of Motor-Eloquent Gliomas Based on TMS-Guided Fiber Tracking. Brain Sciences. 2021; 11(11):1517. https://doi.org/10.3390/brainsci11111517
Chicago/Turabian StyleBelotti, Francesco, Mehmet Salih Tuncer, Tizian Rosenstock, Meltem Ivren, Peter Vajkoczy, and Thomas Picht. 2021. "Predicting the Extent of Resection of Motor-Eloquent Gliomas Based on TMS-Guided Fiber Tracking" Brain Sciences 11, no. 11: 1517. https://doi.org/10.3390/brainsci11111517
APA StyleBelotti, F., Tuncer, M. S., Rosenstock, T., Ivren, M., Vajkoczy, P., & Picht, T. (2021). Predicting the Extent of Resection of Motor-Eloquent Gliomas Based on TMS-Guided Fiber Tracking. Brain Sciences, 11(11), 1517. https://doi.org/10.3390/brainsci11111517







