Flumazenil-Insensitive Benzodiazepine Effects in Recombinant αβ and Neuronal GABAA Receptors
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Expression of GABAA Receptors in HEK293 Cells
2.3. Primary Cortex and Hippocampus Neuronal Cultures
2.4. Immunofluorescence
2.5. Whole-Cell Patch-Clamp Electrophysiology
2.6. Statistical Analysis
3. Results
3.1. GABA Concentration-Dependent Activation of Recombinant αβ Receptors
3.2. α1β2 Receptor Showed Distinct Sensitivities to Different Benzodiazepines
3.3. The Contribution of α and β Subunits to the Sensitivity of αβ Receptor to Diazepam
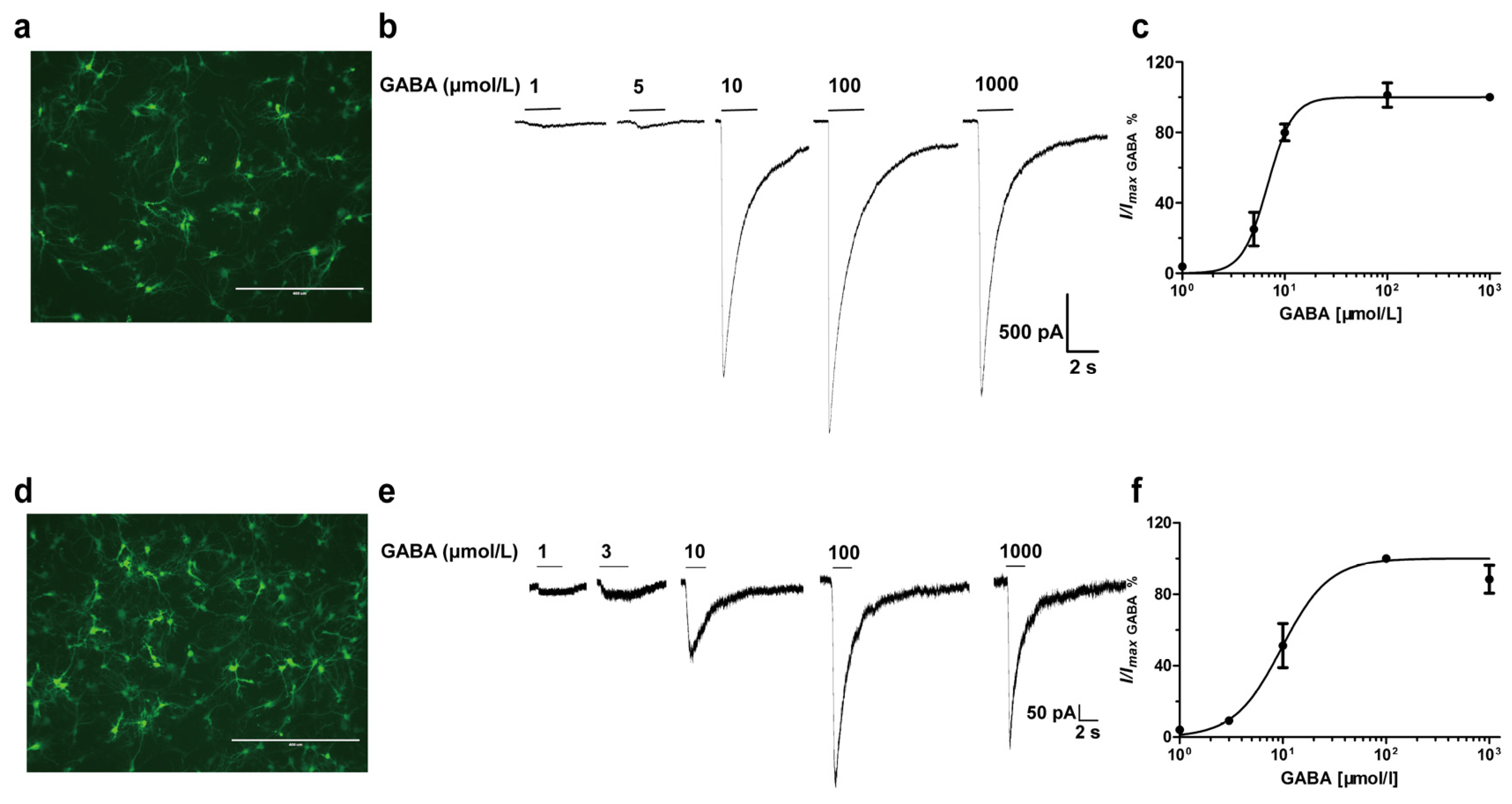
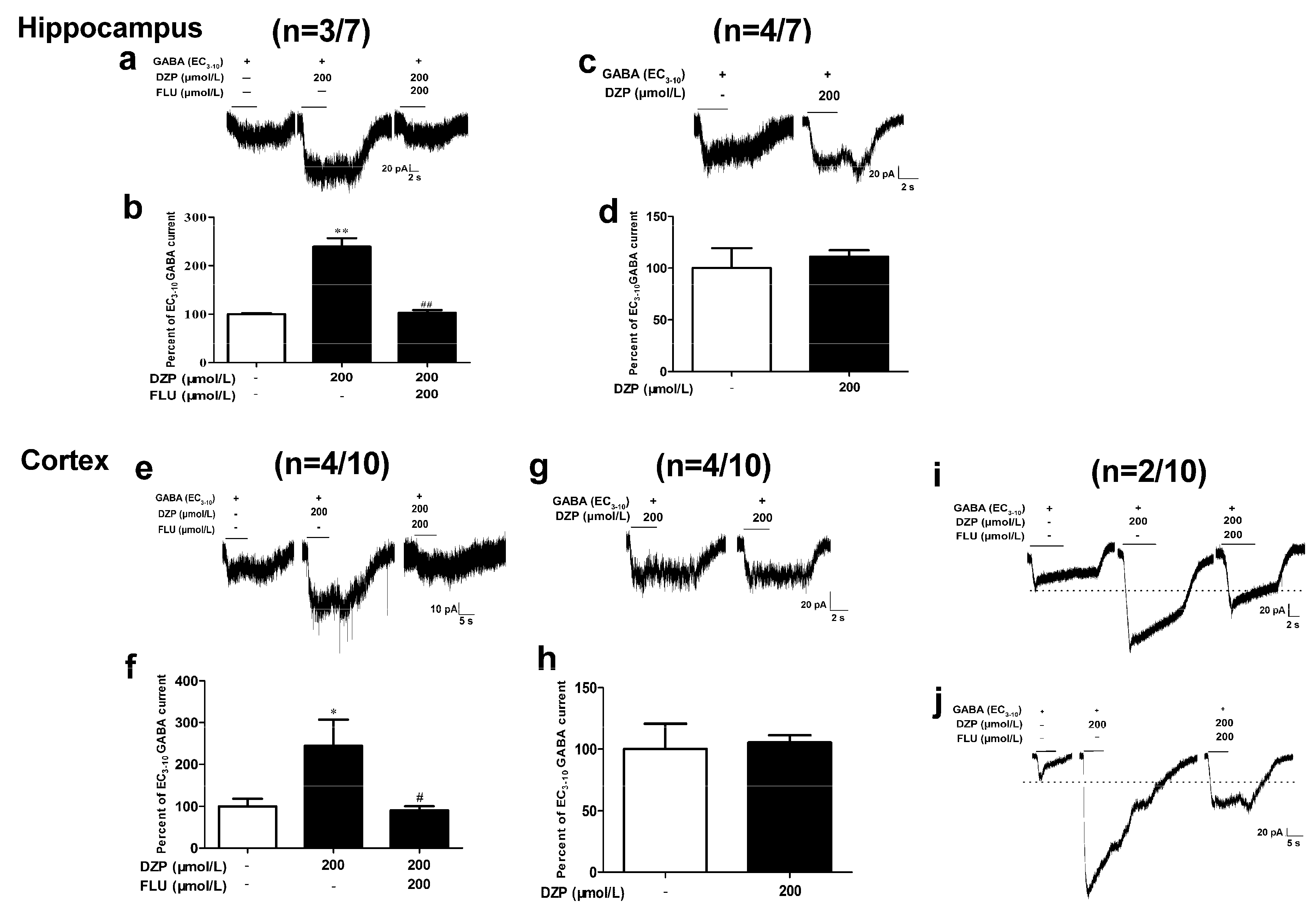
3.4. Effects of Diazepam on Some Cortical Neurons Could Not be Fully Antagonized by Flumazenil
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Nutt, D. GABAA Receptors: Subtypes, regional distribution, and function. J. Clin. Sleep Med. 2006, 2, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Nayeem, N.; Green, T.P.; Martin, I.L.; Barnard, E.A. Quaternary structure of the native GABAA receptor determined by electron microscopic image analysis. J. Neurochem. 1994, 62, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Sieghart, W.; Sperk, G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr. Top. Med. Chem. 2002, 2, 795–816. [Google Scholar] [CrossRef] [PubMed]
