A New Approach to Assess Blinding for Transcranial Direct Current Stimulation Treatment in Patients with Fibromyalgia. A Randomized Clinical Trial
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Participants and Settings
2.3. Sample Size Calculation
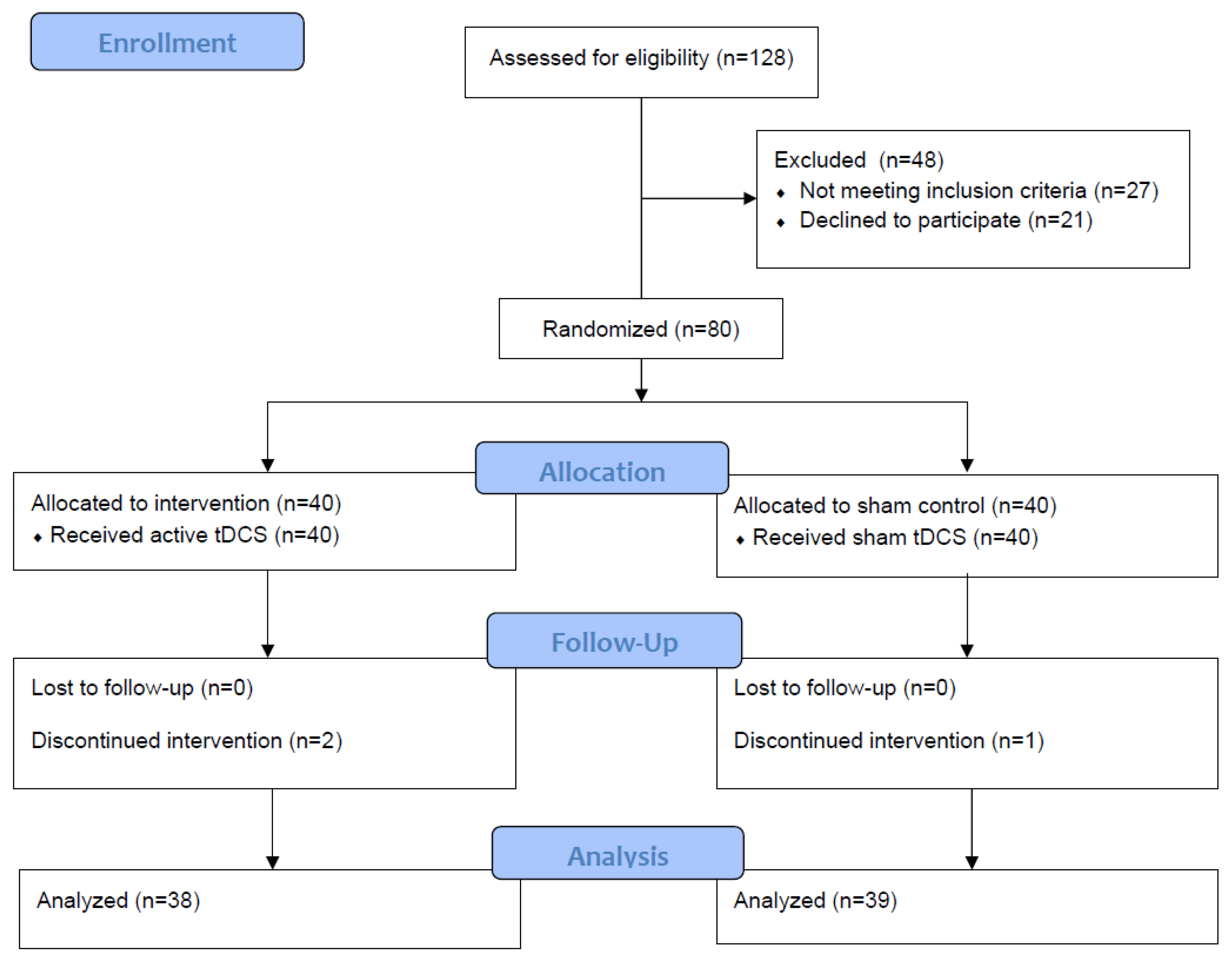
2.4. Randomization and Blinding
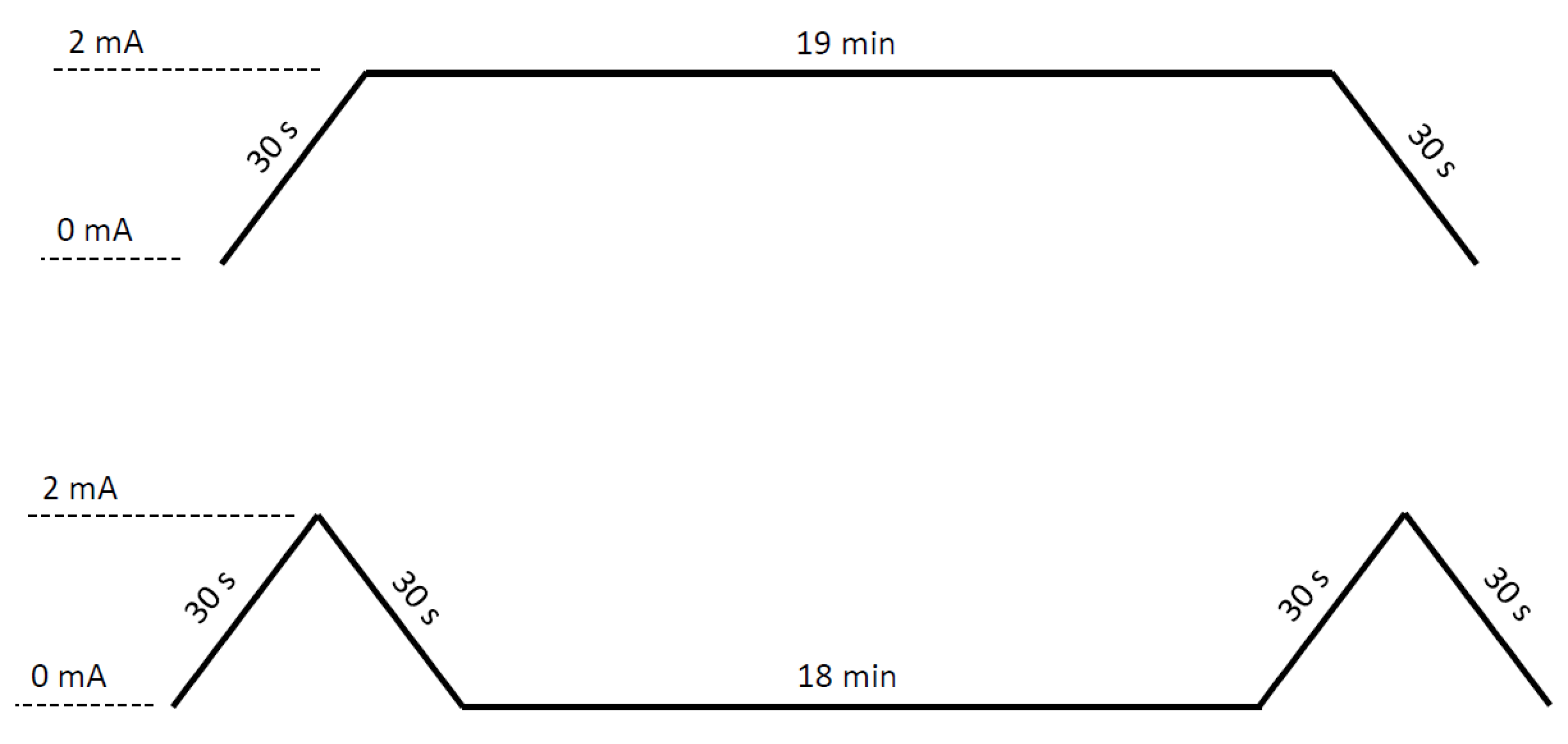
2.5. Intervention
2.6. Outcome Variables
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priori, A.; Berardelli, A.; Rona, S.; Accornero, N.; Manfredi, M. Polarization of the human motor cortex through the scalp. NeuroReport 1998, 9, 2257–2260. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef]
- Woods, A.; Antal, A.; Bikson, M.; Boggio, P.; Brunoni, A.R.; Celnik, P.; Cohen, L.; Fregni, F.; Herrmann, C.; Kappenman, E.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2015, 127, 1031–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.; Valero-Cabré, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2011, 5, 175–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khedr, E.M.; Omran, E.A.; Ismail, N.M.; El-Hammady, D.H.; Goma, S.H.; Kotb, H.; Galal, H.; Osman, A.M.; Farghaly, H.S.; Karim, A.A.; et al. