A Retrospective Analysis of Randomized Controlled Trials on Traumatic Brain Injury: Evaluation of CONSORT Item Adherence
Abstract
:1. Introduction
2. Materials and Methods
Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 18, 56–87. [Google Scholar] [CrossRef] [Green Version]
- Zaloshnja, E.; Miller, T.; Langlois, J.A.; Selassie, A.W. Prevalence of Long-Term Disability from Traumatic Brain Injury in the Civilian Population of the United States, 2005. J. Head Trauma Rehabil. 2008, 23, 394–400. [Google Scholar] [CrossRef] [PubMed]
- A proposal for structured reporting of randomized controlled trials. The Standards of Reporting Trials Group. JAMA 1994, 272, 1926–1931. [CrossRef]
- Working Group on Recommendation for Reporting of Clinical Trials in the Biomedical Literature Call for Comments on a Proposal to Improve Reporting of Clinical Trials in the Biomedical Literature. Ann. Intern. Med. 1994, 121, 894. [CrossRef]
- Altman, D.G. Better reporting of randomised controlled trials: The CONSORT statement. BMJ 1996, 313, 570–571. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.F.; Altman, D.G.; Moher, D.; for the CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010, 7, e1000251. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.K.; Maas, A.I.R. Progress, failures and new approaches for TBI research. Nat. Rev. Neurol. 2015, 11, 71–72. [Google Scholar] [CrossRef]
- Stein, D.G. Embracing failure: What the Phase III progesterone studies can teach about TBI clinical trials. Brain Inj. 2015, 29, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gary, K.W.; Copolillo, A.; Ward, J.; Niemeier, J.P.; Lapane, K.L. Randomized Controlled Trials in Adult Traumatic Brain Injury: A Review of Compliance to CONSORT Statement. Arch. Phys. Med. Rehabil. 2014, 96, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Zafardoost, P.; Ghasemi, A.A.; Salehpour, F.; Piroti, C.; Ziaeii, E. Evaluation of the Effect of Glibenclamide in Patients with Diffuse Axonal Injury Due to Moderate to Severe Head Trauma. Trauma Mon. 2016, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, M.-C.; Wang, Q.-L.; Fang, W.; Jiang, Y.-X.; Li, L.-D.; Sun, P.; Wang, Z.-H. Early Enteral Combined with Parenteral Nutrition Treatment for Severe Traumatic Brain Injury: Effects on Immune Function, Nutritional Status and Outcomes. Chin. Med. Sci. J. 2016, 31, 213–220. [Google Scholar] [CrossRef]
- Rocca, A.; Pignat, J.-M.; Berney, L.; Jöhr, J.; Van De Ville, D.; Daniel, R.T.; Levivier, M.; Hirt, L.; Luft, A.R.; Grouzmann, E.; et al. Sympathetic activity and early mobilization in patients in intensive and intermediate care with severe brain injuries: A preliminary prospective randomized study. BMC Neurol. 2016, 16, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, P.J.; Kolias, A.; Timofeev, I.S.; Corteen, E.A.; Czosnyka, M.; Timothy, J.; Anderson, I.; Bulters, D.; Belli, A.; Eynon, C.A.; et al. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N. Engl. J. Med. 2016, 375, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Bisri, T.; Utomo, B.A.; Fuadi, I. Exogenous lactate infusion improved neurocognitive function of patients with mild traumatic brain injury. Asian J. Neurosurg. 2016, 11, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamani, M.; Namdar, B.; Azizkhani, R.; Ahmadi, O.; Esmailian, M. Comparing the Antiemetic Effects of Ondansetron and Metoclopramide in Patients with Minor Head Trauma. Emergency 2015, 3, 137–140. [Google Scholar]