- Sieghart, W.; Savić, M.M. International Union of Basic and Clinical Pharmacology. CVI: GABAA receptor subtype-and function-selective ligands: Key issues in translation to humans. Pharmacol. Rev. 2018, 70, 836–878. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W.; Sieghart, W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acida receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008, 60, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.; Kelly, E.; Marrion, N.; Marrion, N.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Davies, J.A.; et al. The concise guide to PHARMACOLOGY 2015/16: Ligand-Gated ion channels. Br. J. Pharmacol. 2015, 172, 5870–5903. [Google Scholar] [CrossRef]
- Tretter, V.; Ehya, N.; Fuchs, K.; Sieghart, W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J. Neurosci. 1997, 17, 2728–2737. [Google Scholar] [CrossRef]
- Mortensen, M.; Smart, T.G. Extrasynaptic αβ subunit GABAA receptors on rat hippocampal pyramidal neurons. J. Physiol. 2006, 577, 841–856. [Google Scholar] [CrossRef]
- Brickley, S.G.; Mody, I. Extrasynaptic GABAA receptors: Their function in the CNS and implications for disease. Neuron 2012, 73, 23–34. [Google Scholar] [CrossRef]
- Nielsen, S. Benzodiazepines. Curr. Top. Behav. Neurosci. 2017, 34, 141–159. [Google Scholar]
- Greenblatt, D.J.; Shader, R.I.; Abernethy, D.R. Drug therapy. Current status of benzodiazepines. (Second of two parts). N. Engl. J. Med. 1983, 309, 410–416. [Google Scholar] [PubMed]
- Bachhuber, M.A.; Hennessy, S.; Cunningham, C.O.; Starrels, J.L. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am. J. Public Health 2016, 106, 686–688. [Google Scholar] [CrossRef]
- Sigel, E.; Buhr, A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol. Sci. 1997, 18, 425–429. [Google Scholar] [CrossRef]
- Sieghart, W. Allosteric modulation of GABAA receptors via multiple drug-binding sites. Adv. Pharmacol. 2015, 72, 53–96. [Google Scholar] [PubMed]
- Sigel, E.; Ernst, M. The benzodiazepine binding sites of GABAA receptors. Trends Pharmacol. Sci. 2018, 39, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Q.; Yan, H.; Yu, G.; Su, R.B. Flumazenil-Insensitive benzodiazepine binding sites in GABAA receptors contribute to benzodiazepine-induced immobility in zebrafish larvae. Life Sci. 2019, 239, 117033. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.J.; Hadley, S.H.; Morris, K.D.W.; Amin, J. Benzodiazepines act on GABAA receptors via two distinct and separable mechanisms. Nat. Neurosci. 