Anatomical Features of the Deep Cervical Lymphatic System and Intrajugular Lymphatic Vessels in Humans
Abstract
:1. Introduction
2. Methods
3. Results
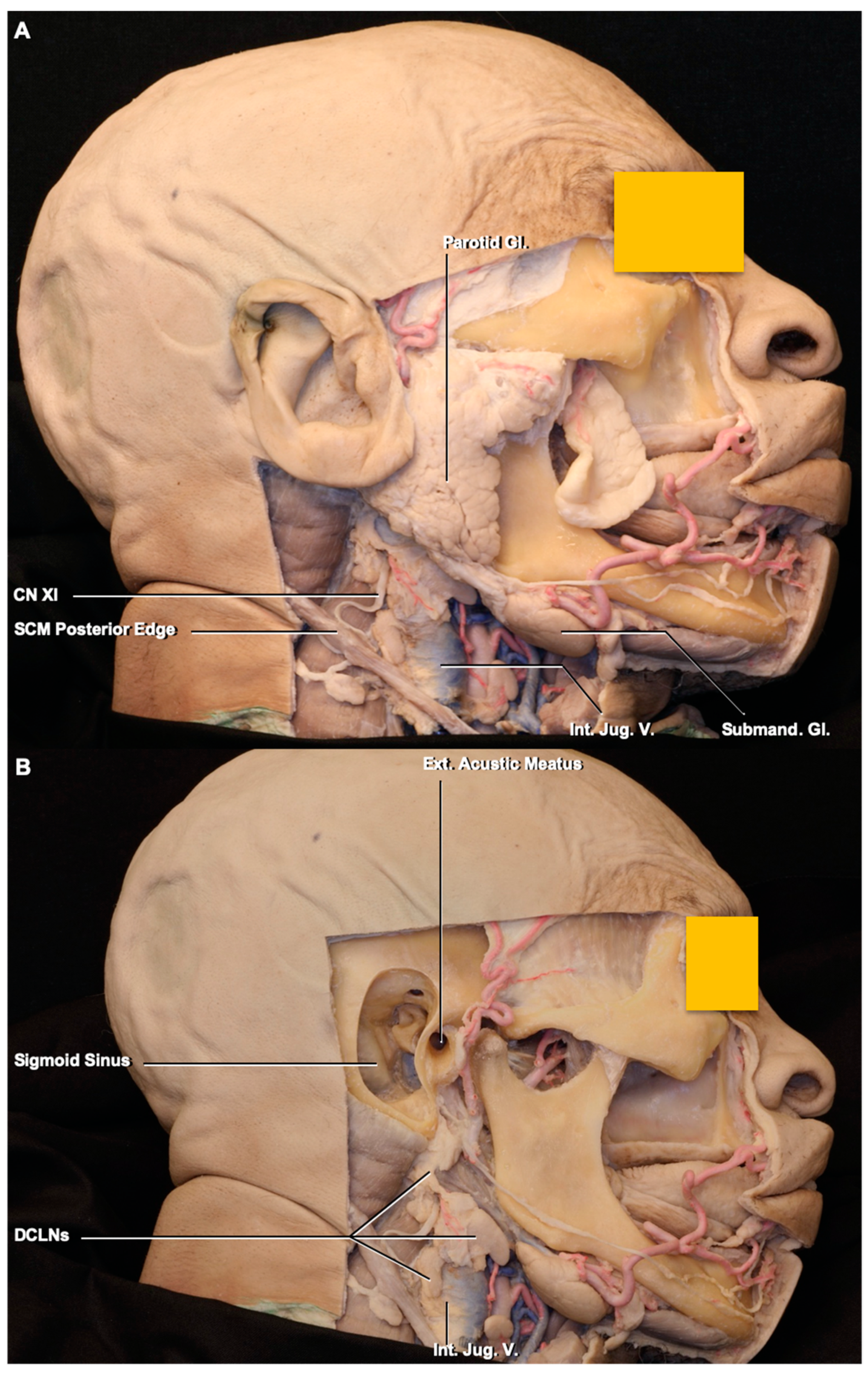
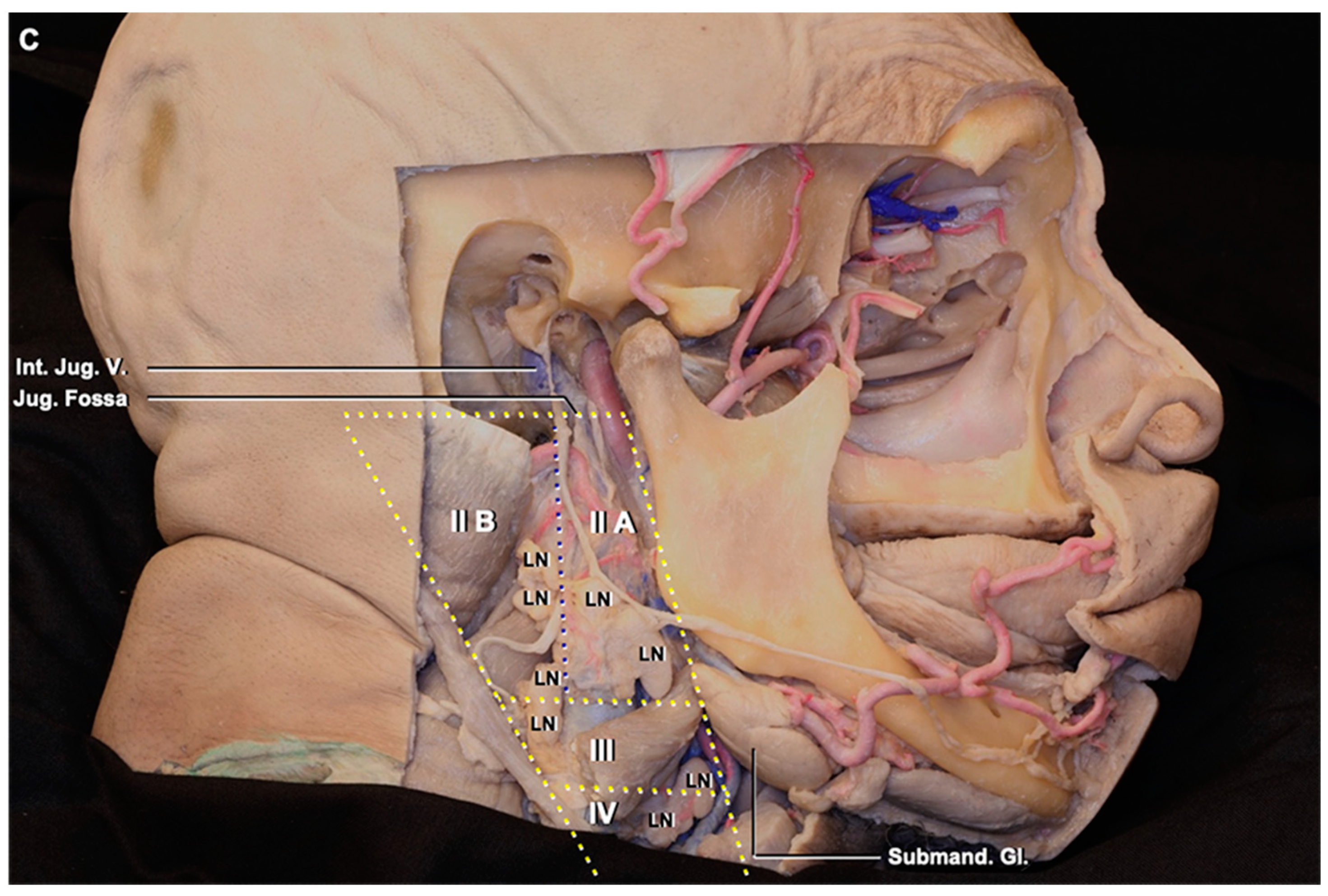
3.1. Lymph Node Location
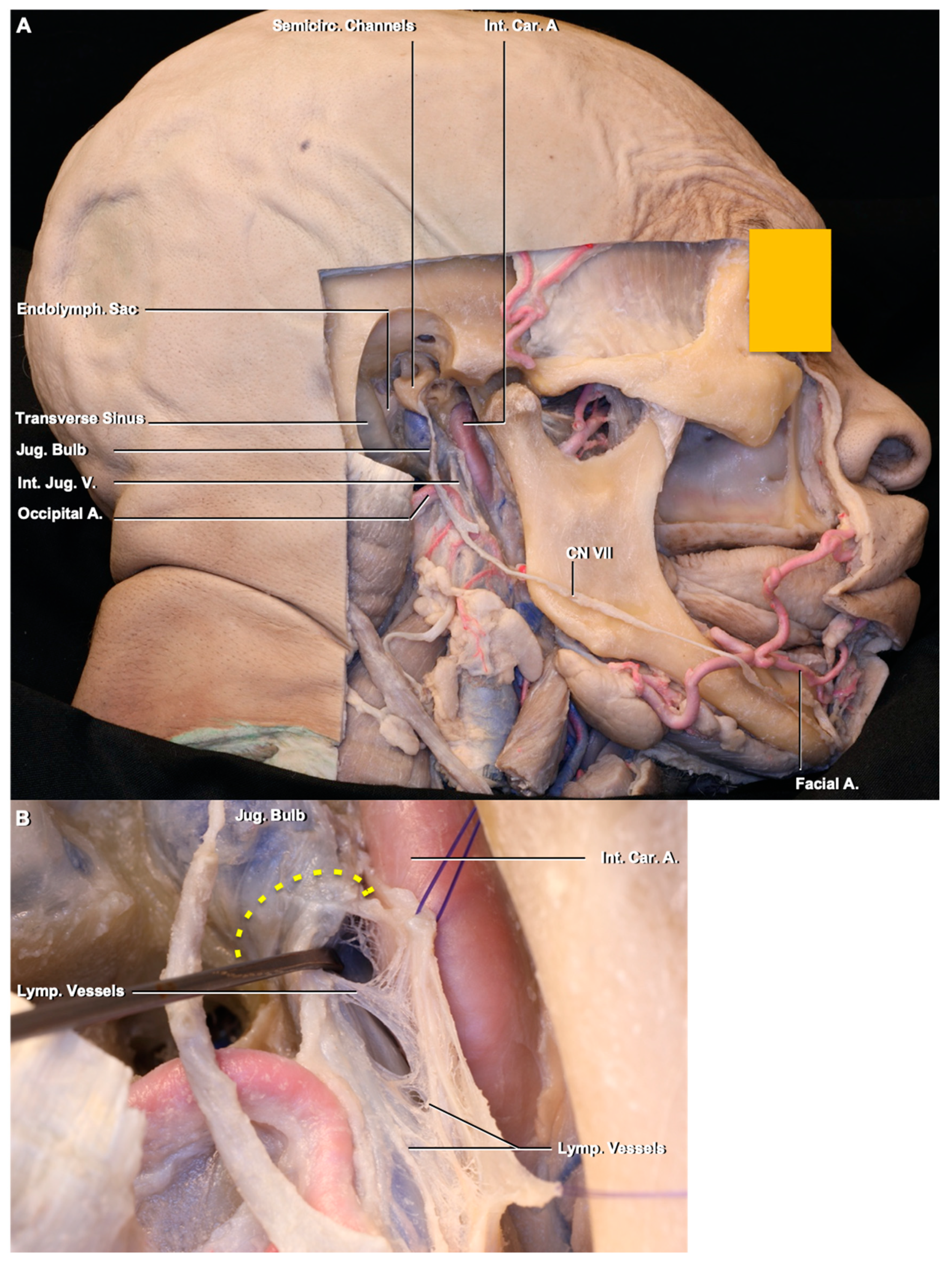
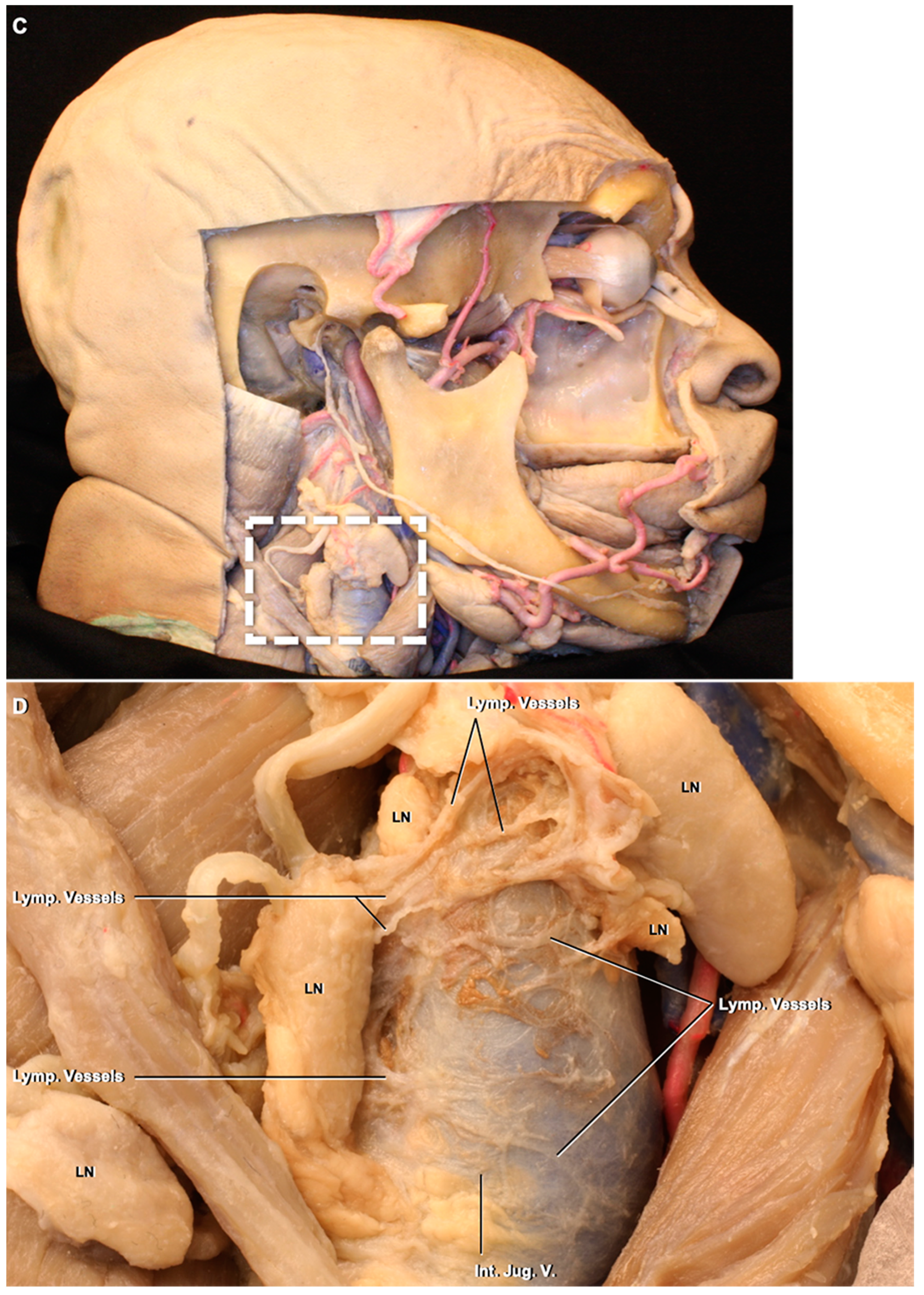
3.2. Lymphatic Pathway
4. Discussion
4.1. Deep Cervical Lymph Node Anatomy, Variations, and Clinical Importance
4.1.1. Lymphatics and the Nervous System
4.1.2. Lymph Node Yield
4.1.3. Lymph Node Biopsy and Dissection
4.1.4. Metastases to Deep Cervical Lymph Nodes
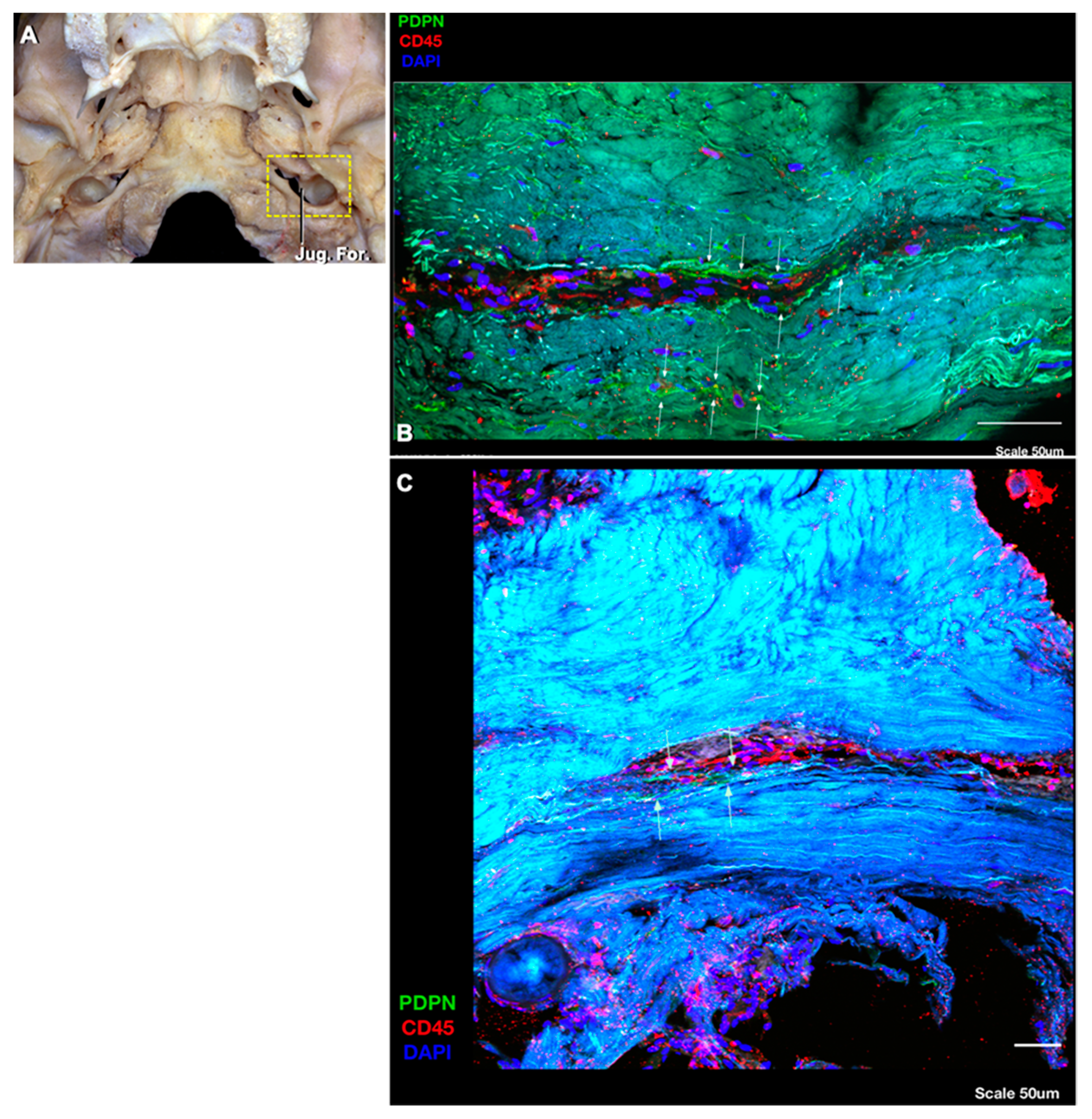
4.2. Deep Cervical Lymph Nodes and Connections to the Intracranial Compartment through the Jugular Foramen
Routes of Lymphatic Drainage
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andres, K.H.; Von Düring, M.; Muszynski, K.; Schmidt, R.F. Nerve fibres and their terminals of the dura mater encephali of the rat. Anat. Embryol. 1987, 175, 289–301. [Google Scholar] [CrossRef]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, H.; Kim, J.H.; Kim, S.H.; Ham, J.S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.-H.; Hong, Y.