Coping Strategies and Stress Related Disorders in Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Ethics
2.3. Scales Interpretation
2.4. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahn, J.S.; McIntosh, K. History and Recent Advances in Coronavirus Discovery. J. Pediatr. Infect. Dis. 2005, 24 (Suppl. 11), 223–227. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Memish, Z.A.; Perlman, S.; Van Kerkhove, M.D.; Zumla, A. Middle East Respiratory Syndrome (MERS). Lancet 2020, 395, 1063–1077. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Huang, Y.; Guo, Y.; Yin, M.; Chen, X.; Xiao, L.; Deng, G. Association of Inflammatory Markers with the Severity of COVID-19: A Meta-Analysis. Int. J. Infect. Dis. 2020, 96, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, X.; Tong, Q.; Li, W.; Wang, B.; Sutter, K.; Trilling, M.; Lu, M.; Dittmer, U.; Yang, D. Overlapping and Discrete Aspects of the Pathology and Pathogenesis of the Emerging Human Pathogenic Coronaviruses SARS-CoV, MERS-CoV, and 2019-NCoV. J. Med. Virol. 2020, 92, 491–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell J. 2020, 181, 271-280.e8. [Google Scholar] [CrossRef]
- Laza, R.; Musta, V.F.; Nicolescu, N.D.; Marinescu, A.R.; Mocanu, A.; Vilceanu, L.; Paczeyka, R.; Cut, T.G.; Lazureanu, V.E. Cutaneous Manifestations in SARS-CoV-2 Infection—A Series of Cases from the Largest Infectious Diseases Hospital in Western Romania. Healthcare 2021, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (ACE2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Devaux, C.A.; Rolain, J.M.; Raoult, D. ACE2 Receptor Polymorphism: Susceptibility to SARS-CoV-2, Hypertension, Multi-Organ Failure, and COVID-19 Disease Outcome. J. Microbiol. Immunol. Infect. 2020, 53, 425–435. [Google Scholar] [CrossRef]
- Meo, S.A.; Al-Khlaiwi, T.; Usmani, A.M.; Meo, A.S.; Klonoff, D.C.; Hoang, T.D. Biological and Epidemiological Trends in the Prevalence and Mortality Due to Outbreaks of Novel Coronavirus COVID-19. J. King Saud Univ. Sci. 2020, 32, 2495–2499. [Google Scholar] [CrossRef] [PubMed]
- Marincu, I.; Bratosin, F.; Vidican, I.; Bostanaru, A.C.; Frent, S.; Cerbu, B.; Turaiche, M.; Tirnea, L.; Timircan, M. Predictive Value of Comorbid Conditions for COVID-19 Mortality. J. Clin. Med. 2021, 10, 2652. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 Pandemic on Mental Health in the General Population: A Systematic Review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Torales, J.; O’Higgins, M.; Castaldelli-Maia, J.M.; Ventriglio, A. The Outbreak of COVID-19 Coronavirus and Its Impact on Global Mental Health. Int. J. Soc. Psychiatry 2020, 66, 317–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veer, I.M.; Riepenhausen, A.; Zerban, M.; Wackerhagen, C.; Puhlmann, L.; Engen, H.; Kober, G.; Bogemann, S.; Weermeijer, S.; Uscilko, A.; et al. Psycho-social factors associated with mental resilience in the Corona lockdown. Transl Psychiatry. 2021, 11, 67. [Google Scholar] [CrossRef]