2001, 3, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Maldifassi, M.C.; Baur, R.; Pierce, D.; Nourmahnad, A.; Forman, S.A.; Sigel, E. Novel positive allosteric modulators of GABAA receptors with anesthetic activity. Sci. Rep. 2016, 6, 25943. [Google Scholar] [CrossRef]
- Has, A.T.C.; Absalom, N.; Nieuwenhuijzen, P.S.V.; Clarkson, A.N.; Ahring, P.K.; Chebib, M. Zolpidem is a potent stoichiometry-selective modulator of α1β3 GABAA receptors: Evidence of a novel benzodiazepine site in the α1-α1 interface. Sci. Rep. 2016, 6, 28674. [Google Scholar] [CrossRef]
- Varagic, Z.; Wimmer, L.; Schnürch, M. Identification of novel positive allosteric modulators and null modulators at the GABAA receptor α + β-interface. Br. J. Pharmacol. 2013, 169, 371–383. [Google Scholar] [CrossRef]
- Ramerstorfer, J.; Furtmuller, R.; Sarto-Jackson, I.; Varagic, Z.; Sieghart, W.; Ernst, M. The GABAA receptor α + β-interface: A novel target for subtype selective drugs. J. Neurosci. 2011, 31, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Ralvenius, W.T.; Benke, D.; AcuńAM, A.; Rudolph, U.; Zeilhoferet, H.U. Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nat. Commun. 2015, 6, 6803. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Ikemoto, Y.; Nielsen, M.; Yano, T. Effects of isoflurane and hexafluorodiethyl ether on human recombinant GABAA receptors expressed in Sf9 cells. Eur. J. Pharmacol. 1999, 378, 223–231. [Google Scholar] [CrossRef]
- Shen, M.L.; Wang, C.H.; Chen, Y.T.; Zhou, N.; Kao, S.T.; Wu, D.C. Luteolin inhibits GABAA receptors in HEK cells and brain slices. Sci. Rep. 2016, 6, 27695. [Google Scholar] [CrossRef] [PubMed]
- Eaton, M.M.; Bracamontes, J.; Shu, H.J.; Li, P.; Mennerick, S.; Steinbach, J.H.; Akk, G. γ-Aminobutyric acid type A α4, β2, and δ subunits assemble to produce more than one functionally distinct receptor type. Mol. Pharmacol. 2014, 86, 647–656. [Google Scholar] [CrossRef]
- Bakas, T.; Van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef]
- Saku, T.; Sinkkonen, B.; Lüddens, H.; Korpi, E.R. Autoradiographic imaging of altered synaptic αβγ2 and extrasynaptic αβ GABAA receptors in a genetic mouse model of anxiety. Neurochem. Int. 2004, 44, 539–547. [Google Scholar]
- Baumann, S.W.; Baur, R.; Sigel, E. Subunit arrangement of γ-aminobutyric acid type A receptors. J. Biol. Chem. 2001, 276, 36275–36280. [Google Scholar] [CrossRef]
- Malminiemi, O.; Korpi, E.R. Diazepam-Insensitive [3H] Ro 15-4513 binding in intact cultured cerebellar granule cells. Eur. J. Pharmacol. 1989, 169, 53–60. [Google Scholar] [CrossRef]
