Infratentorial Stereotactic Biopsy of Brainstem and Cerebellar Lesions
Abstract
:1. Introduction
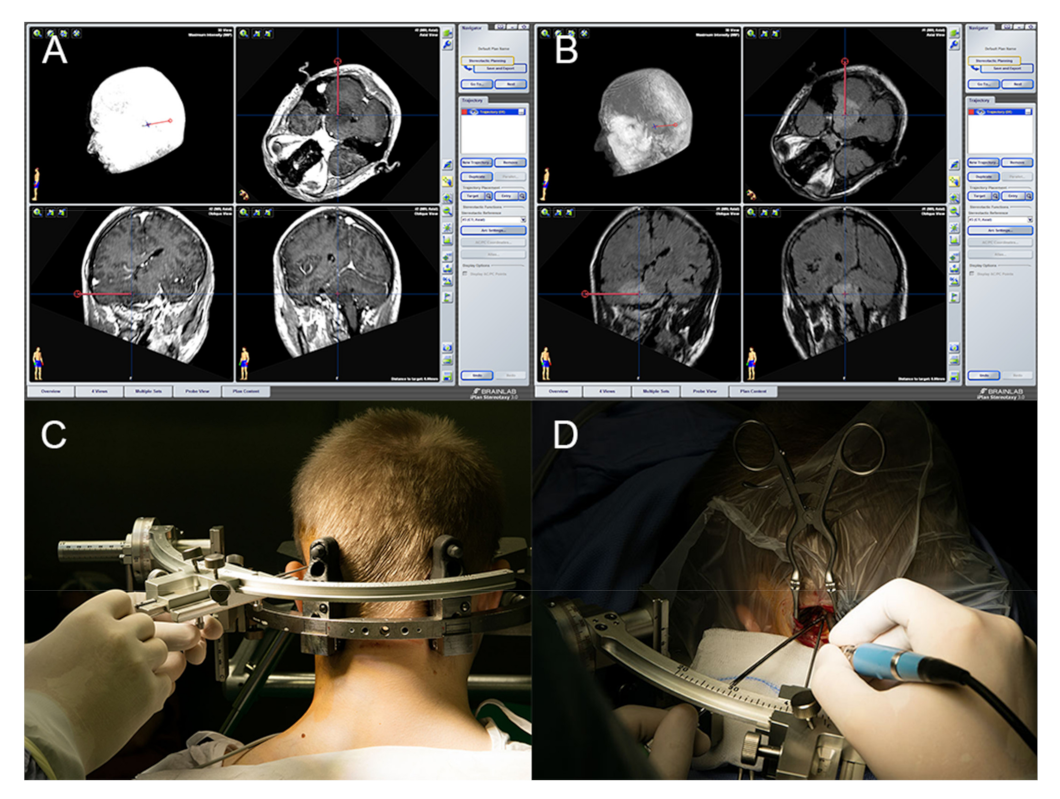
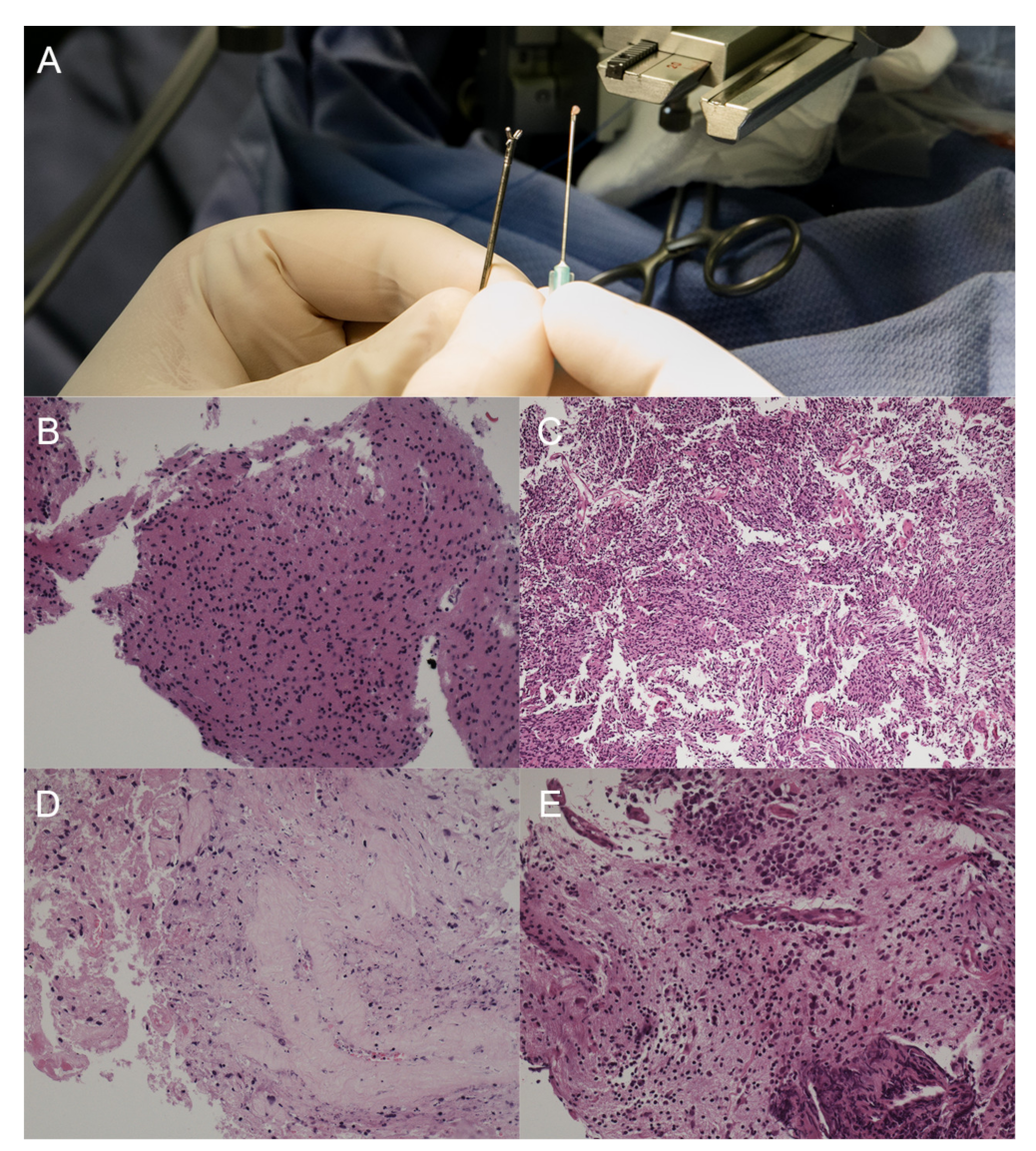
2. Materials and Methods
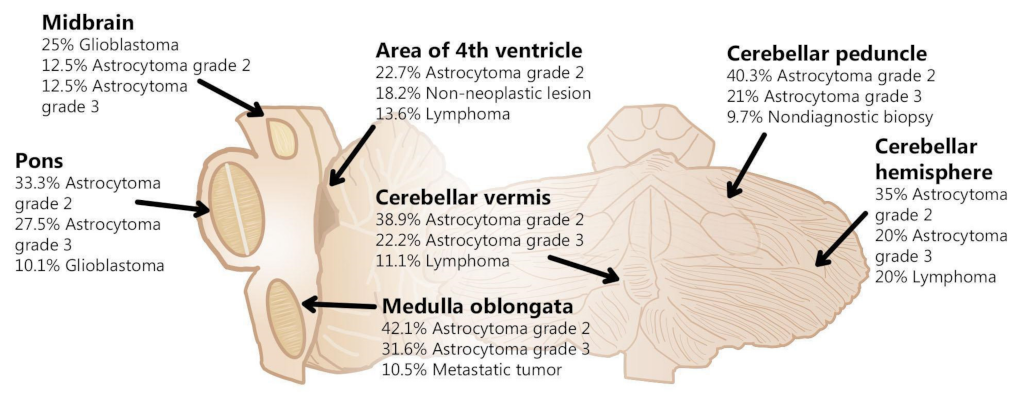
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laigle-Donadey, F.; Doz, F.; Delattre, J.-Y. Brainstem gliomas in children and adults. Curr. Opin. Oncol. 2008, 20, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Rachinger, W.; Grau, S.; Holtmannspötter, M.; Herms, J.; Tonn, J.-C.; Kreth, F.-W. Serial stereotactic biopsy of brainstem lesions in adults improves diagnostic accuracy compared with MRI only. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1134–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Reithmeier, T.; Lopez, W.O.; Doostkam, S.; Machein, M.; Pinsker, M.; Trippel, M.; Nikkhah, G. Intraindividual comparison of histopathological