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: A double blinded, randomized clinical trial. Brain Stimul. 2017, 10, 893–901. [Google Scholar] [CrossRef]
- Silva, A.F.; Zortea, M.; Carvalho, S.; Leite, J.; Torres, I.L.; Fregni, F.; Caumo, W. Anodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates attention and pain in fibromyalgia: Randomized clinical trial. Sci. Rep. 2017, 7, 135. [Google Scholar] [CrossRef]
- Roizenblatt, S.; Fregni, F.; Gimenez, R.; Wetzel, T.; Rigonatti, S.P.; Tufik, S.; Boggio, P.; Valle, A.C. Site-specific Effects of Transcranial Direct Current Stimulation on Sleep and Pain in Fibromyalgia: A Randomized, Sham-controlled Study. Pain Pr. 2007, 7, 297–306. [Google Scholar] [CrossRef]
- Fagerlund, A.J.; Hansen, O.A.; Aslaksen, P.M. Transcranial direct current stimulation as a treatment for patients with fibromyalgia. Pain 2015, 156, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Gimenes, R.; Valle, A.C.; Ferreira, M.J.L.; Rocha, R.R.; Natalle, L.; Bravo, R.; Rigonatti, S.P.; Freedman, S.D.; Nitsche, M.A.; et al. A randomized, sham-controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis Rheum. 2006, 54, 3988–3998. [Google Scholar] [CrossRef]
- Page, S.J.; Persch, A.C.; Clark, F.; Park, D.J.; Burke, J.P. Recruitment, Retention, and Blinding in Clinical Trials. Am. J. Occup. Ther. 2013, 67, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Karanicolas, P.J.; Farrokhyar, F.; Bhandari, M. Blinding: Who, what, when, why, how? Can. J. Surg. 2010, 53, 345–348. [Google Scholar]
- Boutron, I.; Tubach, F.; Giraudeau, B.; Ravaud, P. Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. J. Clin. Epidemiol. 2004, 57, 543–550. [Google Scholar] [CrossRef]
- Boutron, I.; Tubach, F.; Giraudeau, B.; Ravaud, P. Methodological Differences in Clinical Trials Evaluating Nonpharmacological and Pharmacological Treatments of Hip and Knee Osteoarthritis. JAMA 2003, 290, 1062–1070. [Google Scholar] [CrossRef] [Green Version]
- Arandjelovic, O. Assessing Blinding in Clinical Trials. Available online: https://proceedings.neurips.cc/paper/2012/file/d6baf65e0b240ce177cf70da146c8dc8-Paper.pdf (accessed on 25 September 2021).