- Kunzler, A.M.; Röthke, N.; Günthner, L.; Stoffers-Winterling, J.; Tüscher, O.; Coenen, M.; Rehfuess, E.; Schwarzer, G.; Binder, H.; Schmucker, C.; et al. Mental burden and its risk and protective factors during the early phase of the SARS-CoV-2 pandemic: Systematic review and meta-analyses. Glob. Health 2021, 17, 1–29. [Google Scholar] [CrossRef]
- Daviu, N.; Bruchas, M.R.; Moghaddam, B.; Sandi, C.; Beyeler, A. Neurobiological Links between Stress and Anxiety. Neurobiol. Stress 2019, 11, 100191. [Google Scholar] [CrossRef]
- Endler, N.S.; Kocovski, N.L. State and Trait Anxiety Revisited. J. Anxiety Disord. 2001, 15, 231–245. [Google Scholar] [CrossRef]
- Takagi, Y.; Sakai, Y.; Abe, Y.; Nishida, S.; Harrison, B.J.; Martínez-Zalacaín, I.; Soriano-Mas, C.; Narumoto, J.; Tanaka, S.C. A Common Brain Network among State, Trait, and Pathological Anxiety from Whole-Brain Functional Connectivity. Neuroimage 2018, 172, 506–516. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Garofalo, S.; Tortora, F.; Avenanti, A.; di Pellegrino, G. State-Dependent TMS over Prefrontal Cortex Disrupts Fear-Memory Reconsolidation and Prevents the Return of Fear. Curr. Biol. 2020, 30, 3672–3679. [Google Scholar] [CrossRef]
- Borgomaneri, S.; Battaglia, S.; Avenanti, A.; Pellegrino, G.D. Don’t Hurt Me No More: State-dependent Transcranial Magnetic Stimulation for the treatment of specific phobia. J. Affect Disord. 2021, 286, 78–79. [Google Scholar] [CrossRef]
- Herrmann, M.J.; Katzorke, A.; Busch, Y.; Gromer, D.; Polak, T.; Pauli, P.; Deckert, J. Medial prefrontal cortex stimulation accelerates therapy response of exposure therapy in acrophobia. Brain Stimul. 2017, 10, 291–297. [Google Scholar] [CrossRef]
- Association, A.P. Trauma and Stressor-Related Disorders. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Springer Pub. Co.: Heidelberg, Germany, 2013; pp. 271–286. [Google Scholar]
- Usher, K.; Durkin, J.; Bhullar, N. The COVID-19 Pandemic and Mental Health Impacts. Int. J. Ment. Health Nurs. 2020, 29, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Olff, M.; Langeland, W.; Gersons, B.P.R. Effects of Appraisal and Coping on the Neuroendocrine Response to Extreme Stress. Neurosci. Biobehav. Rev. 2005, 29, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Higgins, V.; Sohaei, D.; Diamandis, E.P.; Prassas, I. COVID-19: From an Acute to Chronic Disease? Potential Long-Term Health Consequences. Crit. Rev. Clin. Lab. Sci. 2021, 58, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Shalev, D.; Hsu, I.; Shenoy, A.; Cheung, S.; Nash, S.; Wiener, I.; Fedoronko, D.; Allen, N.; Shapiro, P.A. Depression, Anxiety, and Acute Stress Disorder Among Patients Hospitalized with Coronavirus Disease 2019: A Prospective Cohort Study. J. Acad. Consult. Psychiatry 2020, 62, 211–219. [Google Scholar] [CrossRef]
- Lazarus, R.S.F.S. Cognitive Appraisal Processes. In Stress, Appraisal, and Coping; Springer Pub. Co.: New York, NY, USA, 1984; pp. 22–52. [Google Scholar]