diagnosis obtained by stereotactic serial biopsy to open surgical resection specimen in patients with intracranial tumours. Clin. Neurol. Neurosurg. 2013, 115, 1955–1960. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. World Health Organization Classification of Tumours of the Central Nervous System, 5th ed.; International Agency for Research on Cancer: Paris, France, 2021. [Google Scholar]
- Kickingereder, P.; Willeit, P.; Simon, T.; Ruge, M.I. Diagnostic Value and Safety of Stereotactic Biopsy for Brainstem Tumors: A Systematic Review and Meta-analysis of 1480 Cases. Neurosurgery 2013, 72, 873–882. [Google Scholar] [CrossRef]
- Roujeau, T.; Machado, G.; Garnett, M.R.; Miquel, C.; Puget, S.; Geoerger, B.; Grill, J.; Boddaert, N.; Di Rocco, F.; Zerah, M.; et al. Stereotactic biopsy of diffuse pontine lesions in children. J. Neurosurg. Pediatr. 2007, 107, 1–4. [Google Scholar] [CrossRef]
- Pincus, D.W.; Richter, E.O.; Yachnis, A.T.; Bennett, J.; Bhatti, M.T.; Smith, A. Brainstem stereotactic biopsy sampling in children. J. Neurosurg. Pediatr. 2006, 104, 108–114. [Google Scholar] [CrossRef]
- Śledzińska, P.; Bebyn, M.G.; Furtak, J.; Kowalewski, J.; Lewandowska, M.A. Prognostic and Predictive Biomarkers in Gliomas. Int. J. Mol. Sci. 2021, 22, 10373. [Google Scholar] [CrossRef] [PubMed]
- McGirt, M.J.; Villavicencio, A.T.; Bulsara, K.R.; Friedman, A.H. MRI-guided stereotactic biopsy in the diagnosis of glioma: Comparison of biopsy and surgical resection specimen. Surg. Neurol. 2003, 59, 279–283. [Google Scholar] [CrossRef]
- Accuracy of Frameless and Frame-Based Image-Guided Stereotactic Brain Biopsy in the Diagnosis of Glioma: Comparison of Biopsy and Open Resection Specimen: Neurological Research. Available online: https://www.tandfonline.com/doi/abs/10.1179/016164105X40057 (accessed on 12 June 2021).