- James, K.E.; Bloch, D.A.; Lee, K.K.; Kraemer, H.C.; Fuller, R.K. An Index for Assessing Blindness in a Multi-Centre Clinical Trial: Disulfiram for Alcohol Cessation—A VA Cooperative Study. Stat. Med. 1996, 15, 1421–1434. [Google Scholar] [CrossRef]
- Bang, H.; Ni, L.; Davis, C.E. Assessment of blinding in clinical trials. Control. Clin. Trials 2004, 25, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Rheum. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, A.; Palstam, A.; Löfgren, M.; Ernberg, M.; Bjersing, J.; Bileviciute-Ljungar, I.; Gerdle, B.; Kosek, E.; Mannerkorpi, K. Resistance exercise improves muscle strength, health status and pain intensity in fibromyalgia—a randomized controlled trial. Arthritis Res. Ther. 2015, 17, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaberzadeh, S.; Martin, D.; Knotkova, H.; Woods, A.J. Methodological Considerations for Selection of Transcranial Direct Current Stimulation Approach, Protocols and Devices. In Practical Guide to Transcranial Direct Current Stimulation; Springer: Cham, Switzerland, 2019; pp. 199–223. [Google Scholar] [CrossRef]
- Villamar, M.; Wivatvongvana, P.; Patumanond, J.; Bikson, M.; Truong, D.Q.; Datta, A.; Fregni, F. Focal Modulation of the Primary Motor Cortex in Fibromyalgia Using 4×1-Ring High-Definition Transcranial Direct Current Stimulation (HD-tDCS): Immediate and Delayed Analgesic Effects of Cathodal and Anodal Stimulation. J. Pain 2013, 14, 371–383. [Google Scholar] [CrossRef]
- Valle, A.; Roizenblatt, S.; Botte, S.; Zaghi, S.; Riberto, M.; Tufik, S.; Boggio, P.; Fregni, F. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: Results of a randomized, sham-controlled longitudinal clinical trial. J. Pain Manag. 2009, 2, 353–361. [Google Scholar] [PubMed]
- Riberto, M.; Alfieri, F.M.; Pacheco, K.M.D.B.; Leite, V.D.; Kaihami, H.N.; Fregni, F.; Battistella, L.R. Efficacy of Transcranial Direct Current Stimulation Coupled with a Multidisciplinary Rehabilitation Program for the Treatment of Fibromyalgia. Open Rheumatol. J. 2011, 5, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Mendonca, M.E.; Santana, M.B.; Baptista, A.F.; Datta, A.; Bikson, M.; Fregni, F.; Araujo, C.P. Transcranial DC Stimulation in Fibromyalgia: Optimized Cortical Target Supported by High-Resolution Computational Models. J. Pain 2011, 12, 610–617. [Google Scholar] [CrossRef]
- Bang, H.; Flaherty, S.P.; Kolahi, J.; Park, J. Blinding assessment in clinical trials: A review of statistical methods and a proposal of blinding assessment protocol. Clin. Res. Regul. Aff. 2010, 27, 42–51. [Google Scholar] [CrossRef]
- Kolahi, J.; Bang, H.; Park, J. Towards a proposal for assessment of blinding success in clinical trials: Up-to-date review. Community Dent. Oral Epidemiol. 2009, 37, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, G.G.; Al-Moyed, H.; Chaieb, L.; Sarp, L.; Antal, A.; Paulus, W. The fade-in–Short stimulation–Fade out approach to sham tDCS–Reliable at 1 mA for naïve and experienced subjects, but not investigators. Brain Stimul. 2012, 5, 499–504. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, N.E.; Cossar, J.; Marston, L.; Wand, B.M.; Bunce, D.; Moseley, L.; De Souza, L.H. Rethinking Clinical Trials of Transcranial Direct Current Stimulation: Participant and Assessor Blinding Is Inadequate at Intensities of 2 mA. PLoS ONE 2012, 7, e47514. [Google Scholar] [CrossRef] [Green Version]