- Carver, C.S.; Scheier, M.F. Assessing Coping Strategies: A Theoretically Based Approach. J. Pers. Soc. Psychol. 1989, 56, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Gol, A.R.; Cook, S.W. Exploring the Underlying Dimensions of Coping: A Concept Mapping Approach. J. Soc. Clin. Psychol. 2004, 23, 155–171. [Google Scholar] [CrossRef]
- David, J.P.; Green, P.J.; Martin, R.; Suls, J. Differential Roles of Neuroticism, Extraversion, and Event Desirability for Mood in Daily Life: An Integrative Model of Top-Down and Bottom-Up Influences. J. Pers. Soc. Psychol. 1997, 73, 149–159. [Google Scholar] [CrossRef]
- Louvet, B.; Gaudreau, P.; Menaut, A.; Genty, J.; Deneuve, P. Longitudinal Patterns of Stability and Change in Coping across Three Competitions: A Latent Class Growth Analysis. J. Sport Exerc. Psychol. 2007, 29, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Stanisławski, K. The Coping Circumplex Model: An Integrative Model of the Structure of Coping with Stress. Front. Psychol. 2019, 10, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Shahrour, G.; Dardas, L.A. Acute Stress Disorder, Coping Self-Efficacy and Subsequent Psychological Distress among Nurses amid COVID-19. J. Nurs. Manag. 2020, 28, 1686–1695. [Google Scholar] [CrossRef]
- Carver, C.S.; Connor-Smith, J. Personality and Coping. Annu. Rev. Psychol. 2010, 61, 679–704. [Google Scholar] [CrossRef] [Green Version]
- Kato, T. Frequently Used Coping Scales: A Meta-Analysis. Stress Health 2015, 31, 315–323. [Google Scholar] [CrossRef]
- Crașovan, D.I.; Sava, F.A. Translation, Adaptation and Validation on Romanian Population of COPE Questionnaire for Coping Mechanisms Analysis. Cogn. Brain Behav. 2013, 17, 61–76. [Google Scholar]
- Garofalo, S.; Battaglia, S.; di Pellegrino, G. Individual differences in working memory capacity and cue-guided behavior in humans. Sci. Rep. 2019, 9, 7327. [Google Scholar] [CrossRef]
- Garofalo, S.; Battaglia, S.; Starita, F.; di Pellegrino, G. Modulation of cue-guided choices by transcranial direct current stimulation. Cortex 2021, 137, 124–137. [Google Scholar] [CrossRef]
- Gurvich, C.; Thomas, N.; Thomas, E.H.X.; Hudaib, A.; Sood, L.; Fabiatos, K.; Sutton, K.; Isaacs, A.; Arunogiri, S.; Sharp, G.; et al. Coping Styles and Mental Health in Response to Societal Changes during the COVID-19 Pandemic. Int. J. Soc. Psychiatry 2021, 67, 540–549. [Google Scholar] [CrossRef]
- Holt-Gosselin, B.; Tozzi, L.; Ramirez, C.A.; Ian, H. Coping Strategies, Neural Structure, and Depression and Anxiety during the COVID-19 Pandemic: A Longitudinal Study in a Naturalistic Sample Spanning Clinical Diagnoses and Subclinical Symptoms. Biol. Psychiatry Glob. Open Sci. 2021, 1, 1–24. [Google Scholar] [CrossRef]