- Lin, C.-M.; Lin, M.-C.; Huang, S.-J.; Chang, C.-K.; Chao, D.-P.; Lui, T.-N.; Ma, H.-I.; Liu, M.-Y.; Chung, W.-Y.; Shih, Y.-H.; et al. A Prospective Randomized Study of Brain Tissue Oxygen Pressure-Guided Management in Moderate and Severe Traumatic Brain Injury Patients. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregson, B.A.; Rowan, E.N.; Francis, R.; McNamee, P.; Boyers, D.; Mitchell, P.; McColl, E.; Chambers, I.R.; Unterberg, A.; Mendelow, A.D.; et al. Surgical Trial in Traumatic intraCerebral Haemorrhage (STITCH): A randomised controlled trial of Early Surgery compared with Initial Conservative Treatment. Health Technol. Assess. 2015, 19, 1–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aloizos, S.; Evodia, E.; Gourgiotis, S.; Isaia, E.-C.; Seretis, C.; Baltopoulos, G. Neuroprotective effects of erythropoietin in patients with severe closed brain injury. Turk. Neurosurg. 2015, 25. [Google Scholar] [CrossRef] [Green Version]
- Andrews, P.J.; Sinclair, H.L.; Rodriguez, A.; Harris, B.A.; Battison, C.; Rhodes, J.K.; Murray, G.D. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. N. Engl. J. Med. 2015, 373, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Garner, A.A.; Mann, K.P.; Fearnside, M.; Poynter, E.; Gebski, V. The Head Injury Retrieval Trial (HIRT): A single-centre randomised controlled trial of physician prehospital management of severe blunt head injury compared with management by paramedics only. Emerg. Med. J. 2015, 32, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Mendelow, A.D.; Gregson, B.A.; Rowan, E.N.; Francis, R.; McColl, E.; McNamee, P.; Chambers, I.R.; Unterberg, A.; Boyers, D.; Mitchell, P.M.; et al. Early Surgery versus Initial Conservative Treatment in Patients with Traumatic Intracerebral Hemorrhage (STITCH[Trauma]): The First Randomized Trial. J. Neurotrauma 2015, 32, 1312–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.G.; Apps, J.N.; Hoffmann, R.G.; McCrea, M.; Hammeke, T. Benefits of Strict Rest After Acute Concussion: A Randomized Controlled Trial. Pediatrics 2015, 135, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, T.; Yamashita, S.; Nagao, S.; Hayashi, N.; Ohashi, Y.; on behalf of the Brain-Hypothermia (B-HYPO) Study Group. Prolonged Mild Therapeutic Hypothermia versus Fever Control with Tight Hemodynamic Monitoring and Slow Rewarming in Patients with Severe Traumatic Brain Injury: A Randomized Controlled Trial. J. Neurotrauma 2015, 32, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shokouhi, G.; Haghjoo, A.G.; Sattarnezhad, N.; Asghari, M.; Sattarnezhad, A.; Asghari, A.; Pezeshki, A. Effects of Citicoline on Level of Consciousness, Serum Level of Fetuin-A and Matrix Gla- Protein (MGP) in Trauma Patients with Diffuse Axonal Injury (DAI) and GCS ≤ 8. Turk. J. Trauma Emerg. Surg. 2014, 20, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skolnick, B.E.; Maas, A.I.R.; Narayan, R.K.; Van Der Hoop, R.G.; MacAllister, T.; Ward, J.D.; Nelson, N.R.; Stocchetti, N. A Clinical Trial of Progesterone for Severe Traumatic Brain Injury. N. Engl. J. Med. 2014, 371, 2467–2476. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.W.; Yeatts, S.D.; Silbergleit, R.; Palesch, Y.Y.; Hertzberg, V.S.; Frankel, M.; Goldstein, F.C.; Caveney, A.F.; Howlett-Smith, H.; Bengelink, E.M.; et al. Very Early Administration of Progesterone for Acute Traumatic Brain Injury. N. Engl. J. Med. 2014, 371, 2457–2466. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.-Z.; Li, W.; Liu, K.-G.; Wu, W.; Lu, W.-C.; Zhang, J.-F.; Wang, M.-D. Early Pressure Dressing for the Prevention of Subdural Effusion Secondary to Decompressive Craniectomy in Patients With Severe Traumatic Brain Injury. J. Craniofacial Surg. 2014, 25, 1836–1839. [Google Scholar] [CrossRef] [PubMed]
- Asehnoune, K.; Seguin, P.; Allary, J.; Feuillet, F.; Lasocki, S.; Cook, F.; Floch, H.; Chabanne, R.; Geeraerts, T.; Roger, C.; et al. Hydrocortisone and fludrocortisone for prevention of hospital-acquired pneumonia in patients with severe traumatic brain injury (Corti-TC): A double-blind, multicentre phase 3, randomised placebo-controlled trial. Lancet Respir. Med. 2014, 2, 706–716. [Google Scholar] [CrossRef]
