Electromagnetic Field Stimulation Attenuates Phasic Nociception after Complete Spinal Cord Injury in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Complete Spinal Cord Injury and Postoperative Care
2.2. EMF Stimulation Chamber
2.3. Behavioral Assessment
2.3.1. BBB Score
2.3.2. Tail Flick Latency (TFL)
2.3.3. Threshold of Tail Flick (TTF)
2.3.4. Hind Paw Withdrawal Latency (HPL)
2.3.5. Acetone Test
2.4. Electrophysiological Assessment
2.4.1. H-Reflex
2.4.2. Nociceptive Flexion Reflex (NFR)
2.5. Neurochemical Assessment
2.6. Histological Assessment
2.7. Statistical Analysis
3. Results
3.1. Behavioral Assessment
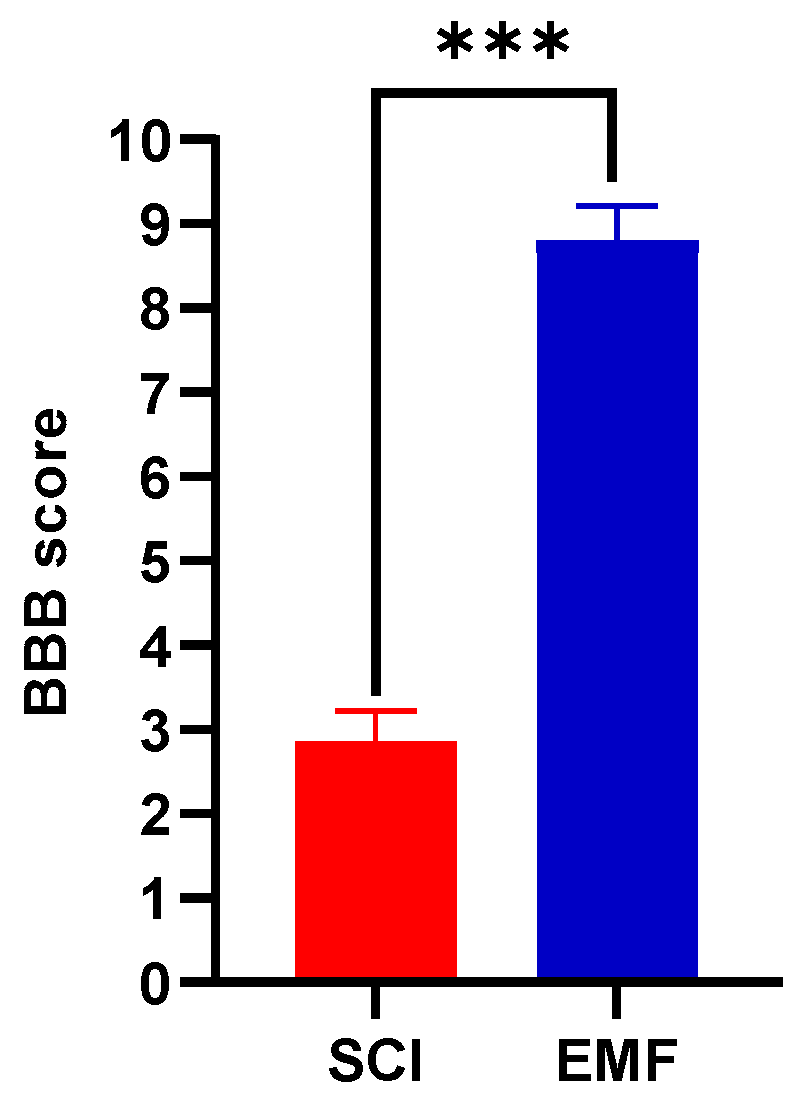
3.1.1. BBB Score
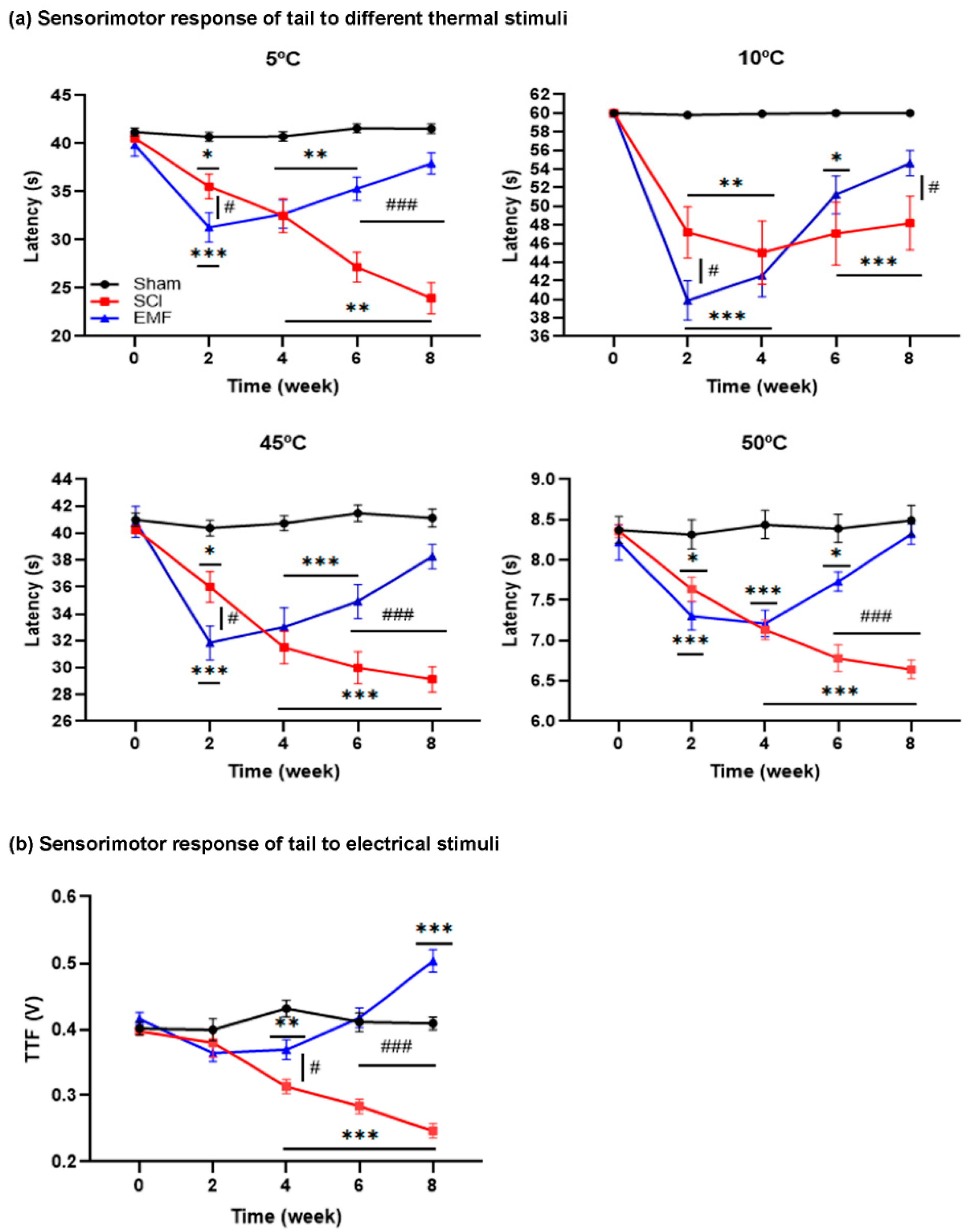
3.1.2. Sensorimotor Response of Tail to Thermal and Electrical Stimulus
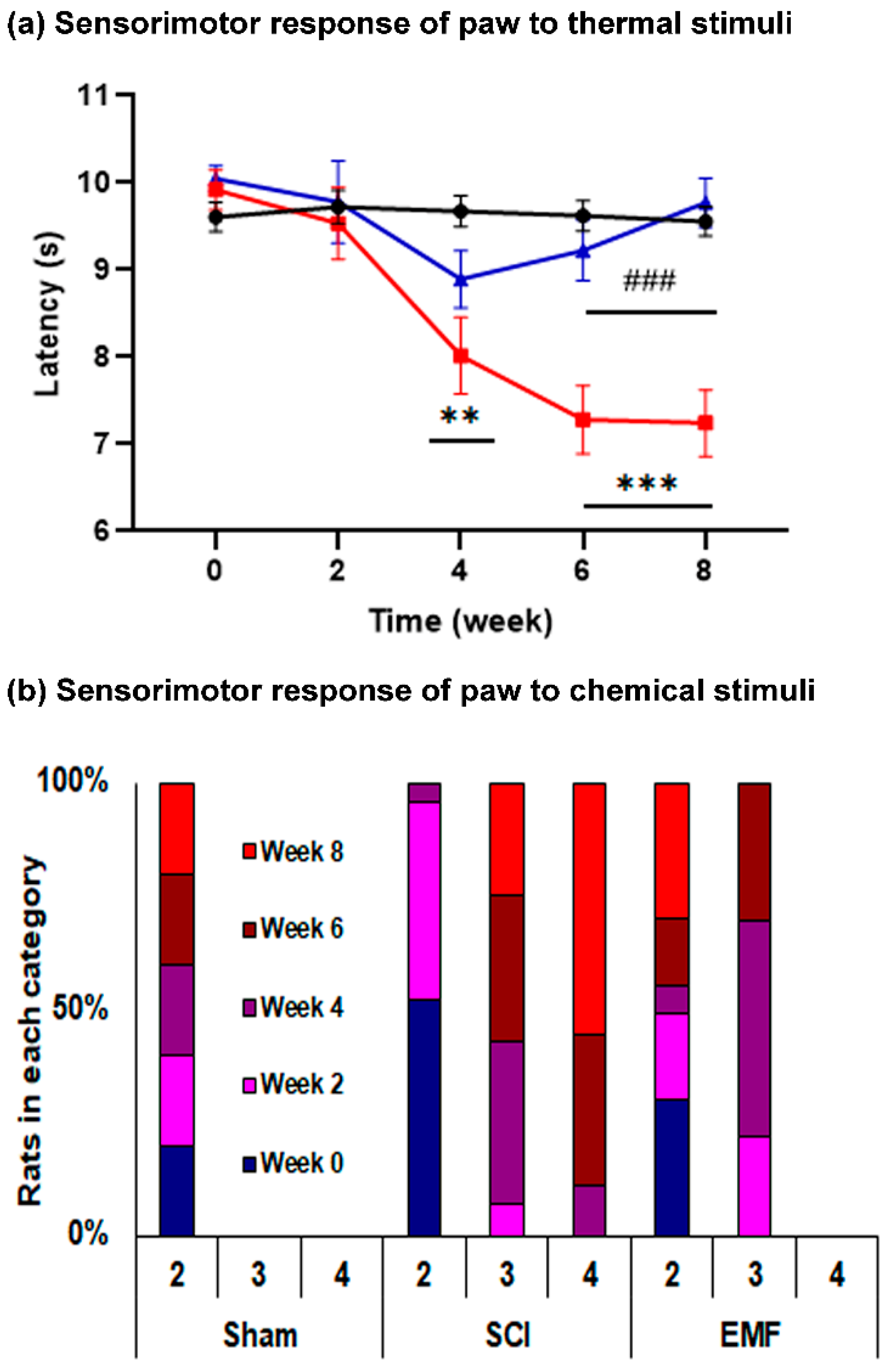
3.1.3. Sensorimotor Response of Paws to Thermal and Chemical Stimulus
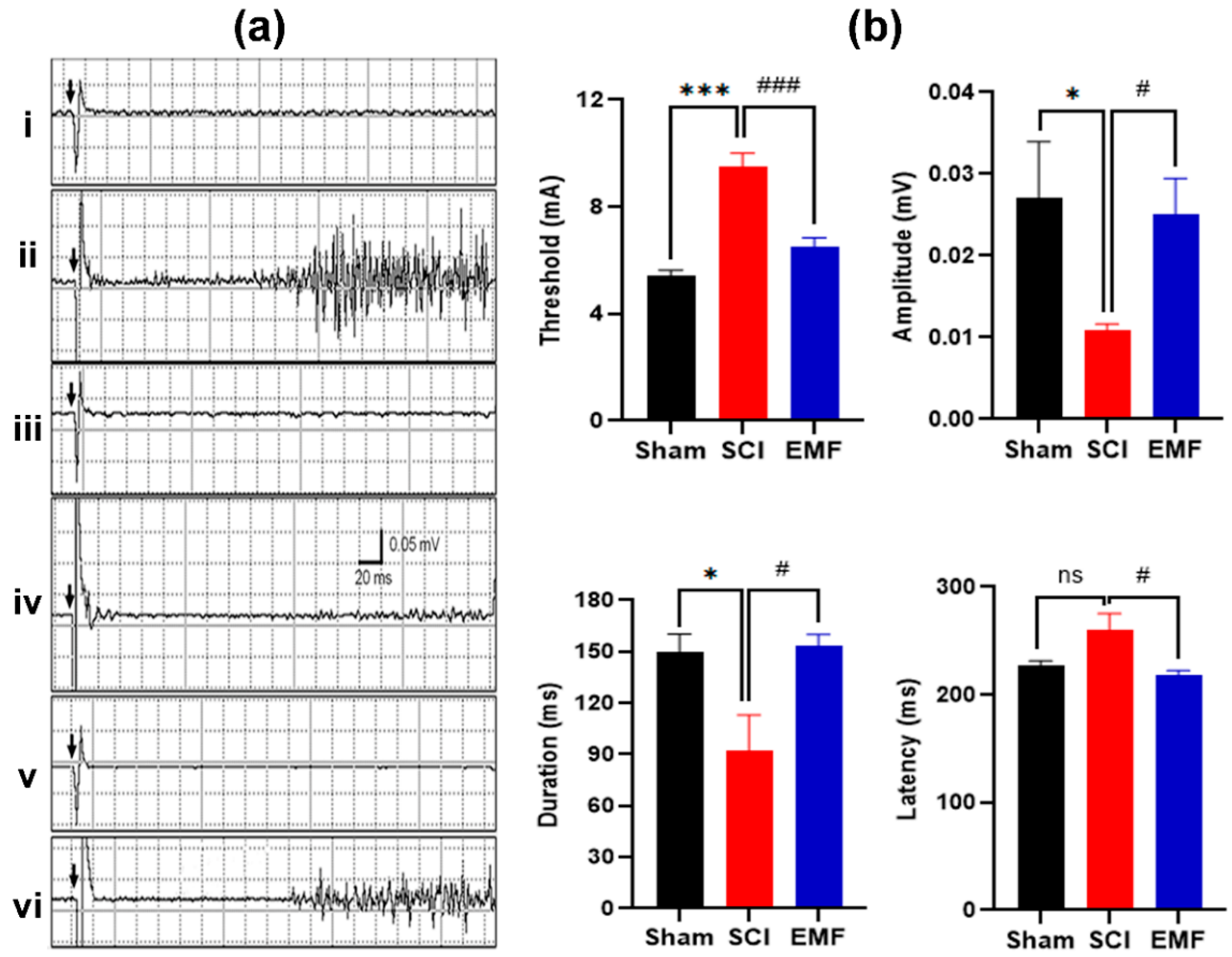
3.2. Electrophysiological Assessment
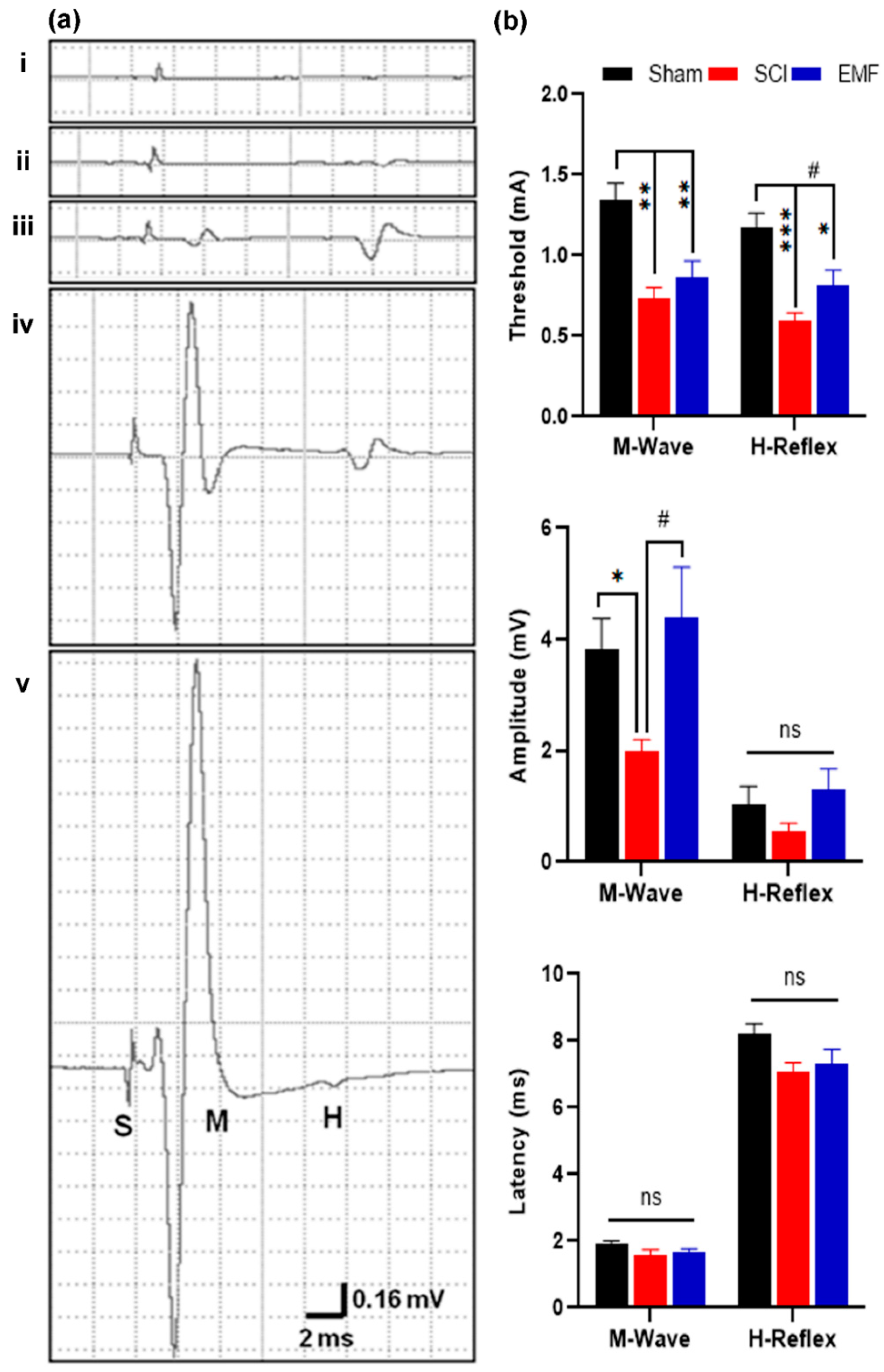
3.2.1. M/H-Reflex
3.2.2. Nociceptive Flexion Reflex
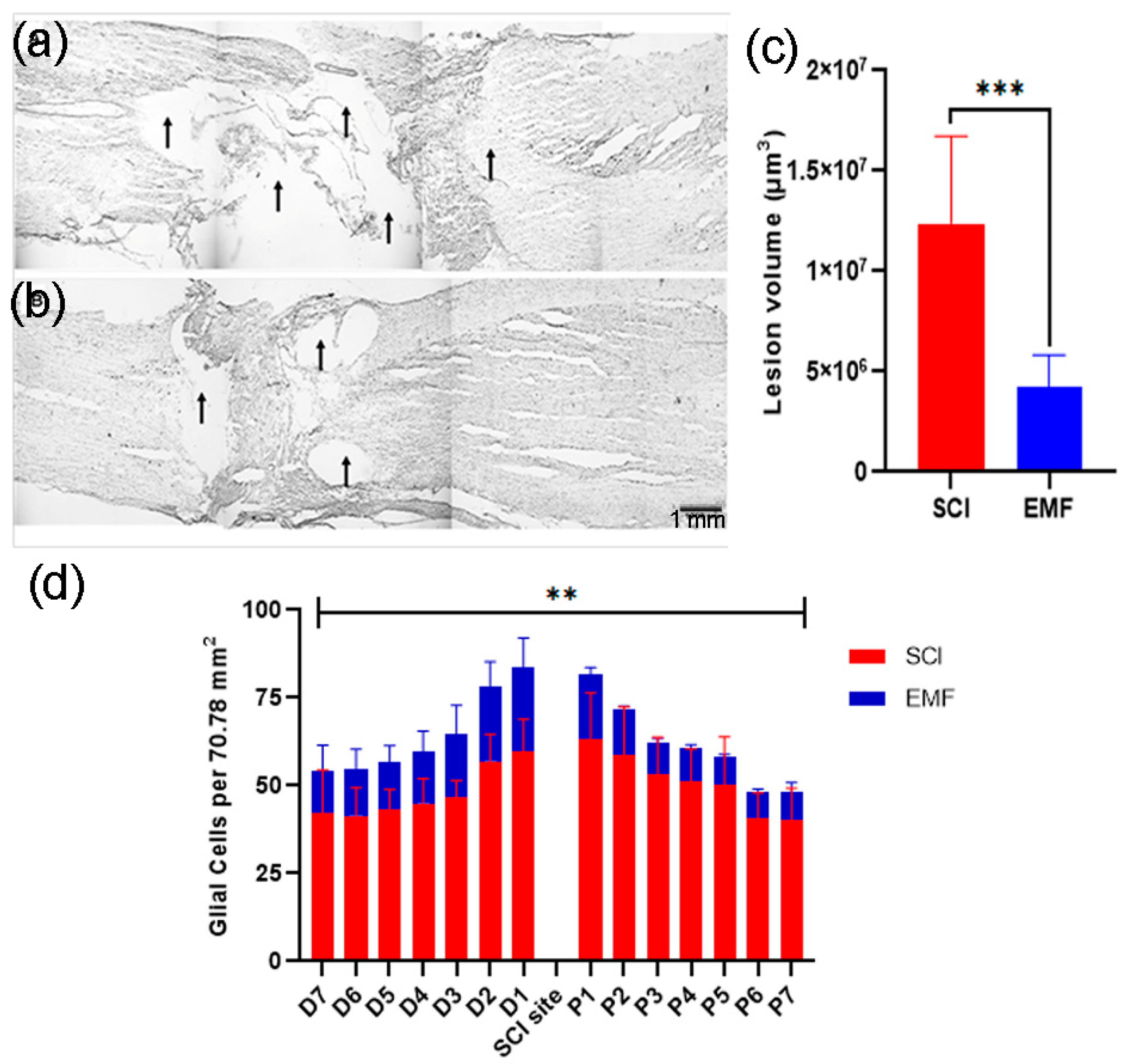
3.3. Histological Assessment
3.4. Neurochemical Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fouad, K.; Popovich, P.G.; Kopp, M.A.; Schwab, J.M. The neuroanatomical-functional paradox in spinal cord injury. Nat. Rev. Neurol. 2021, 17, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.M.; Chakrabarty, S.; Kimura, H.; Martin, J.H. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J. Neurosci. 2012, 32, 12896–12908. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Armada, K.; Martin, J.H. Neuronal activity and microglial activation support corticospinal tract and proprioceptive afferent sprouting in spinal circuits after a corticospinal system lesion. Exp. Neurol. 2019, 321, 113015. [Google Scholar] [CrossRef]