- Palm, U.; Reisinger, E.; Keeser, D.; Kuo, M.-F.; Pogarell, O.; Leicht, G.; Mulert, C.; Nitsche, M.A.; Padberg, F. Evaluation of Sham Transcranial Direct Current Stimulation for Randomized, Placebo-Controlled Clinical Trials. Brain Stimul. 2013, 6, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Durand, S.; Fromy, B.; Bouyé, P.; Saumet, J.L.; Abraham, P. Vasodilatation in response to repeated anodal current application in the human skin relies on aspirin-sensitive mechanisms. J. Physiol. 2002, 540, 261–269. [Google Scholar] [CrossRef]
- Reckow, J.; Rahman-Filipiak, A.; Garcia, S.; Schlaefflin, S.; Calhoun, O.; DaSilva, A.F.; Bikson, M.; Hampstead, B.M. Tolerability and blinding of 4 × 1 high-definition transcranial direct current stimulation (HD-tDCS) at two and three milliamps. Brain Stimul. 2018, 11, 991–997. [Google Scholar] [CrossRef]
- Wallace, D.; Cooper, N.R.; Paulmann, S.; Fitzgerald, P.; Russo, R. Perceived Comfort and Blinding Efficacy in Randomised Sham-Controlled Transcranial Direct Current Stimulation (tDCS) Trials at 2 mA in Young and Older Healthy Adults. PLoS ONE 2016, 11, e0149703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunoni, A.R.; Schestatsky, P.; Lotufo, P.A.; Benseñor, I.M.; Fregni, F. Comparison of blinding effectiveness between sham tDCS and placebo sertraline in a 6-week major depression randomized clinical trial. Clin. Neurophysiol. 2014, 125, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Gbadeyan, O.; Steinhauser, M.; McMahon, K.; Meinzer, M. Safety, Tolerability, Blinding Efficacy and Behavioural Effects of a Novel MRI-Compatible, High-Definition tDCS Set-Up. Brain Stimul. 2016, 9, 545–552. [Google Scholar] [CrossRef] [PubMed]
| Outcome | Active tDCS n = 40 Mean (SD) | Sham tDCS n = 40 Mean (SD) | Between-Groups Comparison p-Value |
|---|---|---|---|
| Age (years) | 50.6 (7.0) | 49.5 (8.7) | 0.55 |
| Gender (women/men) | 38/2 | 39/1 | 1.0 |
| BMI | 27.2 (5.6) | 26.6 (5.6) | 0.59 |
| Duration of illness (years) | 11.9 (7.3) | 11.3 (6.6) | 0.73 |
| Widespread pain index | 13.4 (2.7) | 13.6 (2.5) | 0.73 |
| Symptoms severity scale | 8.7 (2.0) | 8.6 (1.9) | 0.77 |
| Pain (VAS) | 61.1 (14.1) | 59.9 (14.4) | 0.70 |
| Disease impact (FIQ) | 65.2 (14.6) | 62.9 (14.8) | 0.48 |
| Anxiety (STAI-ES) | 33.7 (10.2) | 31.8 (11.5) | 0.43 |
| Catastrophism (PCS) | 24.3 (12.3) | 25.0 (11.0) | 0.79 |
| Depression (BDI-II) | 18.9 (7.2) | 18.9 (7.6) | 1.0 |
| Participant’s Guess | ||||
| Allocation | Main Data, n (%) | |||
| Active tDCS | Sham tDCS | Do not know | Total | |
| Active tDCS | 6 (7.8) | 9 (11.7) | 24 (31.1) | 39 (50.6) |
| Sham tDCS | 10 (13.0) | 7 (9.1) | 21 (27.3) | 38 (49.4) |