- Robertson, C.S.; Hannay, H.J.; Yamal, J.-M.; Gopinath, S.; Goodman, J.C.; Tilley, B.C.; Baldwin, A.; Rivera-Lara, L.; Saucedo-Crespo, H.; Ahmed, O.; et al. Effect of Erythropoietin and Transfusion Threshold on Neurological Recovery After Traumatic Brain Injury. JAMA 2014, 312, 36–47. [Google Scholar] [CrossRef]
- Stover, J.F.; Belli, A.; Boret, H.; Bulters, D.; Sahuquillo, J.; Schmutzhard, E.; Zavala, E.; Ungerstedt, U.; Schinzel, R.; Tegtmeier, F.; et al. Nitric Oxide Synthase Inhibition with the Antipterin VAS203 Improves Outcome in Moderate and Severe Traumatic Brain Injury: A Placebo-Controlled Randomized Phase IIa Trial (NOSTRA). J. Neurotrauma 2014, 31, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.N.; Samini, F.; Nematy, M.; Philippou, E.; Safarian, M.; Tavallaiee, S.; Norouzy, A. Hyperglycemia and antibody titres against heat shock protein 27 in traumatic brain injury patients on parenteral nutrition. Iran. J. Basic Med. Sci. 2014, 17, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Fard, S.A.; Habibabadi, M.R.; Moein, P.; Naderan, M.; Norouzi, R.; Aminmansour, B. The efficacy of cyclosporine-A on diffuse axonal injury after traumatic brain injury. Adv. Biomed. Res. 2014, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Lumba-Brown, A.; Harley, J.; Lucio, S.; Vaida, F.; Hilfiker, M. Hypertonic Saline as a Therapy for Pediatric Concussive Pain. Pediatr. Emerg. Care 2014, 30, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Helmy, A.; Guilfoyle, M.R.; Carpenter, K.; Pickard, J.D.; Menon, D.K.; Hutchinson, P.J. Recombinant Human Interleukin-1 Receptor Antagonist in Severe Traumatic Brain Injury: A Phase II Randomized Control Trial. Br. J. Pharmacol. 2014, 34, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Jagannatha, A.T.; Sriganesh, K.; Devi, B.I.; Rao, G.S.U. An equiosmolar study on early intracranial physiology and long term outcome in severe traumatic brain injury comparing mannitol and hypertonic saline. J. Clin. Neurosci. 2015, 27, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.-P.; Song, Y.-L.; Zhao, Q.-H. Intensive insulin therapy for preventing postoperative infection in patients with traumatic brain injury. Medicine 2017, 96, e6458. [Google Scholar] [CrossRef]
- Moghaddam, O.M.; Lahiji, M.N.; Hassani, V.; Mozari, S. Early administration of selenium in patients with acute traumatic brain injury: A randomized double-blinded controlled trial. Indian J. Crit. Care Med. 2017, 21, 75–79. [Google Scholar] [CrossRef]
- Jokar, A.; Ahmadi, K.; Salehi, T.; Sharif-Alhoseini, M.; Rahimi-Movaghar, V. The effect of tranexamic acid in traumatic brain injury: A randomized controlled trial. Chin. J. Traumatol. 2017, 20, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.; Pilehvari, Z.; Poorolajal, J.; Aghajanloo, M. Effects of Normobaric Hyperoxia in Traumatic Brain Injury: A Randomized Controlled Clinical Trial. Trauma Mon. 2016, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, M.; Kumar, M.; Malviya, D.; Malviya, P.S.; Kumar, V.; Tyagi, A. Influence of two anesthetic techniques on blood sugar level in head injury patients: A comparative study. Anesth. Essays Res. 2016, 10, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Mofid, B.; Soltani, Z.; Khaksari, M.; Shahrokhi, N.; Nakhaee, N.; Karamouzian, S.; Ahmadinejad, M.; Maiel, M.; Khazaeli, P. What are the progesterone-induced changes of the outcome and the serum markers of injury, oxidant activity and inflammation in diffuse axonal injury patients? Int. Immunopharmacol. 2016, 32, 103–110. [Google Scholar] [CrossRef]
- Nichol, A.; French, C.; Little, L.; Haddad, S.; Presneill, J.; Arabi, Y.; Bailey, M.; Cooper, D.J.; Duranteau, J.; Huet, O.; et al. Erythropoietin in traumatic brain injury (EPO-TBI): A double-blind randomised controlled trial. Lancet 2015, 386, 2499–2506. [Google Scholar] [CrossRef]