- Talarowska, M.; Chodkiewicz, J.; Nawrocka, N.; Miniszewska, J.; Bili, P. Mental Health and the SARS-COV-2 Epidemic—Polish Research Study. Int. J. Environ. Res. Public Health 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.Y.Y.; Liu, L.; Tian, Y.S.S.; Yi, Y.W.; Liu, A.Z.S.S.X.; Zhang, Y.; Lin, X.; Shi, L.; Yan, W.; Fazel, S.; et al. Prevalence of Posttraumatic Stress Disorder after Infectious Disease Pandemics in the Twenty-First Century, Including COVID-19: A Meta- Analysis and Systematic Review. Mol. Psychiatry 2021, 4, 1–17. [Google Scholar] [CrossRef]
- De Lorenzo, R.; Conte, C.; Lanzani, C.; Benedetti, F.; Roveri, L.; Mazza, M.G.; Brioni, E.; Giacalone, G.; Canti, V.; Sofia, V.; et al. Residual Clinical Damage after COVID-19: A Retrospective and Prospective Observational Cohort Study. PLoS ONE 2020, 15, e0239570. [Google Scholar] [CrossRef] [PubMed]
- Association, A.M. Posttraumatic Stress Disorder in Patients after Severe COVID-19 Infection. JAMA Psychiatry 2021, 78, 567–569. [Google Scholar] [CrossRef] [Green Version]
- Amir, M.; Kaplan, Z.; Kotler, M. Type of Trauma, Severity of Posttraumatic Stress Disorder Core Symptoms, and Associated Features. J. Gen. Psychol. 1996, 123, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Righy, C.; Rosa, R.G.; Da Silva, R.T.A.; Kochhann, R.; Migliavaca, C.B.; Robinson, C.C.; Teche, S.P.; Teixeira, C.; Bozza, F.A.; Falavigna, M. Prevalence of Post-Traumatic Stress Disorder Symptoms in Adult Critical Care Survivors: A Systematic Review and Meta-Analysis. Crit. Care 2019, 23, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldwin, C.M.; Yancura, L.A. Coping and Health: A Comparison of the Stress and Trauma Literatures. In Physical Health Consequences of Exposure to Extreme Stress; American Psychological Association: Washington, DC, USA, 2004; pp. 2–56. [Google Scholar]
- Bernstein, E.; Rosser-Hogan, R. Trauma Experiences, Posttraumatic Stress, Dissociation and Depression in Cambodian Refugees. Am. J. Psychiatry 1991, 148, 1548–1551. [Google Scholar] [CrossRef]
- Bremner, J.D.; Southwick, S.; Brett, E.; Fontana, A.; Rosenheck, R.; Charney, D.S. Dissociation and Posttraumatic Stress Disorder in Vietnam Combat Veterans. Am. J. Psychiatry 1992, 149, 328–332. [Google Scholar] [CrossRef]
- Shalev, A.Y.; Peri, T. Predictors of PTSD in Injured Trauma Survivors: A Prospective Study. Am. J. Psychiatry 1996, 153, 219–225. [Google Scholar]
- Nussbaum, L.; Hogea, L.; Folescu, R.; Grigoraș, M.; Zamfir, C.; Boancă, M.; Erdelean, D.; Cecilia, E.; Rosca, I.; Nussbaum, L.; et al. Biochemical Modifications Study of Cerebral Metabolites by Spectroscopy in Epilepsy Treatment. Rev. Chim. 2018, 69, 965–970. [Google Scholar] [CrossRef]
- Sherin, J.E.; Nemeroff, C.B. Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011, 13, 263–278. [Google Scholar] [CrossRef]
- Ehring, T. Emotion Regulation Difficulties in Trauma Survivors: The Role of Trauma Type and PTSD Symptom Severity. Behav. Ther. 2010, 41, 587–598. [Google Scholar] [CrossRef]
- Kim, H.Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2018, 73, 143–153. [Google Scholar]