- Kondziolka, D.; Lunsford, L.D. Results and expectations with image-integrated brainstem stereotactic biopsy. Surg. Neurol. 1995, 43, 558–562. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Chen, C.-H.; Sun, M.-H.; Lee, H.-T.; Shen, C.-C. Stereotactic biopsy for brainstem lesion: Comparison of approaches and reports of 10 cases. J. Chin. Med. Assoc. 2011, 74, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Dellaretti, M.; Reyns, N.; Touzet, G.; Dubois, F.; Gusmão, S.; Pereira, J.L.B.; Blond, S. Stereotactic Biopsy for Brainstem Tumors: Comparison of Transcerebellar with Transfrontal Approach. Stereotact. Funct. Neurosurg. 2012, 90, 79–83. [Google Scholar] [CrossRef]
- Kelly, P.J.; Gonçalves-Ferreira, A.J.; Herculano-Carvalho, M.; Pimentel, J. Stereotactic biopsies of focal brainstem lesions. Surg. Neurol. 2003, 60, 311–320. [Google Scholar] [CrossRef]
- Mathon, B.; Amelot, A.; Mokhtari, K.; Bielle, F. Increasing the diagnostic yield of stereotactic brain biopsy using intraoperative histological smear. Clin. Neurol. Neurosurg. 2019, 186, 105544. [Google Scholar] [CrossRef]
- Sabbagh, A.J.; Alaqeel, A.M. Focal brainstem gliomas. Neurosciences 2015, 20, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, H.; Arnaout, O.; Hoshide, R.; Young, I.M.; Yeung, J.T.; Sughrue, M.E.; Teo, C. The Surgical Resection of Brainstem Glioma: Outcomes and Prognostic Factors. World Neurosurg. 2021, 146, e639–e650. [Google Scholar] [CrossRef]
- Cavalheiro, S.; Yagmurlu, K.; da Costa, M.D.S.; Nicácio, J.M.; Rodrigues, T.P.; Chaddad-Neto, F.; Rhoton, A.L. Surgical approaches for brainstem tumors in pediatric patients. Childs Nerv. Syst. 2015, 31, 1815–1840. [Google Scholar] [CrossRef] [Green Version]
- Schuman, L.M.; Choi, N.W.; Gullen, W.H. Relationship of central nervous system neoplasms to Toxoplasma gondii infection. Am. J. Public Health Nations Health 1967, 57, 848–856. [Google Scholar] [CrossRef]
- John Steck, M.D.; William, A.F.M.D. Stereotactic biopsy of brainstem mass lesions. Surg. Neurol. 1995, 43, 563–567. [Google Scholar] [CrossRef]
- Samadani, U.; Judy, K.D. Stereotactic Brainstem Biopsy Is Indicated for the Diagnosis of a Vast Array of Brainstem Pathology. Stereotact. Funct. Neurosurg. 2003, 81, 5–9. [Google Scholar] [CrossRef]
- Jackson, R.J.; Fuller, G.N.; Abi-Said, D.; Lang, F.F.; Gokaslan, Z.L.; Shi, W.M.; Wildrick, D.M.; Sawaya, R. Limitations of stereotactic biopsy in the initial management of gliomas. Neuro-oncology 2001, 3, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Sanai, N.; Wachhorst, S.P.; Gupta, N.M.; McDermott, M.W. Transcerebellar stereotactic biopsy for lesions of the brainstem and peduncles under local anesthesia. Neurosurgery 2008, 63, 460–466, discussion 466–468. [Google Scholar] [CrossRef]
- Tilgner, J.; Herr, M.; Ostertag, C.; Volk, B. Validation of Intraoperative Diagnoses Using Smear Preparations from Stereotactic Brain Biopsies: Intraoperative versus Final Diagnosis—Influence of Clinical Factors. Neurosurgery 2005, 56, 257–265. [Google Scholar] [CrossRef]