-K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Antila, S.; Karaman, S.; Nurmi, H.; Airavaara, M.; Voutilainen, M.H.; Mathivet, T.; Chilov, D.; Li, Z.; Koppinen, T.; Park, J.-H.; et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 2017, 214, 3645–3667. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Fu, Z.; Kipnis, J. The meningeal lymphatic system: A new player in neurophysiology. Neuron 2018, 100, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.C.; Kingsmore, K.M.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185. [Google Scholar] [CrossRef]
- Louveau, A.; Herz, J.; Alme, M.N.; Salvador, A.F.; Dong, M.Q.; Viar, K.E.; Herod, S.G.; Knopp, J.; Setliff, J.C.; Lupi, A.L.; et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci. 2018, 21, 1380. [Google Scholar] [CrossRef]
- Patel, T.K.; Habimana-Griffin, L.; Gao, X.; Xu, B.; Achilefu, S.; Alitalo, K.; McKee, C.A.; Sheehan, P.W.; Musiek, E.S.; Xiong, C.; et al. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol. Neurodegener. 2019, 14, 11. [Google Scholar] [CrossRef]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain—Implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457. [Google Scholar] [CrossRef] [Green Version]
- Földi, M.; Csillik, B.; Zoltán, Ö.T. Lymphatic drainage of the brain. Experientia 1968, 24, 1283–1287. [Google Scholar] [CrossRef]
- Mezey, E.; Palkovits, M. Neuroanatomy: Forgotten findings of brain lymphatics. Nature 2015, 524, 415. [Google Scholar] [CrossRef] [Green Version]
- Wallace, C.J.; Forsyth, P.A.; Edwards, D.R. Lymph node metastases from glioblastoma multiforme. Am. J. Neuroradiol. 1996, 17, 1929–1931. [Google Scholar]
- Yağmurlu, K.; Mooney, M.A.; Almefty, K.K.; Bozkurt, B.; Tanrıöver, N.; Little, A.S.; Preul, M.C. An Alternative Endoscopic Anterolateral Route to Meckel’s Cave: An Anatomic Feasibility Study Using a Sublabial Transmaxillary Approach. World Neurosurg. 2018, 174, 837–844. [Google Scholar] [CrossRef]
- Som, P.M.; Curtin, H.D.; Mancuso, A.A. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. Am. J. Roentgenol. 2000, 174, 837–844. [Google Scholar] [CrossRef]
- Rhoton, A.L., Jr. Jugular foramen. Neurosurgery 2000, 23, 654–661. [Google Scholar] [CrossRef]
- Földi, M.; Földi, E.; Kubik, S. Textbook of Lymphology: For Physicians and Lymphedema Therapists; Urban & Fischer: Munchen, Germany, 2003. [Google Scholar]
- Pan, W.R.; Le Roux, C.M.; Levy, S.M.; Briggs, C.A. The morphology of the human lymphatic vessels in the head and neck. Clin. Anat. 2010, 23, 654–661. [Google Scholar] [CrossRef]