| Total | 16 (20.8) | 16 (20.8) | 45 (58.4) | 77 (100.0) |
| Allocation | Ancillary Data, n (%) | |||
| Active tDCS | Sham tDCS | Not answered | Total | |
| Active tDCS | 8 (17.8) | 16 (35.5) | 0 (0.0) | 24 (53.3) |
| Sham tDCS | 10 (22.2) | 11 (24.5) | 0 (0.0) | 21 (46.7) |
| Total | 18 (40.0) | 27 (60.0) | 0 (0.0) | 45 (100.0) |
| Therapist’s Guess | ||||
| Allocation | Main Data, n (%) | |||
| Active tDCS | Sham tDCS | Do not know | Total | |
| Active tDCS | 19 (24.6) | 11 (14.3) | 9 (11.7) | 39 (50.6) |
| Sham tDCS | 11 (14.3) | 16 (20.8) | 11 (14.3) | 38 (49.4) |
| Total | 30 (39.0) | 27 (35.0) | 20 (26.0) | 77 (100.0) |
| Allocation | Ancillary Data, n (%) | |||
| Active tDCS | Sham tDCS | Not answered | Total | |
| Active tDCS | 4 (20.0) | 5 (25.0) | 0 (0.0) | 9 (45.0) |
| Sham tDCS | 6 (30.0) | 5 (25.0) | 0 (0.0) | 11 (55.0) |
| Total | 10 (50.0) | 10 (50.0) | 0 (0.0) | 20 (100.0) |
| Methods | Index | p-Value | 95% Confidence Interval | Conclusion |
|---|---|---|---|---|
| Participants | ||||
| James | 0.83 | 1.0 | 0.76–0.90 | Blinded |
| Bang—Active/2 × 3 | −0.08 | 0.78 | −0.24–0.09 | Blinded |
| Bang—Active/2 × 3 a | −0.13 | 0.90 | −0.29–0.04 | Blinded |
| Bang—Placebo/2 × 3 | −0.08 | 0.77 | −0.26–0.1 | Blinded |
| Bang—Placebo/2 × 3 a | −0.07 | 0.74 | −0.26–0.11 | Blinded |
| Therapist | ||||
| James | 0.55 | 0.79 | 0.45–0.64 | Blinded |
| Bang—Active/2 × 3 | 0.21 | 0.07 | −0.02–0.43 | Blinded |
| Bang—Active/2 × 3 a | 0.20 | 0.08 | −0.03–0.43 | Blinded |
| Bang—Placebo/2× 3 | 0.13 | 0.17 | −0.09–0.35 | Blinded |
| Bang—Placebo/2 × 3 a | 0.13 | 0.18 | −0.10–0.35 | Blinded |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroyo-Fernández, R.; Avendaño-Coy, J.; Velasco-Velasco, R.; Palomo-Carrión, R.; Bravo-Esteban, E.; Ferri-Morales, A. A New Approach to Assess Blinding for Transcranial Direct Current Stimulation Treatment in Patients with Fibromyalgia. A Randomized Clinical Trial. Brain Sci. 2021, 11, 1335. https://doi.org/10.3390/brainsci11101335
Arroyo-Fernández R, Avendaño-Coy J, Velasco-Velasco R, Palomo-Carrión R, Bravo-Esteban E, Ferri-Morales A. A New Approach to Assess Blinding for Transcranial Direct Current Stimulation Treatment in Patients with Fibromyalgia. A Randomized Clinical Trial. Brain Sciences. 2021; 11(10):1335. https://doi.org/10.3390/brainsci11101335
Chicago/Turabian StyleArroyo-Fernández, Rubén, Juan Avendaño-Coy, Rafael Velasco-Velasco, Rocío Palomo-Carrión, Elisabeth Bravo-Esteban, and Asunción Ferri-Morales. 2021. "A New Approach to Assess Blinding for Transcranial Direct Current Stimulation Treatment in Patients with Fibromyalgia. A Randomized Clinical Trial" Brain Sciences 11, no. 10: 1335. https://doi.org/10.3390/brainsci11101335
APA StyleArroyo-Fernández, R., Avendaño-Coy, J., Velasco-Velasco, R., Palomo-Carrión, R., Bravo-Esteban, E., & Ferri-Morales, A. (2021). A New Approach to Assess Blinding for Transcranial Direct Current Stimulation Treatment in Patients with Fibromyalgia. A Randomized Clinical Trial. Brain Sciences, 11(10), 1335. https://doi.org/10.3390/brainsci11101335