- Wongsamitkul, N.; Maldifassi, M.C.; Simeone, X.; Baur, R.; Ernst, M.; Sigel, E. α subunits in GABAA receptors are dispensable for GABA and diazepam action. Sci. Rep. 2017, 7, 15498. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, R.; Barot, S.; Weiss, D.S. Stoichiometry of a recombinant GABAA receptor. J. Neurosci. 1996, 16, 5415–5424. [Google Scholar] [CrossRef] [PubMed]
- Korpi, E.R.; Mattila, M.J.; Wisden, W.; Lüddens, H. GABAA-receptor subtypes: Clinical efficacy and selectivity of benzodiazepine site ligands. Ann. Med. 1997, 29, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hamor, T.A.; Martin, I.L. The benzodiazepines. Prog. Med. Chem. 1983, 20, 157–223. [Google Scholar] [PubMed]
- Hadjipavloulitina, D.; Hansch, C. Quantitative structure-activity relationships of the benzodiazepines: A review and reevaluation. Chem. Rev. 1995, 26, 1483–1505. [Google Scholar]
- Gunja, N. The clinical and forensic toxicology of Z-drugs. J. Med. Toxicol. 2013, 9, 155–162. [Google Scholar] [CrossRef]
- Rudolph, U.; Knoflach, F. Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2012, 10, 685–697. [Google Scholar] [CrossRef]
