Network Analysis of Induced Neural Plasticity Post-Acceptance and Commitment Therapy for Chronic Pain
Abstract
1. Introduction
2. Materials and Methods
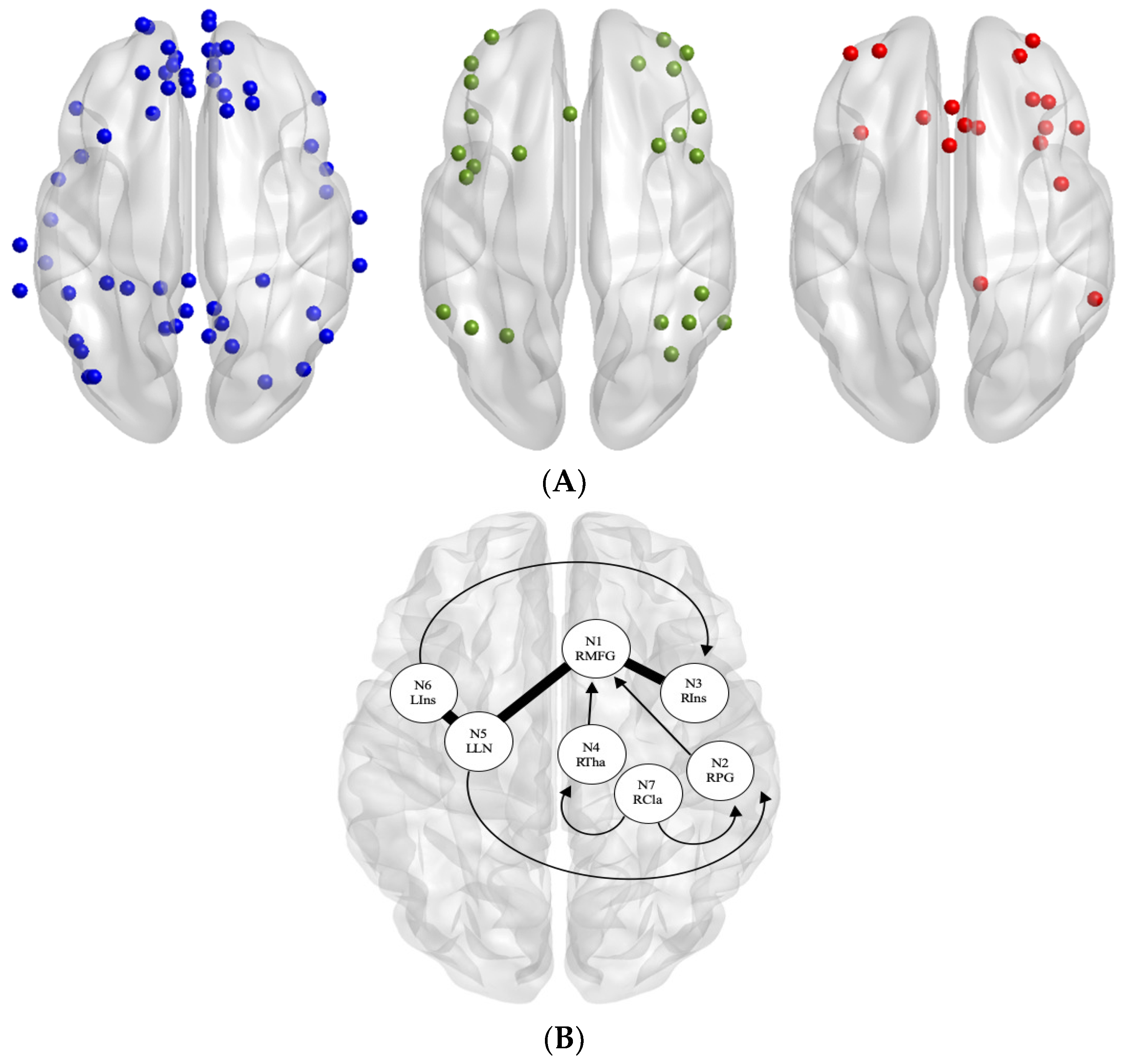
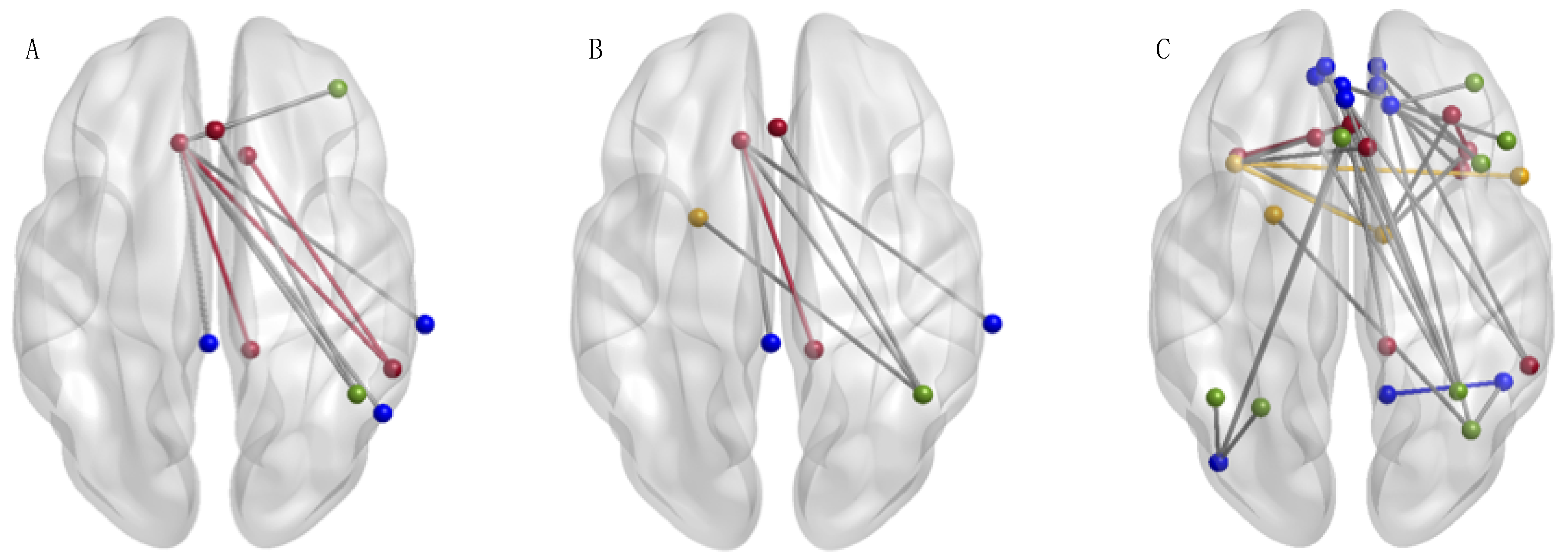
3. Results
rs-fMRI Connectivity Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Helmick, C. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Kotecha, G.; Leibnitz, K.; Matsubara, T.; Sprenger, C.; Nakae, A.; Seymour, B. Classification and characterisation of brain network changes in chronic back pain: A multicenter study. Wellcome Open Res. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Reddan, M.C.; Wager, T.D. Modeling pain using fMRI: From regions to biomarkers. Neurosci. Bull. 2018, 34, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, M.H.; Von Korff, M.; Bushnell, M.C.; Porter, L. Prevalence and profile of high-impact chronic pain in the United States. J. Pain 2019, 20, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Čeko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef]
- Morton, D.; Jones, A.; Sandhu, J. Brain imaging of pain: State of the art. J. Pain Res. 2016, 9, 613–624. [Google Scholar] [CrossRef]
- Mitsi, V.; Zachariou, V. Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience 2016, 338, 81–92. [Google Scholar] [CrossRef]
- Mak, L.E.; Minuzzi, L.; MacQueen, G.; Hall, G.; Kennedy, S.H.; Miley, R. The default mode network in healthy individuals: A systematic review and meta-analysis. Brain Connect. 2017, 7, 25–33. [Google Scholar] [CrossRef]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2017, 18, 20–30. [Google Scholar] [CrossRef]
- Pfannmöller, J.; Lotze, M. Review on biomarkers in the resting-state networks of chronic pain patients. Brain Cognit. 2019, 131, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Hemington, K.S.; Wu, Q.; Kucyi, A.; Inman, R.D.; Davis, K.D. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct. Funct. 2016, 221, 4203–4219. [Google Scholar] [CrossRef] [PubMed]