- Beca, J.; McSharry, B.; Erickson, S.; Yung, M.; Schibler, A.; Slater, A.; Wilkins, B.; Singhal, A.; Williams, G.; Sherring, C.; et al. Hypothermia for Traumatic Brain Injury in Children—A Phase II Randomized Controlled Trial*. Crit. Care Med. 2015, 43, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.E.; Marqueti, R.D.C.; Livino-De-Carvalho, K.; De Araujo, A.E.T.; Castro, J.; Da Silva, V.M.; Vieira, L.; Souza, V.C.; Dantas, L.O.; Cipriano, G., Jr.; et al. Neuromuscular electrical stimulation in critically ill traumatic brain injury patients attenuates muscle atrophy, neurophysiological disorders, and weakness: A randomized controlled trial. J. Intensiv. Care 2019, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Babamohamadi, H.; Ansari, Z.; Nobahar, M.; Mirmohammadkhani, M. The effects of peppermint gel on prevention of pressure injury in hospitalized patients with head trauma in neurosurgical ICU: A double-blind randomized controlled trial. Complement. Ther. Med. 2019, 47, 102223. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, C.T. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial. Lancet 2019, 394, 1713–1723. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-M.; Li, R.; Sun, W.-B.; Wang, X.-P.; Qi, M.-M.; Bai, Y.; Bai, J.; Zheng, W.-C. Low-Dose, Early Fresh Frozen Plasma Transfusion Therapy After Severe Trauma Brain Injury: A Clinical, Prospective, Randomized, Controlled Study. World Neurosurg. 2019, 132, e21–e27. [Google Scholar] [CrossRef] [PubMed]
- Poon, W.; Matula, C.; Vos, P.E.; Muresanu, D.F.; Von Steinbüchel, N.; Von Wild, K.; Hömberg, V.; Wang, E.; Lee, T.M.C.; Strilciuc, S.; et al. Safety and efficacy of Cerebrolysin in acute brain injury and neurorecovery: CAPTAIN I—A randomized, placebo-controlled, double-blind, Asian-Pacific trial. Neurol. Sci. 2019, 41, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wei, L.; Xu, G.; Ren, C.; Zhang, Z.; Liu, Y. Effects of dexmedetomidine vs sufentanil during percutaneous tracheostomy for traumatic brain injury patients. Medicine 2019, 98, e17012. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, A.; Rahimi-Bashar, F.; Torabian, S.; Sohrabi, S.; Makarchian, H.R. Efficacy of Simultaneous Administration of Nimodipine, Progesterone, and Magnesium Sulfate in Patients with Severe Traumatic Brain Injury: A Randomized Controlled Trial. Bull. Emerg. Trauma 2019, 7, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Emami, M.; Asadipour, E.; Kasirzadeh, S.; Rouini, M.-R.; Najafi, A.; Heshmat, R.; Abdollahi, M.; Mojtahedzadeh, M. A randomized controlled trial on the efficacy, safety, and pharmacokinetics of metformin in severe traumatic brain injury. J. Neurol. 2019, 266, 1988–1997. [Google Scholar] [CrossRef]
- Kumar, S.A.; Devi, B.I.; Reddy, M.; Shukla, D. Comparison of equiosmolar dose of hyperosmolar agents in reducing intracranial pressure—A randomized control study in pediatric traumatic brain injury. Child’s Nerv. Syst. 2019, 35, 999–1005. [Google Scholar] [CrossRef]
- Gobatto, A.L.N.; Link, M.A.; Solla, D.J.; Bassi, E.; Tierno, P.F.; Paiva, W.; Taccone, F.S.; Malbouisson, L.M. Transfusion requirements after head trauma: A randomized feasibility controlled trial. Crit. Care 2019, 23, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mowla, A.; Baharvahdat, H.; Ganjeifar, B.; Etemadrezaie, H.; Farajirad, M.; Zabihyan, S. Enoxaparin in the treatment of severe traumatic brain injury: A randomized clinical trial. Surg. Neurol. Int. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Basar, S.; Younus, S.M.; Gauri, S.A.; Khan, A.A.; Imran, M.; Abubakar, S.; Sheikh, D.; Shehbaz, N.; Ashraf, J. Comparison of phenytoin versus levetiracetam in early seizure prophylaxis after traumatic brain injury, at a tertiary care hospital in Karachi, Pakistan. Asian J. Neurosurg. 