- Michopoulos, V.; Vester, A.; Neigh, G. Posttraumatic stress disorder: A metabolic disorder in disguise? Exp. Neurol. 2016, 284 (Pt B), 220–229. [Google Scholar] [CrossRef] [Green Version]
- Eraly, S.A.; Nievergelt, C.M.; Maihofer, A.X.; Barkauskas, D.A.; Biswas, N.; Agorastos, A.; O’Connor, D.T.; Baker, D.G. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry 2014, 71, 423–431. [Google Scholar] [CrossRef]
- Pervanidou, P.; Kolaitis, G.; Charitaki, S.; Margeli, A.; Ferentinos, S.; Bakoula, C.; Lazaropoulou, C.; Papassotiriou, I.; Tsiantis, J.; Chrousos, G.P. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology 2007, 32, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Dhabhar, F.S.; Mellon, S.H.; Yehuda, R.; Grenon, S.M.; Flory, J.D.; Bierer, L.M.; Abu-Amara, D.; Coy, M.; Makotkine, I.; et al. Increased pro-inflammatory milieu in combat related PTSD—A new cohort replication study. Brain Behav. Immun. 2017, 59, 260–264. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.; Driscoll, D.; Smith, L.M.; Ramaswamy, S. The role of inflammation in late-life post-traumatic stress disorder. Mil. Med. 2017, 182, e1815–e1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passos, I.C.; Vasconcelos-Moreno, M.P.; Costa, L.G.; Kunz, M.; Brietzke, E.; Quevedo, J.; Salum, G.; Magalhaes, P.V.; Kapczinski, F.; Kauer-Sant’Anna, M. Inflammatory markers in post-traumatic stress disorder: A systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015, 2, 1002–1012. [Google Scholar] [CrossRef]
- Imai, R.; Hori, H.; Itoh, M.; Lin, M.; Niwa, M.; Ino, K.; Ogawa, S.; Ishida, M.; Sekiguchi, A.; Matsui, M.; et al. Inflammatory markers and their possible effects on cognitive function in women with posttraumatic stress disorder. J. Psychiatry Res. 2018, 102, 192–200. [Google Scholar] [CrossRef]
- de Oliveira, J.F.; Wiener, C.D.; Jansen, K.; Portela, L.V.; Lara, D.R.; Souza, L.D.M.; da Silva, R.A.; Moreira, F.P.; Oses, J.P. Serum levels of interleukins IL-6 and IL-10 in individuals with posttraumatic stress disorder in a population-based sample. Psychiatry 2018, 260, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Katrinli, S.; Lori, A.; Kilaru, V.; Carter, S.; Powers, A.; Gillespie, C.F.; Wingo, A.P.; Michopoulos, V.; Jovanovic, T.; Ressler, K.J.; et al. Association of HLA locus alleles with posttraumatic stress disorder. Brain Behav. Immun. 2019, 81, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, L.M. Immune proteins in brain development and synaptic plasticity. Neuron 2009, 64, 93–109. [Google Scholar] [CrossRef] [Green Version]
- Elmer, B.M.; McAllister, A.K. Major histocompatibility complex class I proteins in brain development and plasticity. Trends Neurosci. 2012, 35, 660–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankar, A.; MacKenzie, R.N.; Foster, J.A. Loss of class I MHC function alters behavior and stress reactivity. J. Neuroimmunol. 2012, 244, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, A.; Costa, E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3α, 5α-tetrahydroprogesterone (allopregnanolone) availability? Biol. Psychiatry 1998, 44, 865–873. [Google Scholar] [CrossRef]