- Sancar, F.; Ericksen, S.S.; Kucken, A.M.; Teissére, J.A.; Czajkowski, C. Structural determinants for high-affinity zolpidem binding to GABAA receptors. Mol. Pharmacol. 2007, 71, 38–46. [Google Scholar] [CrossRef]
- Rush, C.R. Behavioral pharmacology of Zolpidem relative to benzodiazepines: A review. Pharmacol. Biochem. Behav. 1998, 61, 253–269. [Google Scholar] [CrossRef]
- Drexler, B.; Zinser, S.; Hentschke, H.; Antkowiak, B. Diazepam decreases action potential firing of neocortical neurons via two distinct mechanisms. Anesth. Analg. 2010, 111, 1394–1399. [Google Scholar] [CrossRef]
 represents the possible benzodiazepine binding sites.
represents the possible benzodiazepine binding sites.
 represents the possible benzodiazepine binding sites.
represents the possible benzodiazepine binding sites.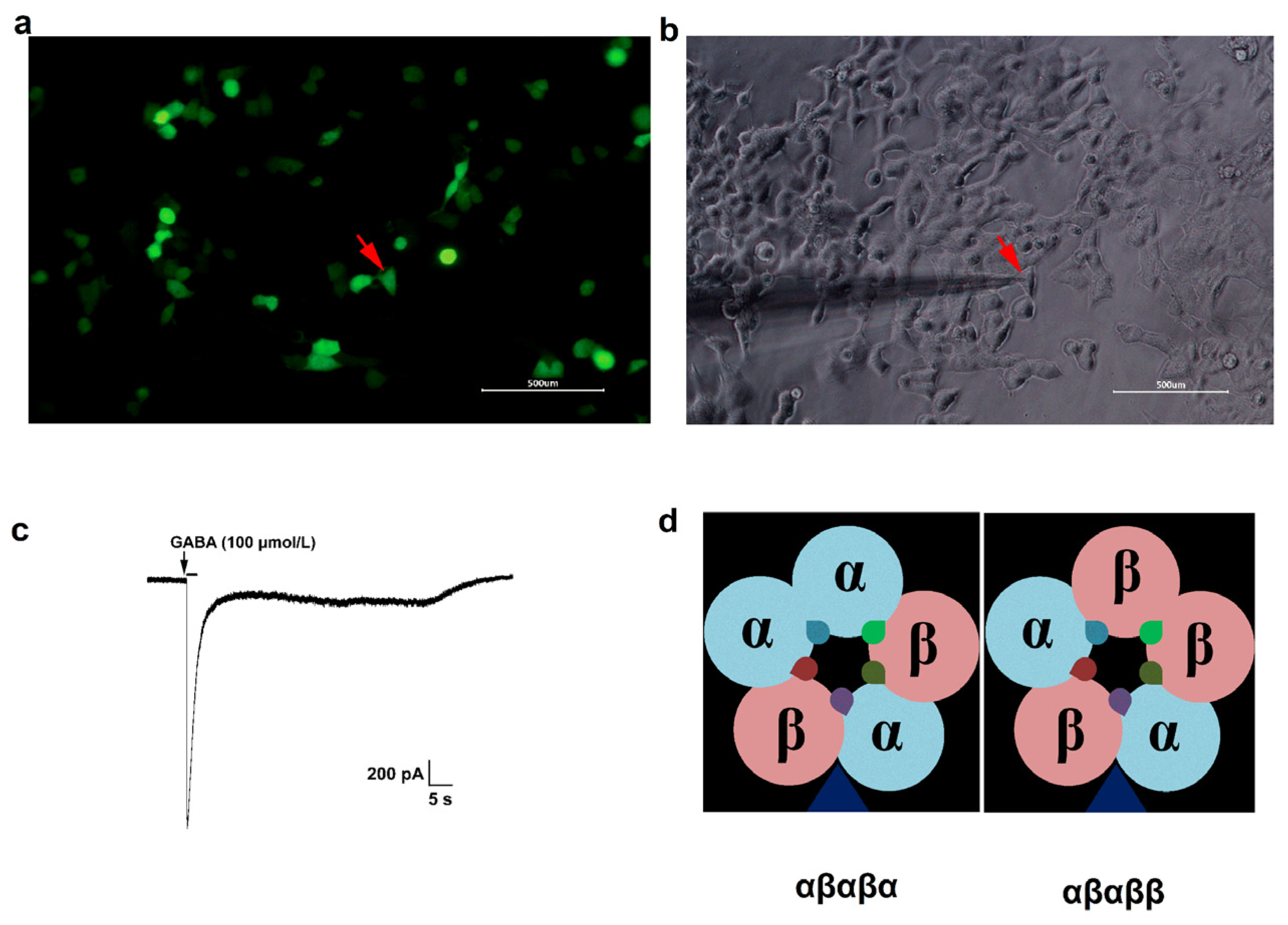
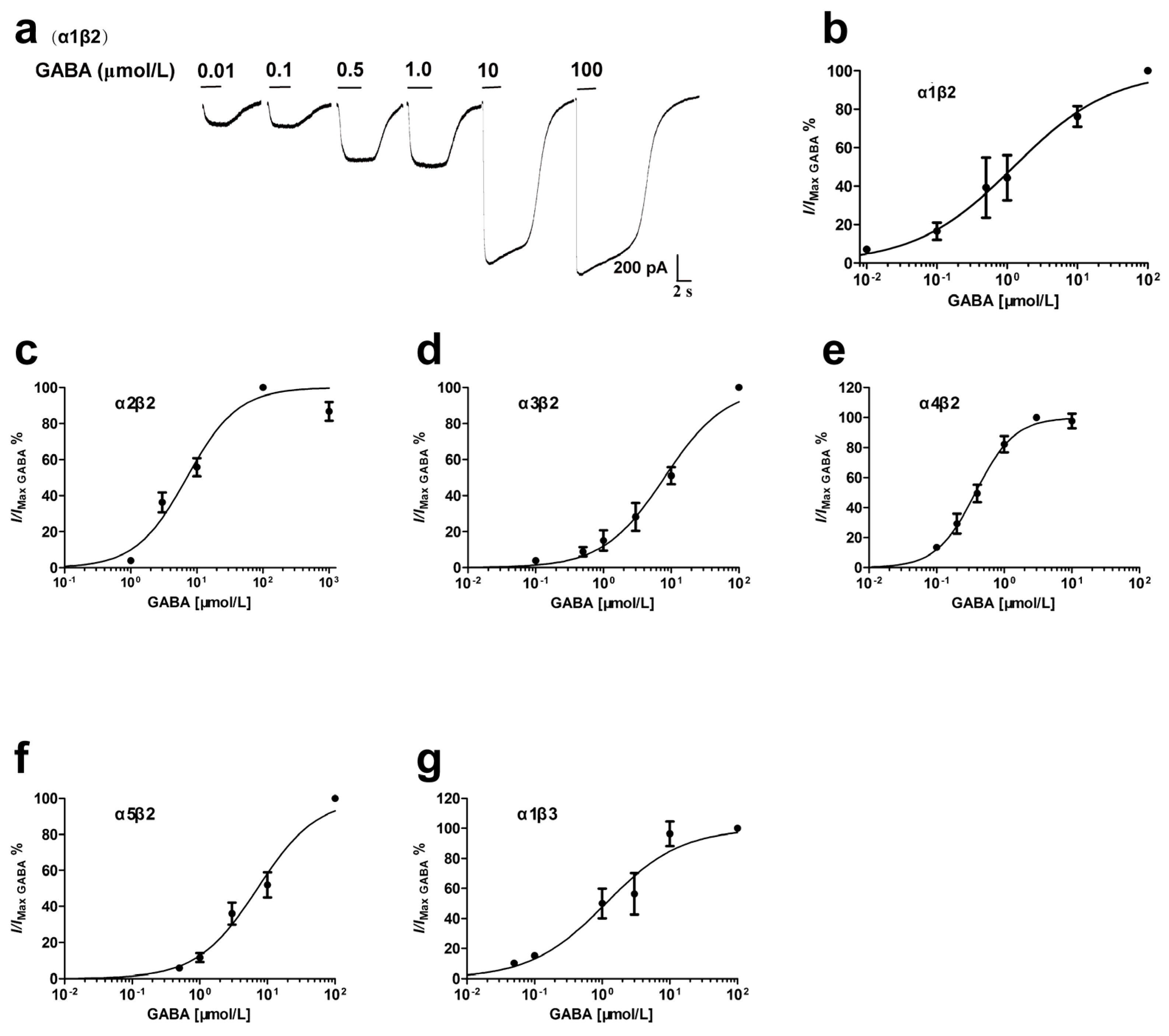

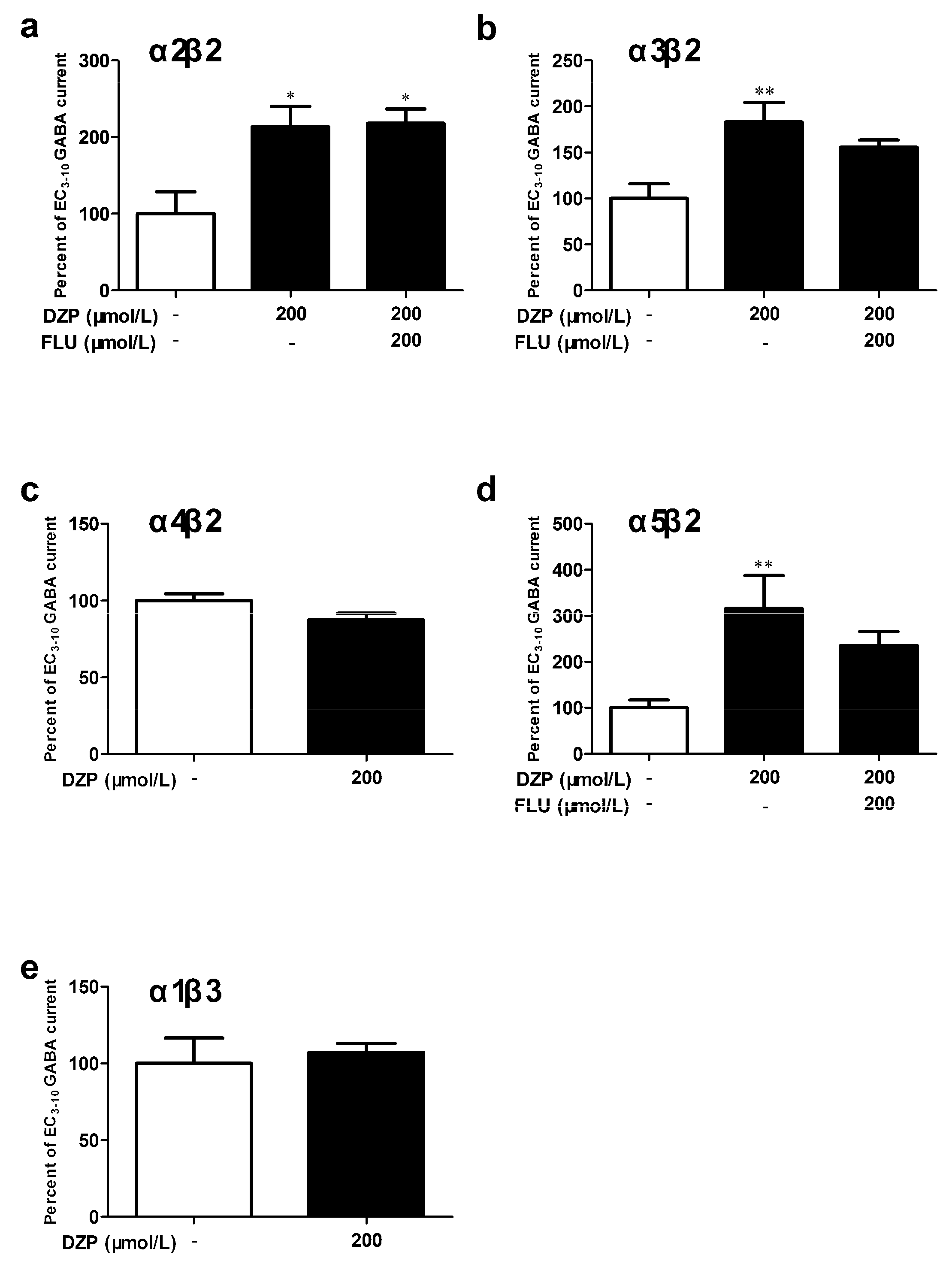
| Receptor | cDNA Ratio | GABA (μmol/L) | Hill Coefficient | Potentiation of GABA Currents (%) | n | |||
|---|---|---|---|---|---|---|---|---|
| EC50 | EC3–10 | (Diazepam) | (Midazolam) | (Zolpidem) | ||||
| α1β2 | 1:1 | 1.23 (0.65–2.33) | 0.1 | 0.62 ± 0.12 | 214.0 ± 31.4 * | 171.8 ± 13.8 * | 118.5 ± 7.0 | 3–5 |
| α2β2 | 1:1 | 7.05 (5.16–9.63) | 1.0 | 1.13 ± 0.19 | 213.3 ± 26.7 * | -- | -- | 3–4 |