2018, 13, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.J.; Nichol, A.; Bailey, M.; Bernard, S.; Cameron, P.A.; Pili-Floury, S.; Forbes, A.; Gantner, D.; Higgins, A.; Huet, O.; et al. Effect of Early Sustained Prophylactic Hypothermia on Neurologic Outcomes Among Patients with Severe Traumatic Brain Injury. JAMA 2018, 320, 2211–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomar, G.S.; Singh, G.P.; Bithal, P.; Upadhyay, A.D.; Chaturvedi, A. Comparison of Effects of Manual and Mechanical Airway Clearance Techniques on Intracranial Pressure in Patients with Severe Traumatic Brain Injury on a Ventilator: Randomized, Crossover Trial. Phys. Ther. 2019, 99, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Fakharian, E.; Abedzadeh-Kalahroudi, M.; Atoof, F. Effect of Tranexamic Acid on Prevention of Hemorrhagic Mass Growth in Patients with Traumatic Brain Injury. World Neurosurg. 2018, 109, e748–e753. [Google Scholar] [CrossRef]
- Okonkwo, D.O.; Shutter, L.; Moore, C.; Temkin, N.R.; Puccio, A.M.; Madden, C.J.; Andaluz, N.; Chesnut, R.; Bullock, M.R.; Grant, G.A.; et al. Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II. Crit. Care Med. 2017, 45, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, E.; Islam, A.A.; Hatta, M.; Widodo, D.; Pattelongi, I. The Profile of MMP-9, MMP-9 mRNA Expression, -1562 C/T Polymorphism and Outcome in High-risk Traumatic Brain Injury: The Effect of Therapeutic Mild Hypothermia. Neurol. Med.-Chir. 2017, 57, 612–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokhtari, M.; Nayeb-Aghaei, H.; Kouchek, M.; Miri, M.M.; Goharani, R.; Amoozandeh, A.; Salamat, S.A.; Sistanizad, M. Effect of Memantine on Serum Levels of Neuron-Specific Enolase and on the Glasgow Coma Scale in Patients with Moderate Traumatic Brain Injury. J. Clin. Pharmacol. 2017, 58, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Farzanegan, G.R.; Derakhshan, N.; Khalili, H.; Ghaffarpasand, F.; Paydar, S. Effects of atorvastatin on brain contusion volume and functional outcome of patients with moderate and severe traumatic brain injury; a randomized double-blind placebo-controlled clinical trial. J. Clin. Neurosci. 2017, 44, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.R.H.; Phang, L.F.; Sia, S.F.; Amir, A.; Veerakumaran, J.S.; Kassim, M.K.A.; Othman, J.M.I.; Tah, P.C.; Loh, P.S.; Jailani, M.I.O.; et al. Effects of immunonutrition on biomarkers in traumatic brain injury patients in Malaysia: A prospective randomized controlled trial. BMC Anesthesiol. 2017, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Salmani, F.; Mohammadi, E.; Rezvani, M.; Kazemnezhad, A. The effects of family-centered affective stimulation on brain-injured comatose patients’ level of consciousness: A randomized controlled trial. Int. J. Nurs. Stud. 2017, 74, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Khazdouz, M.; Mazidi, M.; Ehsaei, M.-R.; Ferns, G.; Kengne, A.P.; Norouzy, A. Impact of Zinc Supplementation on the Clinical Outcomes of Patients with Severe Head Trauma: A Double-Blind Randomized Clinical Trial. J. Diet. Suppl. 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.-Z.; Wang, W.-Y.; Zeng, J.; Zhou, Z.-Y.; Peng, J.; Yang, H.; Deng, P.-C.; Li, S.-J.; Lu, C.D.; Jiang, H. Optimization of brain metabolism using metabolic-targeted therapeutic hypothermia can reduce mortality from traumatic brain injury. J. Trauma Acute Care Surg. 2017, 83, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, H.; Zhang, X.; Ge, L.; Bie, L. The effect of hypertonic saline and mannitol on coagulation in moderate traumatic brain injury patients. Am. J. Emerg. Med. 2017, 35, 1404–1407. [Google Scholar] [CrossRef]