- Furtak, J.; Mielczarek, M.; Szylberg, M.; Harat, M. Biomarker concordance between molecular stereotactic biopsy and open surgical specimens in gliomas. Neurol. Neurochir. Pol. 2019, 53, 435–441. [Google Scholar] [CrossRef]
- Ramakonar, H.H. 220 A Stereotactic Brain Biopsy Needle Integrating an Optical Coherence Tomography (OCT) Probe with Blood Vessel Detection in Human Patients. Neurosurgery 2017, 64, 260. [Google Scholar] [CrossRef]
- Fritsch, M.J.; Leber, M.J.; Gossett, L.; Lulu, B.A.; Hamilton, A.J. Stereotactic Biopsy of Intracranial Brain Lesions. Stereotact. Funct. Neurosurg. 1998, 71, 36–42. [Google Scholar] [CrossRef]
- Fujimaki, T.; Hirata, S.; Terano, N.; Wakiya, K.; Adachi, J.-I.; Nishikawa, R.; Sasaki, A.; Kobayashi, M. Surg-21. stereotactic biopsy for deep seated brain lesions using the leksell stereotactic frame system. Neuro-Oncology 2019, 21, vi244. [Google Scholar] [CrossRef]
- Carruthers, V.B.; Suzuki, Y. Effects of Toxoplasma gondii Infection on the Brain. Schizophr. Bull. 2007, 33, 745–751. [Google Scholar] [CrossRef] [Green Version]
- Hodge, J.M.; Coghill, A.E.; Kim, Y.; Bender, N.; Smith-Warner, S.A.; Gapstur, S.; Teras, L.R.; Grimsrud, T.K.; Waterboer, T.; Egan, K.M. Toxoplasma gondii infection and the risk of adult glioma in two prospective studies. Int. J. Cancer 2021, 148, 2449–2456. [Google Scholar] [CrossRef]
- Jung, B.-K.; Song, H.; Kim, M.-J.; Cho, J.; Shin, E.-H.; Chai, J.-Y. High Toxoplasma gondii Seropositivity among Brain Tumor Patients in Korea. Korean J. Parasitol. 2016, 54, 201–204. [Google Scholar] [CrossRef]
- Vittecoq, M.; Elguero, E.; Lafferty, K.D.; Roche, B.; Brodeur, J.; Gauthier-Clerc, M.; Missé, D.; Thomas, F. Brain cancer mortality rates increase with Toxoplasma gondii seroprevalence in France. Infect. Genet. Evol. 2012, 12, 496–498. [Google Scholar] [CrossRef]
- Ryan, P.; Hurley, S.F.; Johnson, A.M.; Salzberg, M.; Lee, M.W.; North, J.B.; McNeil, J.J.; McMichael, A.J. Tumours of the brain and presence of antibodies to Toxoplasma gondii. Int. J. Epidemiol. 1993, 22, 412–419. [Google Scholar] [CrossRef]
- Muniz-Feliciano, L.; Grol, J.V.; Portillo, J.-A.C.; Liew, L.; Liu, B.; Carlin, C.R.; Carruthers, V.; Matthews, S.; Subauste, C.S. Toxoplasma gondii-Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite. PLoS Pathog. 2013, 9, e1003809. [Google Scholar] [CrossRef] [Green Version]
- Portillo, J.-A.C.; Muniz-Feliciano, L.; Corcino, Y.L.; Lee, S.J.; Van Grol, J.; Parsons, S.J.; Schiemman, W.P.; Subauste, C.S. Toxoplasma gondii induces FAK-Src-STAT3 signaling during infection of host cells that prevents parasite targeting by autophagy. PLoS Pathog. 2017, 13, e1006671. [Google Scholar] [CrossRef] [Green Version]
- Corcino, Y.L.; Portillo, J.-A.C.; Subauste, C.S. Epidermal growth factor receptor promotes cerebral and retinal invasion by Toxoplasma gondii. Sci. Rep. 2019, 9, 669. [Google Scholar] [CrossRef]
- Ginsberg, L.E.; Fuller, G.N.; Hashmi, M.; Leeds, N.E.; Schomer, D.F. The Significance of Lack of MR Contrast Enhancement of Supratentorial Brain Tumors in Adults: Histopathological Evaluation of a Series. Surg. Neurol. 1998, 49, 436–440. [Google Scholar] [CrossRef]