- Tom, B.; Witko, J.; Lemay, M.; Singh, A. Effects of bioengineered scaffold loaded with neurotrophins and locomotor training in restoring H-reflex responses after spinal cord injury. Exp. Brain Res. 2018, 236, 3077–3084. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Kumar, S.; Jain, S.; Nag, T.C.; Mathur, R. Neuroregenerative effects of electromagnetic field and magnetic nanoparticles on spinal cord injury in rats. J. Nanosci. Nanotechnol. 2018, 18, 6756–6764. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Wang, Y.; Wei, Z.; Suo, D.; Ning, G.; Wu, Q.; Feng, S.; Wan, C. Low-frequency pulsed electromagnetic field promotes functional recovery, reduces inflammation and oxidative stress, and enhances HSP70 expression following spinal cord injury. Mol. Med. Rep. 2019, 19, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.U.; Alam, M.; Zheng, Y.P. Experimental spinal cord injury and behavioral tests in laboratory rats. Heliyon 2019, 5, e01324. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Behari, J.; Avelev, V.D.; Mathur, R. Effect of magnetic field on food and water intake and body weight of spinal cord injured rats. Indian J. Exp. Biol. 2010, 48, 982–986. [Google Scholar] [PubMed]
- Manjhi, J.; Kumar, S.; Behari, J.; Mathur, R. Effect of extremely low frequency magnetic field in prevention of spinal cord injury-induced osteoporosis. J. Rehabil. Res. Dev. 2013, 50, 17–30. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, S.; Velpandian, T.; Petrovich Gerasimenko, Y.; D Avelev, V.; Behari, J.; Behari, M.; Mathur, R. Exposure to extremely low-frequency magnetic field restores spinal cord injury-induced tonic pain and its related neurotransmitter concentration in the