- Tang, C.; Bao, Y.; Qi, M.; Zhou, L.; Liu, F.; Mao, J.; Lei, Q.; Qi, S.; Qiu, B. Mild induced hypothermia for patients with severe traumatic brain injury after decompressive craniectomy. J. Crit. Care 2017, 39, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; Derakhshan, N.; Niakan, A.; Ghaffarpasand, F.; Salehi, M.; Eshraghian, H.; Shakibafard, A.; Zahabi, B. Effects of Oral Glibenclamide on Brain Contusion Volume and Functional Outcome of Patients with Moderate and Severe Traumatic Brain Injuries: A Randomized Double-Blind Placebo-Controlled Clinical Trial. World Neurosurg. 2017, 101, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Chakroun-Walha, O.; Samet, A.; Jerbi, M.; Nasri, A.; Talbi, A.; Kanoun, H.; Souissi, B.; Chtara, K.; Bouaziz, M.; Ksibi, H.; et al. Benefits of the tranexamic acid in head trauma with no extracranial bleeding: A prospective follow-up of 180 patients. Eur. J. Trauma Emerg. Surg. 2018, 45, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Ghalaenovi, H.; Fattahi, A.; Koohpayehzadeh, J.; Khodadost, M.; Fatahi, N.; Taheri, M.; Azimi, A.; Rohani, S.; Rahatlou, H. The effects of amantadine on traumatic brain injury outcome: A double-blind, randomized, controlled, clinical trial. Brain Inj. 2018, 32, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chouhan, R.S.; Bindra, A.; Radhakrishna, N. Comparison of effect of dexmedetomidine and lidocaine on intracranial and systemic hemodynamic response to chest physiotherapy and tracheal suctioning in patients with severe traumatic brain injury. J. Anesth. 2018, 32, 518–523. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, Y.; Yadav, G.; Mathur, S.K.; Bhadani, U.K. Role of neomycin polymyxin sulfate solution bladder wash for prevention of catheter associated urinary tract infection in traumatic brain injury patient admitted to Intensive Care Unit: A prospective randomized study. Int. J. Crit. Illn. Inj. Sci. 2018, 8, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.-F.; Gao, Y.-K. Recombinant human erythropoietin for treating severe traumatic brain injury. Medicine 2018, 97, e9532. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.H.; Hassan, W.M.N.W.; Zaini, R.H.M.; Shukeri, W.F.W.M.; Abidin, H.Z.; Eu, C.S. Balanced Fluid Versus Saline-Based Fluid in Post-operative Severe Traumatic Brain Injury Patients: Acid-Base and Electrolytes Assessment. Malays. J. Med. Sci. 2017, 24, 83–93. [Google Scholar] [CrossRef]
- Hassan, W.M.N.W.; Nasir, Y.M.; Zaini, R.H.M.; Shukeri, W.F.W.M. Target-controlled Infusion Propofol Versus Sevoflurane Anaesthesia for Emergency Traumatic Brain Surgery: Comparison of the Outcomes. Malays. J. Med. Sci. 2017, 24, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.P.; Notkina, N.; Timofeev, I.; Hutchinson, P.J.; Finnis, M.E.; Gupta, A.K. Cerebral metabolic effects of strict versus conventional glycaemic targets following severe traumatic brain injury. Crit. Care 2018, 22, 16. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Khan, A.A.; Singh, Y.; Singh, A.K.; Mathur, S.K. To compare the effect of two different doses of dexmedetomidine on the attenuation of airway and pressor response during tracheostomy tube change in traumatic brain injury patients. Anesth. Essays Res. 2017, 11, 964–968. [Google Scholar] [CrossRef]