- Pan, W.R.; Suami, H.; Taylor, G.I. Lymphatic drainage of the superficial tissues of the head and neck: Anatomical study and clinical implications. Plast. Reconstr. Surg. 2008, 121, 1614–1624. [Google Scholar] [CrossRef]
- Pan, W.R.; Suami, H.; Corlett, R.J.; Ashton, M.W. Lymphatic drainage of the nasal fossae and nasopharynx: Preliminary anatomical and radiological study with clinical implications. Head Neck J. Sci. Spec. Head Neck. 2009, 31, 52–57. [Google Scholar] [CrossRef]
- Agrama, M.T.; Reiter, D.; Topham, A.K.; Keane, W.M. Node counts in neck dissection: Are they useful in outcomes research? Otolaryngol. Head Neck Surg. 2001, 124, 433–435. [Google Scholar] [CrossRef]
- Capelli, F.D.; Paes, V.R.; Machado, M.M.; Menezes, C.L.; Silva, P.R.A.D.; Siqueira, S.A.C.; Alves, V.A.F.; Matos, L.L.; Cernea, C.R. Quantitative analysis of lymph nodes in neck dissection specimens. Morphologic study. Acta Cir. Bras. 2016, 31, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Myers, E.N.; Fagan, J.J. Treatment of the N+ neck in squamous cell carcinoma of the upper aerodigestive tract. Otolaryngol. Clin. N. Am. 1998, 31, 671–686. [Google Scholar] [CrossRef]