brain. Electromagn. Biol. Med. 2013, 32, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Singh, A.; Nag, T.C.; Chattopadhyay, P.; Mathur, R.; Jain, S. Iron oxide nanoparticles and magnetic field exposure promote functional recovery by attenuating free radical-induced damage in rats with spinal cord transection. Int. J. Nanomed. 2013, 8, 2259–2272. [Google Scholar]
- Das, S.; Kumar, S.; Jain, S.; Avelev, V.D.; Mathur, R. Exposure to ELF- magnetic field promotes restoration of sensori-motor functions in adult rats with hemisection of thoracic spinal cord. Electromagn. Biol. Med. 2012, 31, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jain, S.; Mohanty, S.; Velpandian, T.; Vishnubhatla, S.; Mathur, R. Rat bone marrow stromal cell transplantation ameliorates complete spinal cord injury induced sensorimotor dysfunctions and associated neurotransmitters. Indian J. Exp. Biol. 2018, 56, 535–546. [Google Scholar]
- Kaur, C.; Ling, E.A.; Wong, W.C. Development of the various glial cell types in the cerebral cortex of postnatal rats. Acta Anat. 1989, 136, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.S.; Sharma, V.; Seidler, F.J.; Slotkin, T.A. Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Brain Res. Dev. Brain Res. 2005, 155, 71–80. [Google Scholar] [CrossRef]
- Hubscher, C.H.; Kaddumi, E.G.; Johnson, R.D. Segmental neuropathic pain does not develop in male rats with complete spinal transections. J. Neurotrauma 2008, 25, 1241–1245. [Google Scholar] [CrossRef]
- Hulsebosch, C.E.; Hains, B.C.; Crown, E.D.; Carlton, S.M. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 2009, 60, 202–213. [Google Scholar] [CrossRef]
- Yezierski, R.P. Spinal cord injury pain: Spinal and supraspinal mechanisms. J. Rehabil. Res. Dev. 2009, 46, 95–107. [Google Scholar] [CrossRef]