- Vieira, E.; Guimarães, T.C.; Faquini, I.; Silva, J.L.; Saboia, T.; Andrade, R.V.C.L.; Gemir, T.L.; Neri, V.C.; Almeida, N.S.; Azevedo-Filho, H.R.C. Randomized controlled study comparing 2 surgical techniques for decompressive craniectomy: With watertight duraplasty and without watertight duraplasty. J. Neurosurg. 2018, 129, 1017–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeppel, T.J.; Sharpe, J.P.; Shahan, C.P.; Clement, L.P.; Magnotti, L.J.; Lee, M.; Muhlbauer, M.; Weinberg, J.A.; Tolley, E.A.; Croce, M.A.; et al. Beta-adrenergic blockade for attenuation of catecholamine surge after traumatic brain injury: A randomized pilot trial. Trauma Surg. Acute Care Open 2019, 4, e000307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, P.J.; Sinclair, H.L.; Rodríguez, A.; Harris, B.; Rhodes, J.; Watson, H.; Murray, G. Therapeutic hypothermia to reduce intracranial pressure after traumatic brain injury: The Eurotherm3235 RCT. Health Technol. Assess. 2018, 22, 1–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauch, K.; Kowark, A.; Coburn, M.; Clusmann, H.; Höllig, A. Randomized Controlled Trials on Intracerebral Hemorrhage: A Cross Sectional Retrospective Analysis of CONSORT Item Adherence. Front. Neurol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kiehna, E.; Starke, R.M.; Pouratian, N.; Dumont, A.S. Standards for reporting randomized controlled trials in neurosurgery. J. Neurosurg. 2011, 114, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Cooper, B.; Shin, S.M.; Kondziolka, D. Randomized controlled trials and neurosurgery: The ideal fit or should alternative methodologies be considered? J. Neurosurg. 2016, 124, 558–568. [Google Scholar] [CrossRef] [Green Version]
- Horton, L.; Rhodes, J.; Wilson, L. Randomized Controlled Trials in Adult Traumatic Brain Injury: A Systematic Review on the Use and Reporting of Clinical Outcome Assessments. J. Neurotrauma 2018, 35, 2005–2014. [Google Scholar] [CrossRef]
- Hewitt, C.; Hahn, S.; Torgerson, D.; Watson, J.; Bland, J.M. Adequacy and reporting of allocation concealment: Review of recent trials published in four general medical journals. BMJ 2005, 330, 1057–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, T.C.; Erueti, C.; Glasziou, P. Poor description of non-pharmacological interventions: Analysis of consecutive sample of randomised trials. BMJ 2013, 347, f3755. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P.; Caplan, A.L.; Dal-Ré, R. Outcome reporting bias in clinical trials: Why monitoring matters. BMJ 2017, 356, j408. [Google Scholar] [CrossRef] [PubMed]
- Simera, I.; Moher, D.; Hirst, A.; Hoey, J.; Schulz, K.F.; Altman, D.G. Transparent and accurate reporting increases reliability, utility, and impact of your research: Reporting guidelines and the EQUATOR Network. BMC Med. 2010, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopewell, S.; Loudon, K.; Clarke, M.J.; Oxman, A.D.; Dickersin, K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database Syst. Rev. 2009, 2010, MR000006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, A.I.; Menon, D.K.; Lingsma, H.F.; Pineda, J.A.; Sandel, M.E.; Manley, G.T. Re-Orientation of Clinical Research in Traumatic Brain Injury: Report of an International Workshop on Comparative Effectiveness Research. J. Neurotrauma 2012, 29, 32–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamseer, L.; Hopewell, S.; Altman, U.G.; Moher, D.; Schulz, K.F. Update on the endorsement of CONSORT by high impact factor journals: A survey of journal “Instructions to Authors” in 2014. Trials 2016, 17, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutron, I.; Altman, D.G.; Moher, D.; Schulz, K.F.; Ravaud, P.; for the CONSORT NPT Group. CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann. Intern. Med. 2017, 167, 40–47. [Google Scholar] [CrossRef] [PubMed]

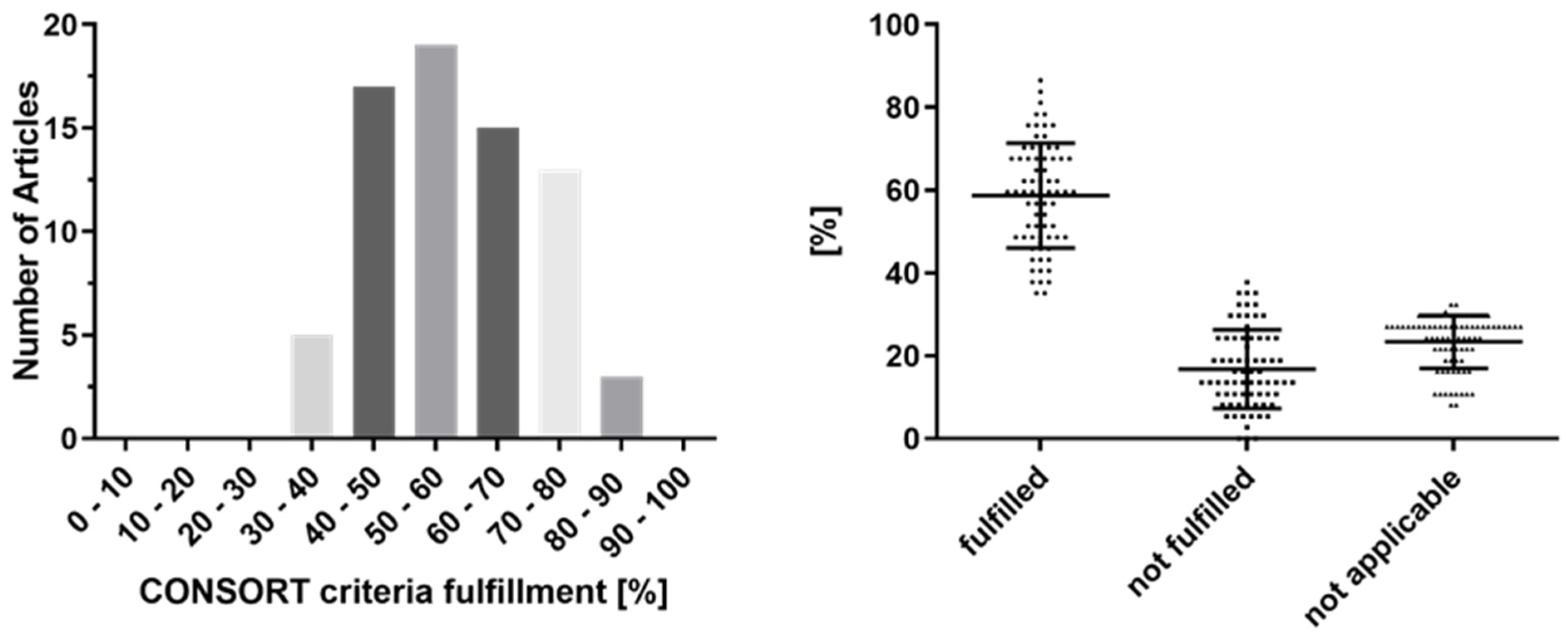
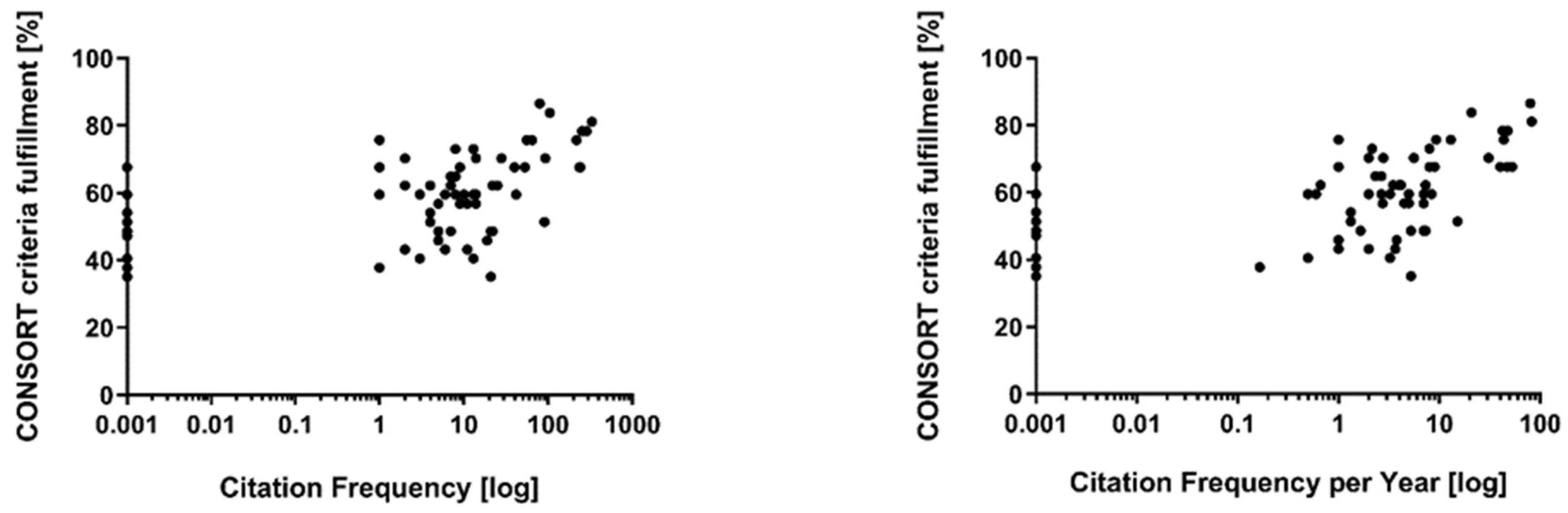
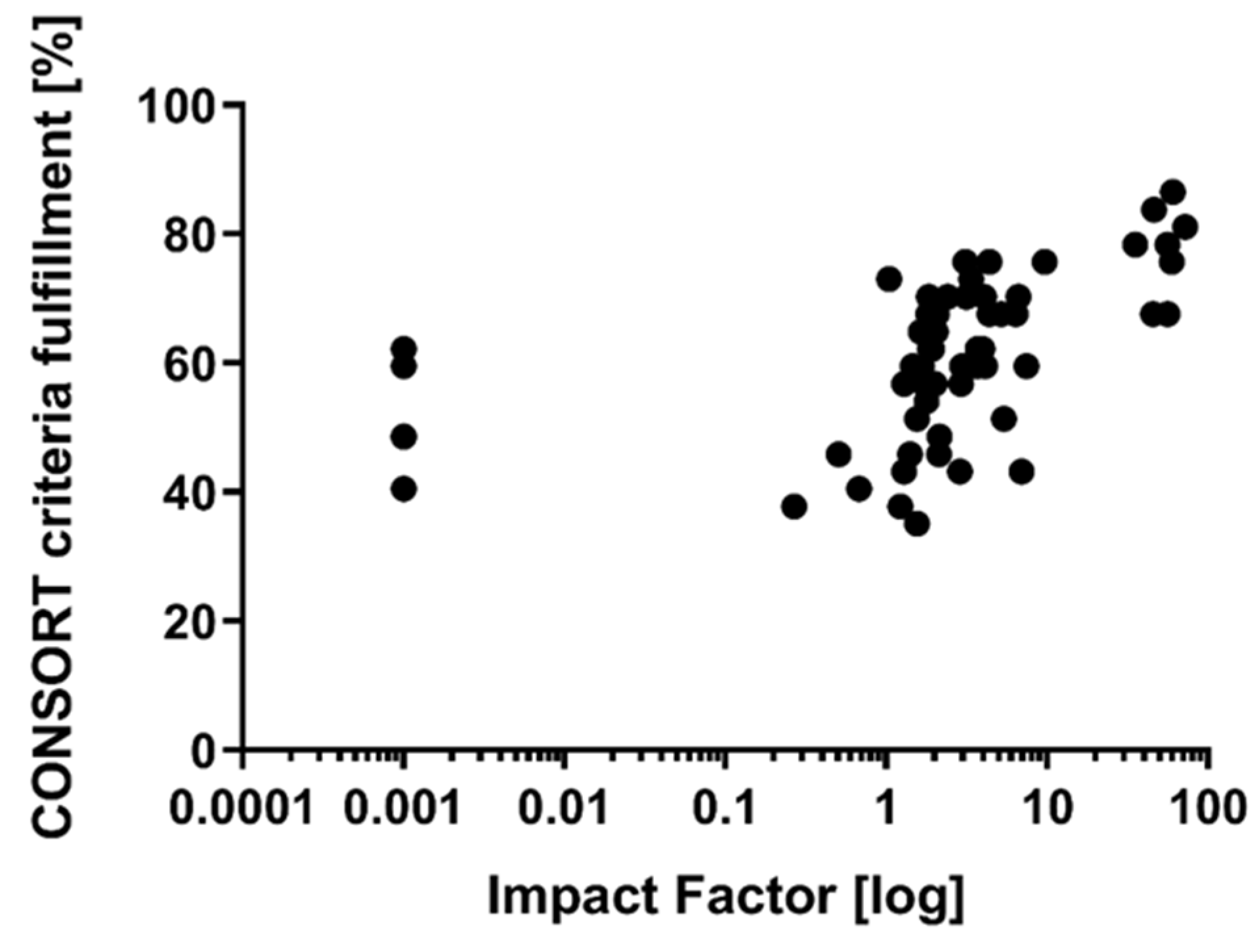
| Item | Item Description | Items Fulfilled n/(%) | |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title. | n = 42; 58.3% | |
| 1b | Structured summary of trial design, methods, results, and conclusions. | n = 70; 98.6% | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale. | n = 72; 100% |