- Norling, R.; Therkildsen, M.H.; Bradley, P.J.; Nielsen, M.B.; Buchwald, C.V. Nodal yield in selective neck dissection. Acta Otolaryngol. 2013, 133, 965–971. [Google Scholar] [CrossRef]
- Ofo, E.; Thavaraj, S.; Cope, D.; Barr, J.; Kapoor, K.; Jeannon, J.P.; Oakley, R.; Lock, C.; Odell, E.; Simo, R. Quantification of lymph nodes in the central compartment of the neck: A cadaveric study. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 2773–2778. [Google Scholar] [CrossRef]
- Wang, H.; Sullivan, P. Metastatic Pituitary Carcinoma to Cervical Lymph Node: Diagnosis by Fine Needle Aspiration. Am. J. Clin. Pathol. 2016, 146 (Suppl. S1). [Google Scholar] [CrossRef] [Green Version]
- Cserr, H.F.; Knopf, P.M. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: A new view. Immunol. Today 1992, 13, 507–512. [Google Scholar] [CrossRef]
- Kida, S.; Pantazis, A.W.; Weller, R.O. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol. 1993, 19, 480–488. [Google Scholar] [CrossRef]
- Weller, R.O.; Djuanda, E.; Yow, H.Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1. [Google Scholar] [CrossRef]
- Davson, H.; Welch, K.; Segal, M.B. Physiology and Pathophysiology of the Cerebrospinal Fluid; Churchill Livingstone: Edinburgh, UK, 1987; p. 189. [Google Scholar]