- Kumru, H.; Murillo, N.; Samso, J.V.; Valls-Sole, J.; Edwards, D.; Pelayo, R.; Valero-Cabre, A.; Tormos, J.M.; Pascual-Leone, A. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil. Neural. Repair. 2010, 24, 435–441. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Gorodnichev, R.; Machueva, E.; Pivovarova, E.; Semyenov, D.; Savochin, A.; Roy, R.R.; Edgerton, V.R. Novel and direct access to the human locomotor spinal circuitry. J. Neurosci. 2010, 30, 3700–3708. [Google Scholar] [CrossRef] [PubMed]
- Avelev, V.D.; Mathur, R.; Behari, D.; Shcherbakova, N.A.; Dorofeev, I.Y.; Savokhin, A.A.; Gerasimenko, Y.P. Initiation of locomotion in decerebrate cats by pulsed magnetic fields projected onto spinal cord segments. Neurosci. Behav. Physiol. 2011, 41, 91–96. [Google Scholar] [CrossRef]
- Crowe, M.J.; Sun, Z.P.; Battocletti, J.H.; Macias, M.Y.; Pintar, F.A.; Maiman, D.J. Exposure to pulsed magnetic fields enhances motor recovery in cats after spinal cord injury. Spine 2003, 28, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Wagdy, M.; Benjamin, M.; Mohamed, S.; Mohamed, H.; Ahmed, S. Therapeutic effects of acrobatic exercise and magnetic field exposure on functional recovery after spinal cord injury in mice. Bioelectromagnetics 2011, 32, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Chalfouh, C.; Guillou, C.; Hardouin, J.; Delarue, Q.; Li, X.; Duclos, C.; Schapman, D.; Marie, J.P.; Cosette, P.; Guérout, N. The Regenerative Effect of Trans-spinal Magnetic Stimulation after Spinal Cord Injury: Mechanisms and Pathways Underlying the Effect. Neurotherapeutics 2020, 17, 2069–2088. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, D.; Pang, R.; Deng, Q.; Sun, N.; Zheng, J.; Liu, J.; Xiang, W.; Chen, Z.; Lu, J.; et al. Effects of repetitive magnetic stimulation on motor function and GAP43 and 5-HT expression in rats with spinal cord injury. J. Int. Med. Res. 2020, 48, 300060520970765. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Sahu, S.; Kaur, S.; Jain, S. Effect of Low Intensity Magnetic Field Stimulation on Calcium-Mediated Cytotoxicity After Mild Spinal Cord Contusion Injury in Rats. Ann. Neurosci. 2020, 27, 49–56. [Google Scholar] [CrossRef]
- Dey, S.; Bose, S.; Kumar, S.; Rathore, R.; Mathur, R.; Jain, S. Extremely low frequency magnetic field protects injured spinal cord from the microglia- and iron-induced tissue damage. Electromagn. Biol. Med. 2017, 36, 330–340. [Google Scholar] [CrossRef]
- Liu, D.; McAdoo, D.J. Methylprednisolone reduces excitatory amino acid release following experimental spinal cord injury. Brain Res. 1993, 609, 293–297. [Google Scholar] [CrossRef]
- McAdoo, D.J.; Xu, G.Y.; Robak, G.; Hughes, M.G. Changes in amino acid concentrations over time and space around an impact injury and their diffusion through the rat spinal cord. Exp. Neurol. 1999, 159, 538–544. [Google Scholar] [CrossRef]
- Tanhoffer, R.A.; Yamazaki, R.K.; Nunes, E.A.; Pchevozniki, A.I.; Pchevozniki, A.M.; Nogata, C.; Aikawa, J.; Bonatto, S.J.; Brito, G.; Lissa, M.D.; et al. Glutamine concentration and immune response of spinal cord-injured rats. J. Spinal Cord Med. 2007, 30, 140–146. [Google Scholar] [CrossRef]
- Aira, Z.; Buesa, I.; Gallego, M.; del Caño, G.G.; Mendiable, N.; Mingo, J.; Rada, D.; Bilbao, J.; Zimmermann, M.; Azkue, J.J. Time-dependent cross talk between spinal serotonin 5-HT2A receptor and mGluR1 subserves spinal hyperexcitability and neuropathic pain after nerve injury. J. Neurosci. 2012, 32, 13568–13581. [Google Scholar] [CrossRef]