| 2b | Specific objectives or hypotheses. | n = 68; 94.4% | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio. | n = 70; 97.2% |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons. | n = 3; 4.2% | |
| Participants | 4a | Eligibility criteria for participants. | n = 72; 100% |
| 4b | Settings and locations where the data were collected. | n = 67; 93.1% | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered. | n = 71; 98.6% |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed. | n = 70; 97.2% |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons. | n = 2; 2.8% | |
| Sample size | 7a | How sample size was determined. | n = 38; 52.8% |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines. | n = 8; 11.1% | |
| Randomisation: | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence. | n = 59; 81.9% |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size). | n = 35; 48.6% | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned. | n = 30; 41.7% |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions. | n = 10; 13.9% |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how. | n = 44; 61.1% |
| 11b | If relevant, description of the similarity of interventions. | n = 3; 4.2% | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes. | n = 71; 98.6% |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses. | n = 11; 15.3% | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome. | n = 43; 59.7% |
| 13b | For each group, losses and exclusions after randomisation, together with reasons. | n = 45; 62.5% | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up. | n = 60; 83.3% |
| 14b | Why the trial ended or was stopped. | n = 7; 9.7% | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group. | n = 68; 94.4% |
| 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups. | n = 61; 84.7% | |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval). | n = 70; 97.2% |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended. | n = 2; 2.8% | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory. | n = 16; 22.2% |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms). | n = 40; 55.6% |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses. | n = 57; 79.2% |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings. | n = 14; 19.4% |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence. | n = 72; 100% |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry. | n = 40; 55.6% |
| Protocol | 24 | Where the full trial protocol can be accessed, if available. | n = 12; 16.7% |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders. | n = 39; 54.2% |
| mean | 58.7% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elcivan, M.; Kowark, A.; Coburn, M.; Hamou, H.A.; Kremer, B.; Clusmann, H.; Höllig, A. A Retrospective Analysis of Randomized Controlled Trials on Traumatic Brain Injury: Evaluation of CONSORT Item Adherence. Brain Sci. 2021, 11, 1504. https://doi.org/10.3390/brainsci11111504
Elcivan M, Kowark A, Coburn M, Hamou HA, Kremer B, Clusmann H, Höllig A. A Retrospective Analysis of Randomized Controlled Trials on Traumatic Brain Injury: Evaluation of CONSORT Item Adherence. Brain Sciences. 2021; 11(11):1504. https://doi.org/10.3390/brainsci11111504
Chicago/Turabian StyleElcivan, Meltem, Ana Kowark, Mark Coburn, Hussam Aldin Hamou, Benedikt Kremer, Hans Clusmann, and Anke Höllig. 2021. "A Retrospective Analysis of Randomized Controlled Trials on Traumatic Brain Injury: Evaluation of CONSORT Item Adherence" Brain Sciences 11, no. 11: 1504. https://doi.org/10.3390/brainsci11111504
APA StyleElcivan, M., Kowark, A., Coburn, M., Hamou, H. A., Kremer, B., Clusmann, H., & Höllig, A. (2021). A Retrospective Analysis of Randomized Controlled Trials on Traumatic Brain Injury: Evaluation of CONSORT Item Adherence. Brain Sciences, 11(11), 1504. https://doi.org/10.3390/brainsci11111504