- Cauda, F.; Palermo, S.; Costa, T.; Torta, R.; Duca, S.; Vercelli, U.; Torta, D.M.E. Gray matter alterations in chronic pain: A network-oriented meta-analytic approach. NeuroImage Clin. 2014, 4, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Cottam, W.J.; Iwabuchi, S.J.; Drabek, M.M.; Reckziegel, D.; Auer, D.P. Altered connectivity of the right anterior insula drives the pain connectome changes in chronic knee osteoarthritis. Pain 2018, 159, 929–938. [Google Scholar] [CrossRef]
- Doll, A.; Hölzel, B.K.; Boucard, C.C.; Wohlschläger, A.M.; Sorg, C. Mindfulness is associated with intrinsic functional connectivity between default mode and salience networks. Front. Hum. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Napadow, V.; LaCount, L.; Park, K.; As-Sanie, S.; Clauw, D.J.; Harris, R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010, 62, 2545–2555. [Google Scholar] [CrossRef]
- Van Ettinger-Veenstra, H.; Lundberg, P.; Alföldi, P.; Södermark, M.; Graven-Nielsen, T.; Sjörs, A.; Gerdle, B. Chronic widespread pain patients show disrupted cortical connectivity in default mode and salience networks, modulated by pain sensitivity. J. Pain Res. 2019, 12, 1743–1755. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, T.; Tang, C.; Ni, K.; Pan, X.; Yan, C.; Luo, Y. Altered resting-state intra- and inter- network functional connectivity in patients with persistent somatoform pain disorder. PLoS ONE 2017, 12, e0176494. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cognit. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Menon, V. The Triple Network Model, Insight, and Large-Scale Brain Organization in Autism. Biol. Psychiatry 2018, 84, 236–238. [Google Scholar] [CrossRef]
- Aytur, S.A.; Ray, K.L.; Meier, S.K.; Campbell, J.; Gendron, B.; Robin, D.A. Neural mechanisms of acceptance and commitment therapy for chronic pain: A network-based fMRI approach. MedRxiv 2020. [Google Scholar] [CrossRef]
- Hayes, S.C.; Luoma, J.B.; Bond, F.W.; Masuda, A.; Lillis, J. Acceptance and Commitment Therapy: Model, processes and outcomes. Behav. Res. Ther. 2006, 44, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; Lundgren, T. Acceptance and commitment therapy in the treatment of chronic pain. In Mindfulness-Based Treatment Approaches: Clinician’s Guide to Evidence Base and Applications; Baer, R.A., Ed.; Elsevier Academic Press: Cambridge, MA, USA, 2006; pp. 285–306. [Google Scholar] [CrossRef]