- Johanson, C.E.; Duncan, J.A.; Klinge, P.M.; Brinker, T.; Stopa, E.G.; Silverberg, G.D. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cereb. Fluid Res. 2008, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Absinta, M.; Ha, S.K.; Nair, G.; Sati, P.; Luciano, N.J.; Palisoc, M.; Louveau, A.; Zaghloul, K.A.; Pittaluga, S.; Kipnis, J.; et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife 2017, 6, e29738. [Google Scholar] [CrossRef]
- Ma, Q.; Ineichen, B.V.; Detmar, M.; Proulx, S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017, 8, 1434. [Google Scholar] [CrossRef] [Green Version]
| DCLN Groups | Lenght (mm) Mean, SD, raNge | Width (mm) Mean, SD, Range | Weight (mm) Mean, SD, Range |
|---|---|---|---|
| IIA | 17.09 ± 4.20 (14.42–22.05) | 6.50 ± 1.38 (4.47–8.21) | 4.25 ± 0.86 (3.00–5.29) |
| IIB | 7.99 ± 5.33 (3.12–17.27) | 6.30 ± 1.98 (2.71–9.01) | 3.28 ± 1.03 (2.19–4.48) |
| III | 12.30 ± 5.42 (4.11–20.41) | 8.72 ± 2.43 (5.41–13.79) | 4.48 ± 2.06 (0.91–7.91) |