- Pibiri, F.; Nelson, M.; Guidotti, A.; Costa, E.; Pinna, G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc. Natl. Acad. Sci. USA 2008, 105, 5567–5572. [Google Scholar] [CrossRef] [Green Version]
- Mahar, I.; Bambico, F.R.; Mechawar, N.; Nobrega, J.N. Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 2014, 38, 173–192. [Google Scholar] [CrossRef]
- Hassell, J.E., Jr.; Collins, V.E.; Li, H.; Rogers, J.T.; Austin, R.C.; Visceau, C.; Renner, K.J. Local inhibition of uptake2 transporters augments stress-induced increases in serotonin in the rat central amygdala. Neurosci. Lett. 2019, 701, 119–124. [Google Scholar] [CrossRef]
- Lu, Q.; Mouri, A.; Yang, Y.; Kunisawa, K.; Teshigawara, T.; Hirakawa, M.; Saito, K. Chronic unpredictable mild stress-induced behavioral changes are coupled with dopaminergic hyperfunction and serotonergic hypofunction in mouse models of depression. Behav. Brain Res. 2019, 372, 112053. [Google Scholar] [CrossRef] [PubMed]
- Katrinli, S.; Smith, A.K. Immune system regulation and role of the human leukocyte antigen in posttraumatic stress disorder. Neurobiol. Stress 2021. [Google Scholar] [CrossRef] [PubMed]
| Sample Characteristics | Disease Category | p-Value | ||
|---|---|---|---|---|
| Acute (n = 45) | Remitted (n = 45) | |||
| Age (mean) | 61 ± 14 | 55 ± 15 | 0.073 | |
| Sex | 0.205 | |||
| Male | 20(42.5%) | 27(57.5%) | ||
| Female | 25(58.1%) | 18(41.9%) | ||
| Civil Status | 0.398 | |||
| Married | 34(75.6%) | 35(77.8%) | ||
| Single | 4(8.9%) | 3(6.7%) | ||
| Divorced | 1(2.2%) | 4(8.9%) | ||
| Widowed | 6(13.3%) | 3(6.7%) | ||
| Children | 0.334 | |||
| Yes | 38(84.4%) | 41(91.1%) | ||
| No | 7(15.6%) | 4(8.9%) | ||
| Income | 0.010 | |||
| Low | 28(62.2%) | 17(37.8%) | ||
| Medium | 12(26.7%) | 11(24.4%) | ||
| High | 5(11.1%) | 17(37.8%) | ||
| Disease Severity | 0.837 | |||
| Mild | 9(20.0%) | 7(15.6%) | ||
| Moderate | 18(40.0%) | 20(44.4%) | ||
| Severe | 18(40.0%) | 18(40.0%) | ||
| Comorbidities | ||||
| Cardiovascular | 25(55.6%) | 18(40.0%) | 0.103 | |
| Diabetes | 4(8.9%) | 13(28.9%) | 0.015 | |
| Metabolic | 7(15.6%) | 8(17.8%) | 0.500 | |
| Neurologic | 3(6.7%) | 1(2.2%) | 0.308 | |
| Neoplasia | 4(8.9%) | 1(2.2%) | 0.180 | |
| Survey/Scale | NSESSS-Total (>14) | PCL-Total (>44) | <0.009 | |
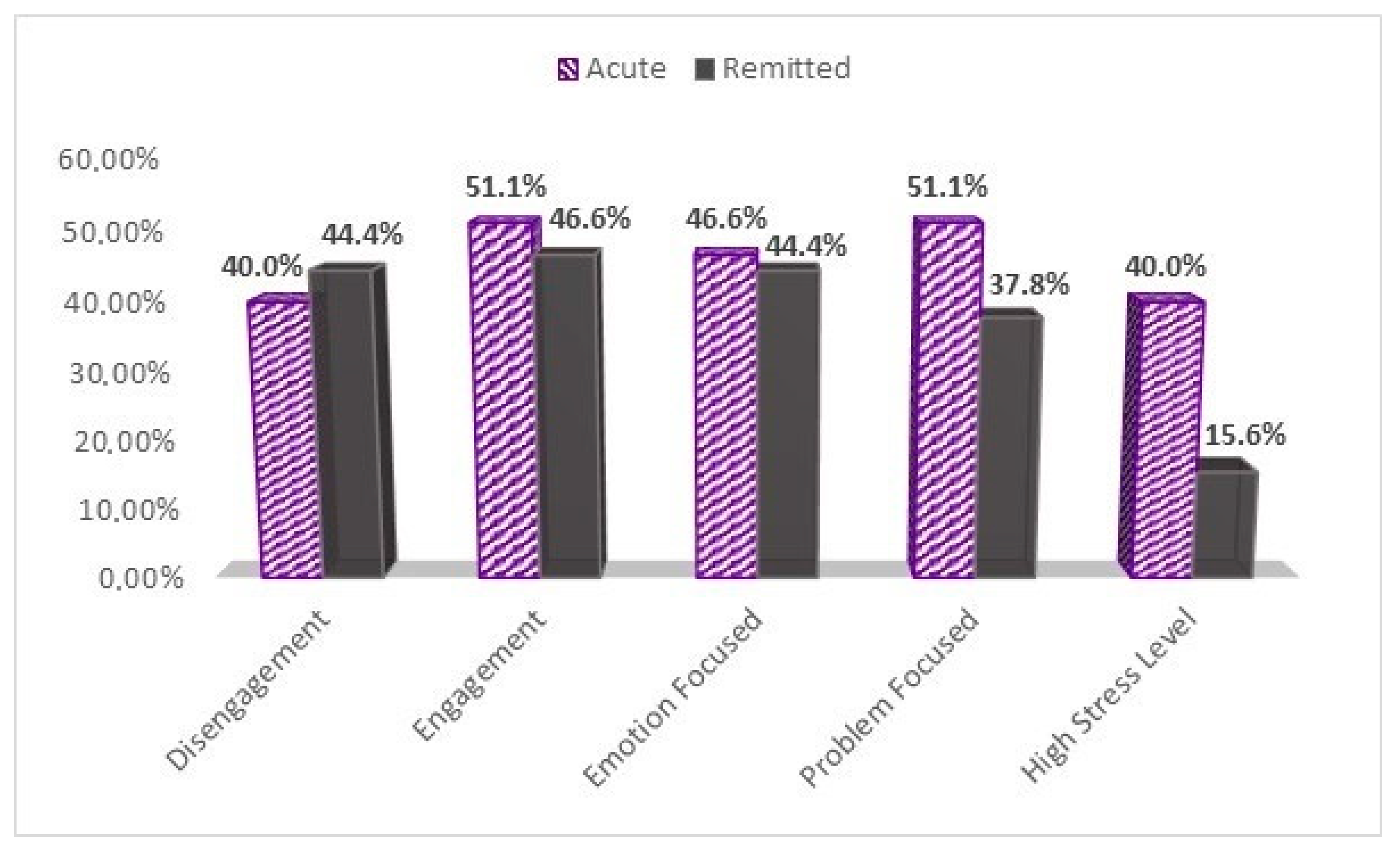
| Low-stress | 27(60.0%) | 38(84.4%) | ||
| High-Stress | 18(40.0%) | 7(15.6%) | ||
| COPE-60 | ||||
| Disengagement (2) | >Median | 18(40%) | 20(44.4%) | 0.046 |
| ≤Median | 27(60%) | 25(55.6%) | ||
| Engagement (3) | >Median | 23(51.1%) | 21(46.6%) | 0.044 |
| ≤Median | 22(48.8%) | 24(53.3%) | ||
| Emotion Focused (2) | >Median | 21(46.6%) | 20(44.4%) | <0.000 |