- Zalesky, A.; Fornito, A.; Bullmore, E.T. Network-based statistic: Identifying differences in brain networks. NeuroImage 2010, 53, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vidaurre, D.; Beckmann, C.F.; Glasser, M.F.; Jenkinson, M.; Miller, K.L.; Van Essen, D.C. Functional connectomics from resting-state fMRI. Trends Cognit. Sci. 2013, 17, 666–682. [Google Scholar] [CrossRef]
- Sporns, O. Graph theory methods: Applications in brain networks. Dialog. Clin. Neurosci. 2018, 20, 111–121. [Google Scholar] [CrossRef]
- Farahani, F.V.; Karwowski, W.; Lighthall, N.R. Application of graph theory for identifying connectivity patterns in human brain networks: A systematic review. Front. Neurosci. 2019, 13, 585. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Hu, X.; Nguyen, V.T.; Guo, L.; Han, J.; Guo, C.C. Test-retest reliability of functional connectivity networks during naturalistic fMRI paradigms. Hum. Brain Mapp. 2017, 38, 2226–2241. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pain Inventory. Annals 1994, 23, 129–138. [Google Scholar]
- Penny, W.; Friston, K.; Ashburner, J.; Kiebel, S.; Nichols, T. Statistical Parametric Mapping: The Analysis of Functional Brain Images, 1st ed.; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- MATLAB, version 9.9(R2017b); The MathWorks Inc.: Natick, MA, USA, 2010.
- Kochunov, P.; Lancaster, J.; Thompson, P.; Toga, A.W.; Brewer, P.; Hardies, J.; Fox, P. An optimized individual target brain in the Talairach coordinate system. NeuroImage 2002, 17, 922–927. [Google Scholar] [CrossRef]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Petersen, S.E. Functional network organization of the human brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef]
- Duff, E.P.; Cunnington, R.; Egan, G.F. REX: Response exploration for neuroimaging datasets. Neuroinformatics 2011, 5, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Waller, N.C.; Ray, K.L.; Meier, S.K.; Aytur, S.A.; Robin, D.A. Regional brain activation in chronic pain: A functional connectivity meta-analysis with healthy controls and chronic pain patients. unpublished results.