- Sheldon, A.L.; Robinson, M.B. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem. Int. 2007, 51, 333–355. [Google Scholar] [CrossRef]
- Hunanyan, A.S.; Petrosyan, H.A.; Alessi, V.; Arvanian, V.L. Repetitive spinal electromagnetic stimulation opens a window of synaptic plasticity in damaged spinal cord: Role of NMDA receptors. J. Neurophysiol. 2012, 107, 3027–3039. [Google Scholar] [CrossRef]
- Petrosyan, H.A.; Alessi, V.; Hunanyan, A.S.; Sisto, S.A.; Arvanian, V.L. Spinal electro-magnetic stimulation combined with transgene delivery of neurotrophin NT-3 and exercise: Novel combination therapy for spinal contusion injury. J. Neurophysiol. 2015, 114, 2923–2940. [Google Scholar] [CrossRef]
- Petrosyan, H.; Liang, L.; Tesfa, A.; Sisto, S.A.; Fahmy, M.; Arvanian, V.L. Modulation of H-reflex responses and frequency-dependent depression by repetitive spinal electromagnetic stimulation: From rats to humans and back to chronic spinal cord injured rats. Eur. J. Neurosci. 2020, 52, 4875–4889. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, J. Time course of segmental reflex changes after chronic spinal cord hemisection in the rat. Acta Physiol. Scand. 1983, 119, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cabré, A.; Forés, J.; Navarro, X. Reorganization of reflex responses mediated by different afferent sensory fibers after spinal cord transection. J. Neurophysiol. 2004, 91, 2838–2848. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Gaughan, C.L.; Lehning, E.J.; Kaneko, Y.; Kelly, T.M.; Blight, A. Experimental spinal cord injury: Spatiotemporal characterization of elemental concentrations and water contents in axons and neuroglia. J. Neurophysiol. 1999, 82, 2143–2153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Bandaru, S.P.; Liu, S.; Waxman, S.G.; Tan, A.M. Dendritic spine dysgenesis contributes to hyperreflexia after spinal cord injury. J. Neurophysiol. 