- Pallud, J.; Capelle, L.; Taillandier, L.; Fontaine, D.; Mandonnet, E.; Guillevin, R.; Bauchet, L.; Peruzzi, P.; Laigle-Donadey, F.; Kujas, M.; et al. Prognostic significance of imaging contrast enhancement for WHO grade II gliomas. Neuro-Oncology 2009, 11, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| N | % | ||
|---|---|---|---|
| Gender | 190 | 100.00% | |
| Female | 96 | 50.50% | |
| Male | 94 | 49.50% | |
| Age (years), mean ± SD | 41 | 16 | |
| KPS, mean ± SD | 84 | 14 | |
| Diagnosis | 190 | 100% | |
| Astrocytoma grade 2 | 68 | 35.80% | |
| Astrocytoma grade 3 | 37 | 19.50% | |
| Lymphoma | 17 | 8.90% | |
| Glioblastoma | 15 | 7.90% | |
| Non-neoplastic lesion | 14 | 7.40% | |
| Metastatic tumor | 10 | 5.30% | |
| Non-diagnostic biopsy | 8 | 4.20% | |
| NA | 5 | 2.60% | |
| Ependymoma | 4 | 2.10% | |
| Pilocytic astrocytoma | 4 | 2.10% | |
| Posterior fossa ependymoma grade 3 | 3 | 1.60% | |
| Oligodendroglioma grade 3 | 2 | 1.10% | |
| Medulloblastoma | 2 | 1.10% | |
| Meningioma grade 1 | 1 | 0.50% | |
| Side | 117 | 100% | |
| Left | 55 | 47.00% | |
| Right | 50 | 42.70% | |
| Both | 12 | 10.30% | |
| Approach | 145 | 100% | |
| Left | 76 | 52.40% | |
| Right | 69 | 47.60% | |
| Symptoms | 118 | 100.00% | |
| Blurred vision | 49 | 41.50% | |
| Balance disorders | 45 | 38.10% | |
| Paresthesia | 37 | 31.30% | |
| Vertigo | 33 | 27.90% | |
| Paresis | 25 | 21.20% | |
| Speech disorders | 21 | 17.80% | |
| Headache | 14 | 11.80% | |
| Epilepsy | 9 | 7.60% | |
| Dysphagia | 7 | 5.90% | |
| Multifocal tumors | 15 | 100% | |
| Lymphoma | 6 | 40% | |
| Astrocytoma grade 2 | 3 | 20% | |
| Astrocytoma grade 3 | 2 | 13.30% | |
| Glioblastoma | 2 | 13.30% | |
| Oligodendroglioma grade 3 | 1 | 6.70% | |
| Benign lesion | 1 | 6.70% | |
| Complications | |||
| Without complications | 179 | 94.50% | |
| Non-significant intracranial bleeding on CT | 4 | 2.00% | |
| Pain during biopsy | 2 | 1.00% | |
| Cerebrospinal fluid leak | 1 | 0.50% | |
| Temporary ataxia | 1 | 0.50% | |
| Worsening of paresis | 1 | 0.50% | |
| CN VII palsy | 1 | 0.50% | |
| Death | 1 | 0.50% | |
| Location | N | % | |
|---|---|---|---|
| Brainstem | 60 | 31.70% | |
| Pons | 22 | 11.60% | |
| Brainstem | 21 | 11.10% | |
| Pons + Medulla oblongata | 10 | 5.30% | |
| Midbrain | 3 | 1.60% | |
| Midbrain + Pons | 2 | 1.10% | |
| Medulla oblongata | 1 | 0.50% | |
| Midbrain + Pons + Medulla oblongata | 1 | 0.50% | |
| Cerebellum | 62 | 32.70% | |
| Cerebellar peduncle | 26 | 13.70% | |
| Cerebellar hemisphere | 25 | 13.20% | |
| Cerebellar vermis | 8 | 4.20% | |
| Cerebellar hemisphere + Cerebellar vermis | 2 | 1.10% | |
| Cerebellar peduncle + Cerebellar vermis | 1 | 0.50% | |
| Brainstem + Cerebellum | 46 | 24.30% | |
| Pons + Cerebellar peduncle | 21 | 11.10% | |
| Pons + Medulla oblongata + Cerebellar peduncle | 7 | 3.70% | |
| Pons + Cerebellar hemisphere | 5 | 2.60% | |
| Pons + Cerebellar peduncle + Cerebellar hemisphere | 4 | 2.10% | |
| Pons + Cerebellar vermis | 3 | 1.60% | |
| Midbrain + Pons + Cerebellar peduncle | 2 | 1.10% | |