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation revisited. NeuroImage 2012, 59, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Laird, A.R.; Glahn, D.C.; Lovallo, W.R.; Fox, P.T. Meta-analytic connectivity modeling: Delineating the functional connectivity of the human amygdala. Hum. Brain Mapp. 2010, 31, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Wang, J.; He, Y. BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef] [PubMed]
- Seminowicz, D.A.; Wideman, T.H.; Naso, L.; Hatami-Khoroushahi, Z.; Fallatah, S.; Ware, M.A.; Stone, L.S. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 2011, 31, 7540–7550. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, F.; Martucci, K.T.; Kraft, R.A.; Gordon, N.S.; McHaffie, J.G.; Coghill, R.C. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J. Neurosci. 2011, 31, 5540–5548. [Google Scholar] [CrossRef]
- Karafin, M.S.; Chen, G.; Wandersee, N.J.; Brandow, A.M.; Hurley, R.W.; Simpson, P.; Field, J.J. Chronic pain in adults with sickle cell disease is associated with alterations in functional connectivity of the brain. PLoS ONE 2019, 14, e0216994. [Google Scholar] [CrossRef]
- Kornelsen, J.; Sboto-Frankenstein, U.; McIver, T.; Gervai, P.; Wacnik, P.; Berrington, N.; Tomanek, B. Default mode network functional connectivity altered in failed Back surgery syndrome. J. Pain 2013, 14, 483–491. [Google Scholar] [CrossRef]
- Wakaizumi, K.; Jabakhanji, R.; Ihara, N.; Kosugi, S.; Terasawa, Y.; Morisaki, H.; Baliki, M.N. Altered functional connectivity associated with time discounting in chronic pain. Sci. Rep. 2019, 9, 8154. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, Z.; Pan, L.; Ling, Z.; Liu, X.; Zhang, J.; Yu, X. Frequency-specific alterations in cortical rhythms and functional connectivity in trigeminal neuralgia. Brain Imaging Behav. 2019, 13, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Kutch, J.J.; Labus, J.S.; Harris, R.E.; Martucci, K.T.; Farmer, M.A.; Fenske, S.; Mayer, E.A. Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: A MAPP network study. Pain 2017, 158, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Woo, C.-W.; Yao, Z.; Goldstein, P.; Atlas, L.Y.; Roy, M.; Wager, T.D. Pain-evoked reorganization in functional brain networks. Cereb. Cortex 2020, 30, 2804–2822. [Google Scholar] [CrossRef] [PubMed]
- Androulakis, X.M.; Krebs, K.A.; Jenkins, C.; Maleki, N.; Finker, A.G.; Rorden, C.; Newman, R. Central executive and default mode network intra-network functional connectivity patterns in chronic migraine. J. Neurol. Disord. 2018, 6. [Google Scholar] [CrossRef]
- Bishop, J.H.; Shpaner, M.; Kubicki, A.; Clements, S.; Watts, R.; Naylor, M.R. Structural network differences in chronic muskuloskeletal pain: Beyond fractional anisotropy. NeuroImage 2018, 182, 441–455. [Google Scholar] [CrossRef]
- Seeley, W.W. The salience network: A neural system for perceiving and responding to homeostatic demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meier, S.K.; Ray, K.L.; Waller, N.C.; Gendron, B.C.; Aytur, S.A.; Robin, D.A. Network Analysis of Induced Neural Plasticity Post-Acceptance and Commitment Therapy for Chronic Pain. Brain Sci. 2021, 11, 10. https://doi.org/10.3390/brainsci11010010
Meier SK, Ray KL, Waller NC, Gendron BC, Aytur SA, Robin DA. Network Analysis of Induced Neural Plasticity Post-Acceptance and Commitment Therapy for Chronic Pain. Brain Sciences. 2021; 11(1):10. https://doi.org/10.3390/brainsci11010010
Chicago/Turabian StyleMeier, Sarah K., Kimberly L. Ray, Noah C. Waller, Barry C. Gendron, Semra A. Aytur, and Donald A. Robin. 2021. "Network Analysis of Induced Neural Plasticity Post-Acceptance and Commitment Therapy for Chronic Pain" Brain Sciences 11, no. 1: 10. https://doi.org/10.3390/brainsci11010010
APA StyleMeier, S. K., Ray, K. L., Waller, N. C., Gendron, B. C., Aytur, S. A., & Robin, D. A. (2021). Network Analysis of Induced Neural Plasticity Post-Acceptance and Commitment Therapy for Chronic Pain. Brain Sciences, 11(1), 10. https://doi.org/10.3390/brainsci11010010