2015, 113, 1598–1615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Carlo, A.L.; White, N.C.; Litovitz, T.A. Mechanical and electromagnetic induction of protection against oxidative stress. Bioelectrochemistry 2001, 53, 87–95. [Google Scholar] [CrossRef]
- Fanelli, C.; Coppola, S.; Barone, R.; Colussi, C.; Gualandi, G.; Volpe, P. Magnetic fields increase cell survival by inhibiting apoptosis via modulation of Ca2+ influx. FASEB J. 1999, 13, 95–102. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.C.; Prato, F.S.; Thomas, A.W. A literature review: The effects of magnetic field exposure on blood flow and blood vessels in the microvasculature. Bioelectromagnetics 2007, 28, 81–98. [Google Scholar] [CrossRef]
- Milgram, J.; Shahar, R.; Levin-Harrus, T.; Kass, P. The effect of short, high intensity magnetic field pulses on the healing of skin wounds in rats. Bioelectromagnetics 2004, 25, 271–277. [Google Scholar] [CrossRef]
- Hou, J.; Nelson, R.; Nissim, N.; Parmer, R.; Thompson, F.J.; Bose, P. Effect of combined treadmill training and magnetic stimulation on spasticity and gait impairments after cervical spinal cord injury. J. Neurotrauma 2014, 31, 1088–1106. [Google Scholar] [CrossRef]
- László, J.; Gyires, K. 3 T homogeneous static magnetic field of a clinical MR significantly inhibits pain in mice. Life Sci. 2009, 84, 12–17. [Google Scholar] [CrossRef]
- Bao, X.; Shi, Y.; Huo, X.; Song, T. A possible involvement of beta-endorphin, substance P, and serotonin in rat analgesia induced by extremely low frequency magnetic field. Bioelectromagnetics 2006, 27, 467–472. [Google Scholar] [CrossRef]
| Spinal Cord Regions | Glutamate Concentration * (Median with Range) | p-Value | ||||