| ≤Median | 24(53.3%) | 25(55.6%) | ||
| Problem Focused (3) | >Median | 23(51.1%) | 17(37.8%) | 0.125 |
| ≤Median | 22(48.8%) | 28(62.2%) | ||
| Disease Category (Acute/Remitted) | Disease Severity | |||
|---|---|---|---|---|
| Correlation | p-Value | Correlation | p-Value | |
| Sex(male) | 0.156 | 0.143 | 0.321 | 0.002 |
| Age | −0.186 | 0.080 | 0.386 | <0.001 |
| Civil Status | −0.034 | 0.750 | −0.226 | 0.032 |
| Children | 0.102 | 0.340 | 0.047 | 0.663 |
| Income | 0.300 | 0.004 | −0.117 | 0.270 |
| Cardiovascular | −0.156 | 0.143 | 0.246 | 0.019 |
| Diabetes | 0.255 | 0.015 | 0.236 | 0.025 |
| Metabolic Disease | 0.030 | 0.780 | 0.043 | 0.684 |
| Neurologic | −0.108 | 0.312 | 0.076 | 0.475 |
| Neoplasia | −0.146 | 0.171 | 0.123 | 0.247 |
| Stress | −0.398 | <0.001 | 0.035 | 0.743 |
| Disengagement | −0.078 | 0.468 | 0.047 | 0.661 |
| Engagement | −0.057 | 0.591 | −0.152 | 0.154 |
| Emotion-Focused | −0.111 | 0.296 | −0.173 | 0.103 |
| Problem Focus | −0.112 | 0.293 | −0.063 | 0.558 |
| Disengagement | Engagement | Emotion-Focused | Problem Focus | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | p | CI | R2 | p | CI | R2 | p | CI | R2 | p | CI | |||||
| Disease Category | 0.69 | −0.62 | 0.94 | 0.58 | −1.01 | 0.56 | 0.83 | −0.86 | 0.69 | 0.21 | −1.28 | 0.28 | ||||
| Disease Severity | 0.09 | 0.69 | −0.39 | 0.59 | 0.03 | 0.11 | −0.90 | 0.09 | 0.11 | 0.04 | −1.01 | −0.09 | 0.02 | 0.48 | −0.67 | 0.31 |
| Stress Level | (0.04) * | 0.01 | 0.32 | 2.02 | (0.44) * | 0.85 | −0.74 | 0.90 | (0.02) * | 0.02 | 0.17 | 1.85 | (0.56) * | 0.53 | −1.08 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehelean, L.; Papava, I.; Musat, M.I.; Bondrescu, M.; Bratosin, F.; Bucatos, B.O.; Bortun, A.-M.C.; Mager, D.V.; Romosan, R.S.; Romosan, A.-M.; et al. Coping Strategies and Stress Related Disorders in Patients with COVID-19. Brain Sci. 2021, 11, 1287. https://doi.org/10.3390/brainsci11101287
Dehelean L, Papava I, Musat MI, Bondrescu M, Bratosin F, Bucatos BO, Bortun A-MC, Mager DV, Romosan RS, Romosan A-M, et al. Coping Strategies and Stress Related Disorders in Patients with COVID-19. Brain Sciences. 2021; 11(10):1287. https://doi.org/10.3390/brainsci11101287
Chicago/Turabian StyleDehelean, Liana, Ion Papava, Madalina Iuliana Musat, Mariana Bondrescu, Felix Bratosin, Bianca Oana Bucatos, Ana-Maria Cristina Bortun, Daniela Violeta Mager, Radu Stefan Romosan, Ana-Maria Romosan, and et al. 2021. "Coping Strategies and Stress Related Disorders in Patients with COVID-19" Brain Sciences 11, no. 10: 1287. https://doi.org/10.3390/brainsci11101287
APA StyleDehelean, L., Papava, I., Musat, M. I., Bondrescu, M., Bratosin, F., Bucatos, B. O., Bortun, A.-M. C., Mager, D. V., Romosan, R. S., Romosan, A.-M., Paczeyka, R., Cut, T. G., Pescariu, S. A., & Laza, R. (2021). Coping Strategies and Stress Related Disorders in Patients with COVID-19. Brain Sciences, 11(10), 1287. https://doi.org/10.3390/brainsci11101287