| IV | 9.65 ± 1.54 (8.08–12.21) | 4.50 ± 1.28 (3.00–6.54) | 3.37 ± 1.20 (1.71–5.00) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yağmurlu, K.; Sokolowski, J.D.; Çırak, M.; Urgun, K.; Soldozy, S.; Mut, M.; Shaffrey, M.E.; Tvrdik, P.; Kalani, M.Y.S. Anatomical Features of the Deep Cervical Lymphatic System and Intrajugular Lymphatic Vessels in Humans. Brain Sci. 2020, 10, 953. https://doi.org/10.3390/brainsci10120953
Yağmurlu K, Sokolowski JD, Çırak M, Urgun K, Soldozy S, Mut M, Shaffrey ME, Tvrdik P, Kalani MYS. Anatomical Features of the Deep Cervical Lymphatic System and Intrajugular Lymphatic Vessels in Humans. Brain Sciences. 2020; 10(12):953. https://doi.org/10.3390/brainsci10120953
Chicago/Turabian StyleYağmurlu, Kaan, Jennifer D. Sokolowski, Musa Çırak, Kamran Urgun, Sauson Soldozy, Melike Mut, Mark E. Shaffrey, Petr Tvrdik, and M. Yashar S. Kalani. 2020. "Anatomical Features of the Deep Cervical Lymphatic System and Intrajugular Lymphatic Vessels in Humans" Brain Sciences 10, no. 12: 953. https://doi.org/10.3390/brainsci10120953
APA StyleYağmurlu, K., Sokolowski, J. D., Çırak, M., Urgun, K., Soldozy, S., Mut, M., Shaffrey, M. E., Tvrdik, P., & Kalani, M. Y. S. (2020). Anatomical Features of the Deep Cervical Lymphatic System and Intrajugular Lymphatic Vessels in Humans. Brain Sciences, 10(12), 953. https://doi.org/10.3390/brainsci10120953