|---|---|---|---|---|---|---|
| Sham Group (n = 8) | SCI Group (n = 6) | EMF Group (n = 7) | Sham vs. SCI | SCI vs. EMF | Sham vs. SCI | |
| Cervical | 20,045.9 (933.3–25,762.7) | 13,607.43 (3000–31,354.2) | 18,198.42 (4907.4–70,602.4) | 1.000 | 1.000 | 1.000 |
| Thoracic | 10,480 (2112.6–26,250) | 19,575.1 (8262.3–67,272.7) | 18,198.42 (4907.4–70,602.4) | 0.058 | 1.000 | 0.378 |
| Injury | 5989.58 (90–23,760) | 61,958.3 (11,303.0–173,333.3) | 12,314.3 (2884.2–61,600) | 0.001 | 0.044 | 0.644 |
| Lumbar | 16,297.2 (6785.7–23,418.8) | 20,542.1 (3633.3–46,800) | 22,810.6 (19075.6–30,731.7) | 1.000 | 1.000 | 0.324 |
| Sacral | 15,271.1 (6735.6–26,753.2) | 13277.1 (3571.4–43,846.1) | 24,714.8 (21,212.1–31,159.4) | 1.000 | 0.185 | 0.319 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Pal, A.; Jain, S.; Velpandian, T.; Mathur, R. Electromagnetic Field Stimulation Attenuates Phasic Nociception after Complete Spinal Cord Injury in Rats. Brain Sci. 2021, 11, 1431. https://doi.org/10.3390/brainsci11111431
Kumar S, Pal A, Jain S, Velpandian T, Mathur R. Electromagnetic Field Stimulation Attenuates Phasic Nociception after Complete Spinal Cord Injury in Rats. Brain Sciences. 2021; 11(11):1431. https://doi.org/10.3390/brainsci11111431
Chicago/Turabian StyleKumar, Suneel, Ajay Pal, Suman Jain, Thirumurthy Velpandian, and Rashmi Mathur. 2021. "Electromagnetic Field Stimulation Attenuates Phasic Nociception after Complete Spinal Cord Injury in Rats" Brain Sciences 11, no. 11: 1431. https://doi.org/10.3390/brainsci11111431
APA StyleKumar, S., Pal, A., Jain, S., Velpandian, T., & Mathur, R. (2021). Electromagnetic Field Stimulation Attenuates Phasic Nociception after Complete Spinal Cord Injury in Rats. Brain Sciences, 11(11), 1431. https://doi.org/10.3390/brainsci11111431