| Pons + Cerebellar hemisphere + Cerebellar vermis | 2 | 1.10% | |
| Medulla oblongata + Cerebellar peduncle | 1 | 0.50% | |
| Pons + Cerebellar peduncle + Cerebellar vermis | 1 | 0.50% | |
| Area of 4th ventricle | 22 | 11.60% | |
| Area of 4th ventricle | 17 | 8.90% | |
| Cerebellar vermis + Area of 4th ventricle | 3 | 1.60% | |
| Pons + Medulla oblongata + Area of 4th ventricle | 2 | 1.10% |
| IDH1 Mutation | MGMT Promoter Methylation | Codeletion of 1p19q | ||||
|---|---|---|---|---|---|---|
| Diagnosis | Wildtype | Mutant | Unmethylated | Methylated | Non-Codeleted | Codeleted |
| Astrocytoma grade 3 | 10 | 1 | 3 | 7 | 10 | 0 |
| Astrocytoma grade 2 | 8 | 2 | 5 | 5 | 10 | 0 |
| Glioblastoma | 2 | 0 | 0 | 2 | 2 | 0 |
| Pilocytic astrocytoma | 1 | 0 | 1 | 0 | 1 | 0 |
| Grade | Pearson Chi-Square | Phi Coefficient | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LGG | HGG | Total | Value | df | p | Value | p | ||
| Toxoplasma gondii | Negative | 20 | 6 | 26 | 14.679 | 1 | <0.001 * | 0.565 | <0.001 |
| Positive | 4 | 16 | 20 | ||||||
| Total | 24 | 22 | 46 | ||||||
| Epstein–Barr virus | Negative | 2 | 3 | 5 | 0.402 | 1 | 0.526 a | −0.94 | 0.526 |
| Positive | 22 | 18 | 40 | ||||||
| Total | 24 | 21 | 45 | ||||||
| MRI contrast enhancement | No | 26 | 10 | 36 | 7.844 | 1 | 0.005 * | 0.313 | 0.005 |
| Yes | 18 | 26 | 44 | ||||||
| Total | 44 | 36 | 80 | ||||||
| Infratentorial | Supratentorial | ||||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Diagnostic material for histopathological evaluation | 181/190 | 95.30% | 61/63 | 96.80% | |
| Complications | |||||
| Without complications | 179 | 94.50% | 62 | 98.40% | |
| Non-significant intracranial bleeding on CT | 4 | 2.00% | |||
| Pain during biopsy | 2 | 1.00% | 1 | 1.60% | |
| Cerebrospinal fluid leak | 1 | 0.50% | |||
| Temporary ataxia | 1 | 0.50% | |||
| Worsening of paresis | 1 | 0.50% | |||
| CN VII palsy | 1 | 0.50% | |||
| Diagnostic material for molecular evaluation | 26/29 | 89.70% | 5/6 | 83.30% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtak, J.; Śledzińska, P.; Bebyn, M.G.; Szylberg, T.; Krajewski, S.; Birski, M.; Harat, M. Infratentorial Stereotactic Biopsy of Brainstem and Cerebellar Lesions. Brain Sci. 2021, 11, 1432. https://doi.org/10.3390/brainsci11111432
Furtak J, Śledzińska P, Bebyn MG, Szylberg T, Krajewski S, Birski M, Harat M. Infratentorial Stereotactic Biopsy of Brainstem and Cerebellar Lesions. Brain Sciences. 2021; 11(11):1432. https://doi.org/10.3390/brainsci11111432
Chicago/Turabian StyleFurtak, Jacek, Paulina Śledzińska, Marek G. Bebyn, Tadeusz Szylberg, Stanisław Krajewski, Marcin Birski, and Marek Harat. 2021. "Infratentorial Stereotactic Biopsy of Brainstem and Cerebellar Lesions" Brain Sciences 11, no. 11: 1432. https://doi.org/10.3390/brainsci11111432
APA StyleFurtak, J., Śledzińska, P., Bebyn, M. G., Szylberg, T., Krajewski, S., Birski, M., & Harat, M. (2021). Infratentorial Stereotactic Biopsy of Brainstem and Cerebellar Lesions. Brain Sciences, 11(11), 1432. https://doi.org/10.3390/brainsci11111432