| α3β2 | 1:1 | 7.94 (5.82–10.84) | 0.5 | 0.96 ± 0.12 | 183.2 ± 21.1 ** | -- | -- | 3–5 |
| α4β2 | 1:1 | 0.38 (0.32–0.44) | 0.01 | 1.51 ± 0.16 | 87.4 ± 4.3 | -- | -- | 4 |
| α5β2 | 1:1 | 7.13 (5.16–9.83) | 0.5 | 0.98 ± 0.13 | 316.2 ± 75.2 ** | -- | -- | 3–6 |
| α1β3 | 1:1 | 1.13 (0.63–2.00) | 0.1 | 0.78 ± 0.15 | 107.2 ± 5.8 | -- | -- | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, J.-J.; Cao, Y.-Q.; Li, Y.-L.; Yu, G.; Su, R.-B. Flumazenil-Insensitive Benzodiazepine Effects in Recombinant αβ and Neuronal GABAA Receptors. Brain Sci. 2020, 10, 150. https://doi.org/10.3390/brainsci10030150
Lian J-J, Cao Y-Q, Li Y-L, Yu G, Su R-B. Flumazenil-Insensitive Benzodiazepine Effects in Recombinant αβ and Neuronal GABAA Receptors. Brain Sciences. 2020; 10(3):150. https://doi.org/10.3390/brainsci10030150
Chicago/Turabian StyleLian, Jing-Jing, Yan-Qing Cao, Yu-Lei Li, Gang Yu, and Rui-Bin Su. 2020. "Flumazenil-Insensitive Benzodiazepine Effects in Recombinant αβ and Neuronal GABAA Receptors" Brain Sciences 10, no. 3: 150. https://doi.org/10.3390/brainsci10030150
APA StyleLian, J.-J., Cao, Y.-Q., Li, Y.-L., Yu, G., & Su, R.-B. (2020). Flumazenil-Insensitive Benzodiazepine Effects in Recombinant αβ and Neuronal GABAA Receptors. Brain Sciences, 10(3), 150. https://doi.org/10.3390/brainsci10030150




